1. Introduction
Alunogen is a hydrated aluminium sulfate with the formula, Al
2(SO
4)
3×17H
2O, regarded as grandfathered mineral species because known since 1832 [Beudant, 1832]. Alunogen is rather widely distributed mineral reported as acid mine drainage mineral and low-temperature fumarolic mineral or pseudofumarolic at coal fires [Košek et al., 2018; Bariand et al., 1977; Biagioni et al., 2020; Zhitova et al., 2018; Menchetti, Sabelli, 1974]. The early study of optical properties has shown that they considerably vary depending on H
2O content in the mineral [Larsen, Steiger, 1928]. Rather detailed description of alunogen crystal forms appeared as a result of the Chilian mineralogical expedition of 1938 [Gordon, 1942]. In this study [Gordon, 1942] authors found crystals of alunogen in small cavities where alums were dissolved and note that the true alunogen is colourless and triclinic which readily dehydrated to white monoclinic mineral designated as meta-alunogen. The first crystal structure refinement of alunogen [Menchetti, Sabelli, 1974] was carried out on crystals from Grotta dell'Allume (or "Alum Cave"), Vulcano Island, Italy (symmetry and unit-cell parameters are given in
Table 1) and allowed determination of Al, S and O positions (two Al sites; three S sites and twenty-nine O sites). For clarity we need to note that Grotta dell'Allume locality was representing a tectonic cavity in the volcanic tuff in close proximity to the sea and on the island where the active Vulcano volcano (Italy) is located [Forti et al., 1994]. The conditions of the mineral formation there are more exotic than usually and include a reaction of volcanic fluid with volcanic tuff in the contact with sea water [Forti et al., 1994]. The first structural study of alunogen [Menchetti, Sabelli, 1974] confirmed variation of the H
2O content and converged to the structural formula, Al
2(SO
4)
3×16.4H
2O. The symmetry of the mineral (
Table 1) and the position of the main atomic sites (for Al, S and O atoms) were confirmed in the result of subsequent structural refinement, performed a couple of years later [Fang, Robinson, 1976]. The refinement converged to the formula, Al
2(SO
4)
3×17H
2O [Fang, Robinson, 1976]. The crystal structure of alunogen consists of isolated SO
4 tetrahedra and isolated Al(H
2O)
6 polyhedra interconnected by hydrogen bonding. The H
2O molecules can be separated to two types: (i) those occurring in cavities and being of “zeolitic” nature (5 per formula unit) and (ii) those coordinating Al octahedra (12 per formula unit: 6 for each of two Al sites). In both studies, only oxygen atoms are localized for H
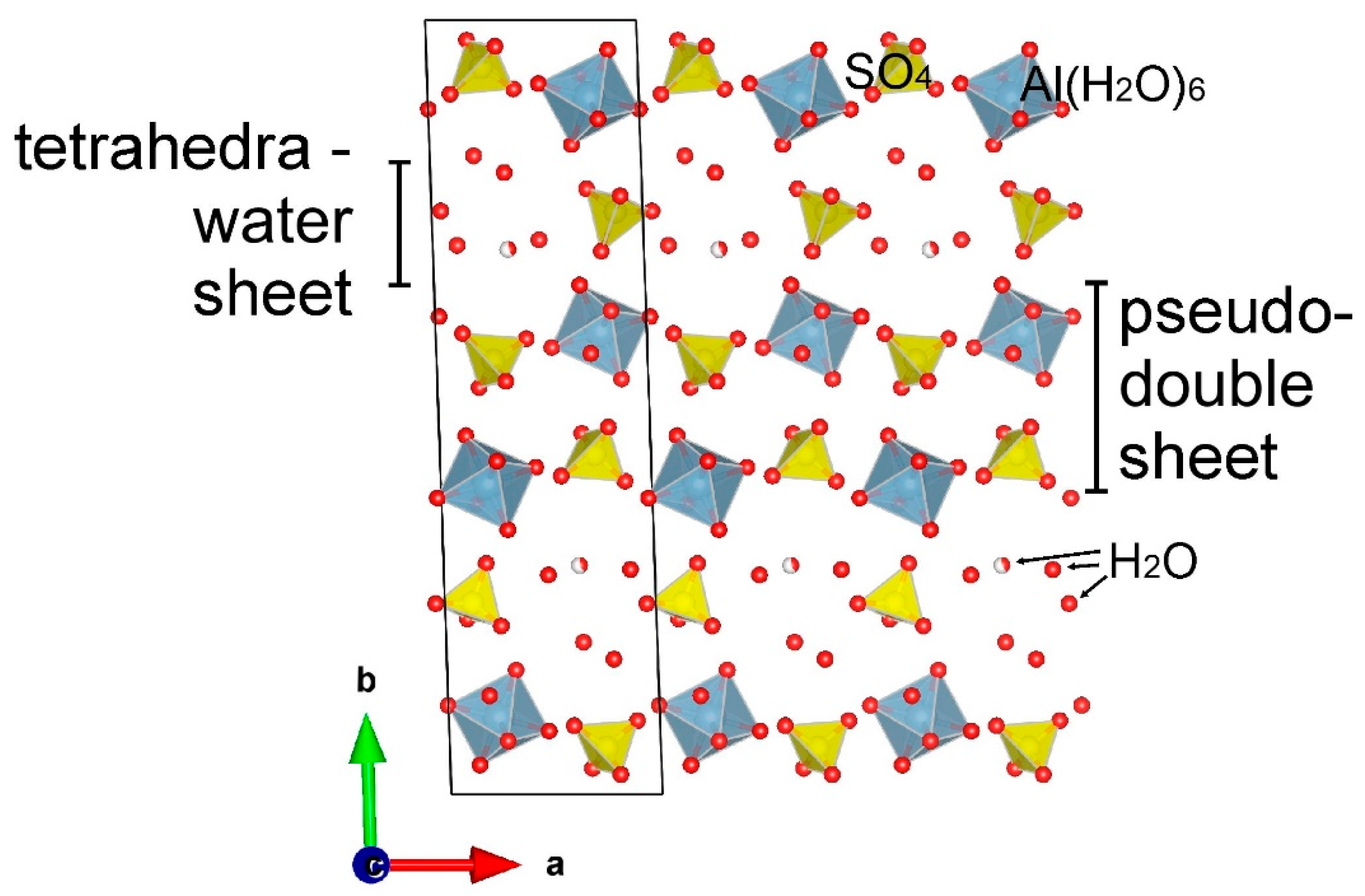
2O molecules. The crystal structure of alunogen is described [Fang, Robinson, 1976] as consisting of pseudo-double sheets parallel to (010) alternating with tetrahedra-water sheet composed of one SO
4 tetrahedra surrounded by H
2O molecules. This leads to perfect cleavage in sheet stacking direction and formation of tabular crystals.
Figure 1.
The crustal structure of alunogen obtained by [Menchetti, Sabelli, 1974].
Figure 1.
The crustal structure of alunogen obtained by [Menchetti, Sabelli, 1974].
Fang and Robinson [Fang, Robinson, 1976] noted that O sites of H
2O molecules are restricted to 5 leading to 17 H
2O molecules (12 H
2O molecules coordinating Al and other 5 H
2O “zeolite” molecules occurring in sulfate-water sheet) per formula unit. Then the crystal structure of alunogen was obtained on synthetic material [Sun et al., 2015] and topologically agreed with previous observation although the unit-cell setting was different leading to the difference in the unit-cell parameters (
Table 1). The crystal structure of synthetic material was supplemented by localization of H atoms [Sun et al., 2015] but according to our analysis geometries of some H
2O molecules do not seem crystal chemically reasonable. Assumptions that Al sulfates are one of the main minerals of Martian Al-bearing soils [Bibring et al. 2006; Swayze et al. 2008; Kounaves et al. 2010; Bish et al. 2013] triggered structure research of alunogen under low- and high-temperature conditions and different moistures [Kahlenberg et al., 2015, 2017] carried out on synthetic samples. This research has shown that under Mars relevant conditions (low temperature) alunogen undergoes a phase transition to low-temperature monoclinic modification (
Table 1), while at slightly elevated temperature of ~ 90 °C partly dehydrated triclinic alunogen-like (or meta-alunogen) material with the formula, Al
2(SO
4)
3(H
2O)
12·1.8H
2O, occurred (
Table 1). Finally, we note, that selenate analogue of alunogen, Al
2(SeO
4)
3(H
2O)
16, was reported in monoclinic space group
P2
1 (
Table 1) [Krivovichev, 2006].
Thus, we can conclude that all previous structural studies carried out for natural and synthetic alunogen have shown the variability of the alunogen framework in relation to the number of “zeolite” H2O molecules, as well as the possibility of changing the symmetry and unit-cell parameters of the mineral depending on the composition, mainly determined by the H2O content, which presumably may even correspond not to alunogen, but to its meta-forms. At the same time, alunogen is a common mineral in geothermal fields, which often act as a proxy for Martian conditions. In particular, alunogen is widely distributed in the geothermal fields of Kamchatka [Zhitova et al., 2018, 2022, 2023a,b], which is one of the most active volcanic areas in the world and belongs to the zone of the Pacific Ring of Fire. At the same time, the crystal structure of alunogen from geothermal fields worldwide or from Kamchatka has not been previously studied. Our work is intended to fill this gap and analyse the crystal structure of natural alunogen from the standpoint of modern X-ray diffraction analysis.
2. Materials and Methods
2.1. Materials
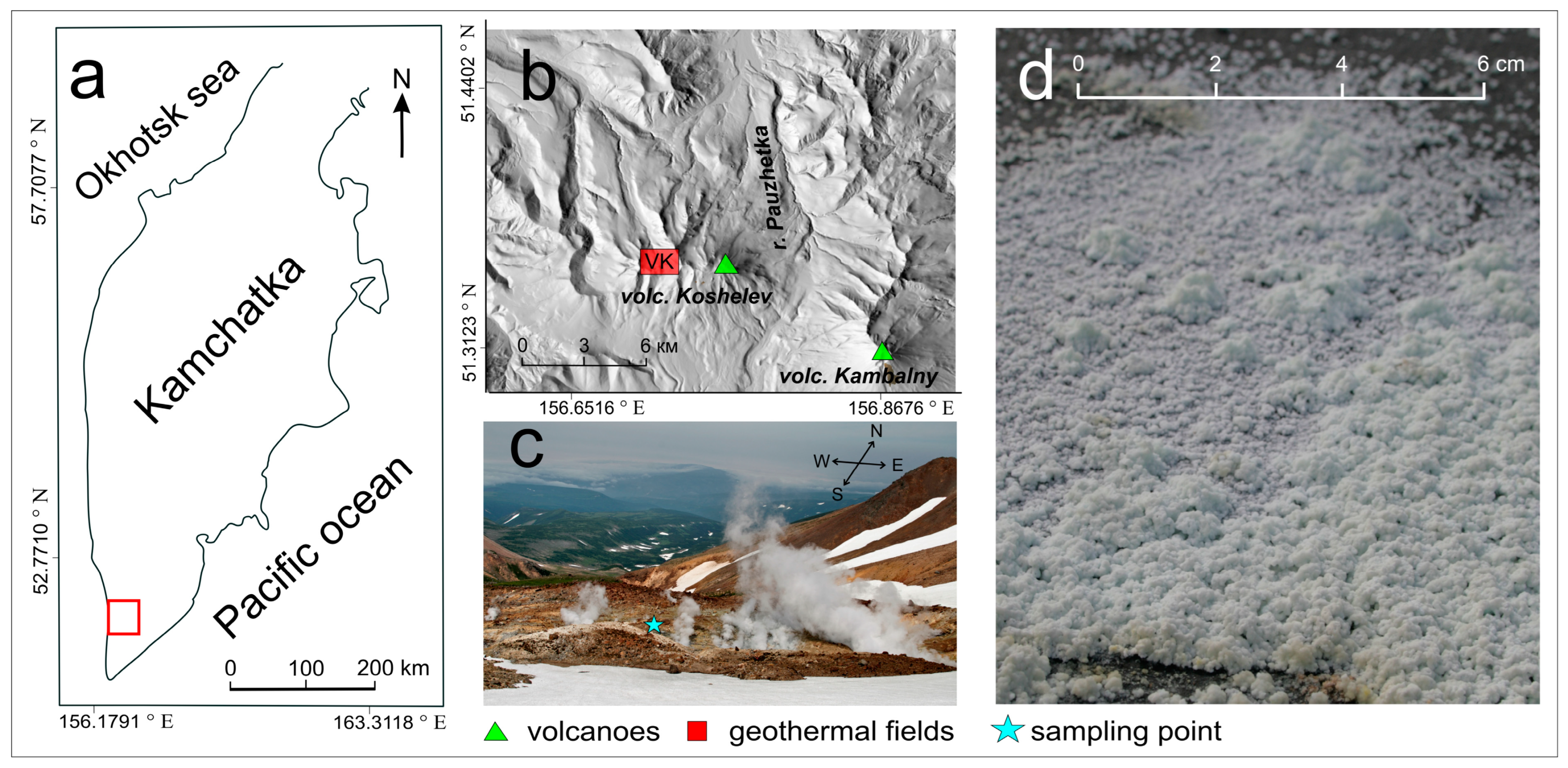
In this study, we investigated the sample of alunogen collected from the surface of Verkne-Koshelevsky geothermal field associated with Koshelev volcano (South Kamchatka) (
Figure 2a). South Kamchatka is a part of the Kuril-Kamchatka Island arc system, it is characterized by high tectonic-magmatic activity, which is expressed on the surface by modern volcanism. The Verkhne-Koshelevsky geothermal field is located in the central part of the Koshelev volcanic complex (
Figure 2b) in an erosion crater at absolute heights of 1200–1250 meters. On the area of the field, especially in its northern part, large-block deluvial deposits are developed and, almost throughout the entire area of the field, argillic rocks are widely developed, which are the product of hydrothermal transformation of basaltic andesites. Thermal manifestations are represented by steam-gas jets (
Figure 2c), water-mud boilers, hot springs and lakes, steaming ground. Thermal waters are characterized by temperature up to 95 °C, pH = 1.9–4.9, Eh = +60…+325 mV [Vakin et al., 1976; Kalacheva et al., 2016].
The sample was collected from the surface of 40 × 40 cm. The temperature of the ground in the sampling point was slightly elevated (retrospectively rated as ~ 40 °C) since the measured temperature at the depths of 30 cm was 80 °C. The sample was represented by white polymineral efflorescent crust up to 1.5 cm thick (
Figure 2d) composed of different hydrated sulfate minerals. In the crust colourless tabular crystals of alunogen were separated using binocular microscope and subjected to structure study. The associated minerals include Fe-Al and Fe sulfates: halotrichite, metavoltine and voltaite-group mineral. The efflorescent was covering pyrite-rich zone of the argillizites (
Figure 2d). The description of halotrichite from this locality is provided recently [Zhitova et al., 2023a,b].
2.1. Methods
2.1.1. Scanning electron microscopy and Energy-dispersive X-ray spectroscopy
The chemical composition of alunogen was analyzed at the “Geomodel’" Resource Center of the Scientific Park of St. Petersburg State University on a Hitachi S-3400N scanning electron microscope, equipped with an Oxford X-Max 20 energy-dispersive spectrometer, at an accelerating voltage of 20 kV, a probe current of 0.5 nA with various electron beam diameters of minimum 5 μm due to fast dehydration of alunogen under the electron beam. The spectrometer was calibrated against the set of natural standards (MAC standards). The alunogen plates were deposited on carbon tape, carbon-coated and analyzed by SEM and EDS. The previous studies of hydrated metal sulfates and fumarolic minerals has indicated an advantage of using the energy-dispersive mode instead of the wave-dispersive mode for their analysis [Balic-Žunic et al., 2016; Kruszewski, 2013; Zhitova et al., 2022] due to lower probe current and shorter time of analysis that both contribute to the preservation of material under study. In addition, energy-dispersive spectroscopy (EDS) allows analysing small-size grains of distinct minerals found in intimate association and in situ control of the sample condition during the spectrum acquisition.
2.1.2. Single-crystal X-ray diffraction
Single-crystal X-ray diffraction analysis was carried out for alunogen using a four-circle diffractometer Rigaku XtaLAB Synergy-S (Oxford Diffraction, Japan) operated with a monochromated Mo
Kα radiation (source: Mo
Kα, λ = 0.71073 Å) at 50 kV and 1.0 mA and equipped with a CCD HyPix-6000HE detector. The scan width was 1.0°, exposition was 130 s. Long exposure time is due to extremely small crystal thickness and relatively weak diffraction. The CrysAlisPro [CrysAlisPro, 2015] software package was used to process the data; an empirical absorption correction was calculated based on spherical harmonics implemented in the SCALES ABSPACK algorithm. Numerous attempts were undertaken in order to collect the single-crystal X-ray diffraction data of reasonable quality for structure refinement and hydrogen localization. These are because alunogen crystals are thin, maybe intergrown, twinned or low-crystalline. A photograph of the crystal on a nylon loop was taken during the analysis and given in the Supplementary File (
Figure S1a), as well as one of the frames of collected single-crystal X-Ray diffraction data (
Figure S1b). The parameters of data collection are listed in
Table 2.
The structure was solved and refined using the ShelX program package [Sheldrick, 2015] incorporated into the Olex2 software shell [Dolomanov et al., 2009] to R1 = 0.068 based on 5112 unique observed reflections with I > 2σ(I).
2.1.3. Structure complexity
The structural complexity of alunogen and related phases were estimated using the approach of numerical evaluation of structural complexity developed by Krivovichev [Krivovichev 2012, 2013]. The complexity of crystal structure can be quantitatively characterized by the amount of Shannon information measured in bits (binary digits) per atom (IG, bits/atom) and per unit cell (IG,total, bits/cell), respectively, according to the following equations:
where
k is the number of different crystallographic orbits (independent crystallographic Wyckoff sites) in the structure and
pi is the random choice probability for an atom from the
i-th crystallographic orbit, that is:
where
mi is a multiplicity of a crystallographic orbit (i.e. the number of atoms of a specific Wyckoff site in the reduced unit cell), and
v is the total number of atoms in the reduced unit cell. It worth to note, that the
IG value provides a negative contribution to the configurational entropy (
Scfg) of crystalline solids in accordance with the general principle that the increase in structural complexity corresponds to the decrease of configurational entropy [Krivovichev, 2016]. Structural complexity was calculated using previously published crystal structure models (
cif-files) listed in
Table 1 and program package ToposPro [Blatov et al., 2014].
3. Results
3.1. Chemical composition
The chemical composition of alunogen is given in
Table 3. The EDS spectra of alunogen shows presence of Al, S and O in its compositions. The contents of other elements with atomic numbers higher than that of C are below their detection limits. Alunogen dehydrates under vacuum conditions which is manifested by the cracking of crystals and a decrease in water content that is, an increase in the measured amount of Al
2O
3 and SO
3 oxides. The contents of Al
2O
3 and SO
3 oxides were measured. The H
2O content has been calculated taking into account the crystal structure data (see below). The analyses were normalized to 100 wt. %. The analyses (
Table 3) show a good agreement with alunogen stoichiometry since Al:S ~ 2:3
3.2. Crystal structure
The obtained data were processed in
P-1 space group,
a = 7.4194(3),
b = 26.9763(9),
c = 6.0549(2) Å, α = 90.043(3), β = 97.703(3), γ = 91.673(3) °,
V = 1200.41(7) Å
3,
Z = 2. Atom coordinates, site occupancies and isotropic displacement parameters are given in
Table 4. Selected bond lengths are listed in
Table 5. Hydrogen bonding scheme is given in
Table 6. Anisotropic displacement parameters are in Supplementary File (
Table S1). The crystallographic information file (cif) has been deposited via the joint Cambridge Crystal Data Centre CCDC/FIZ Karlsruhe deposition service.
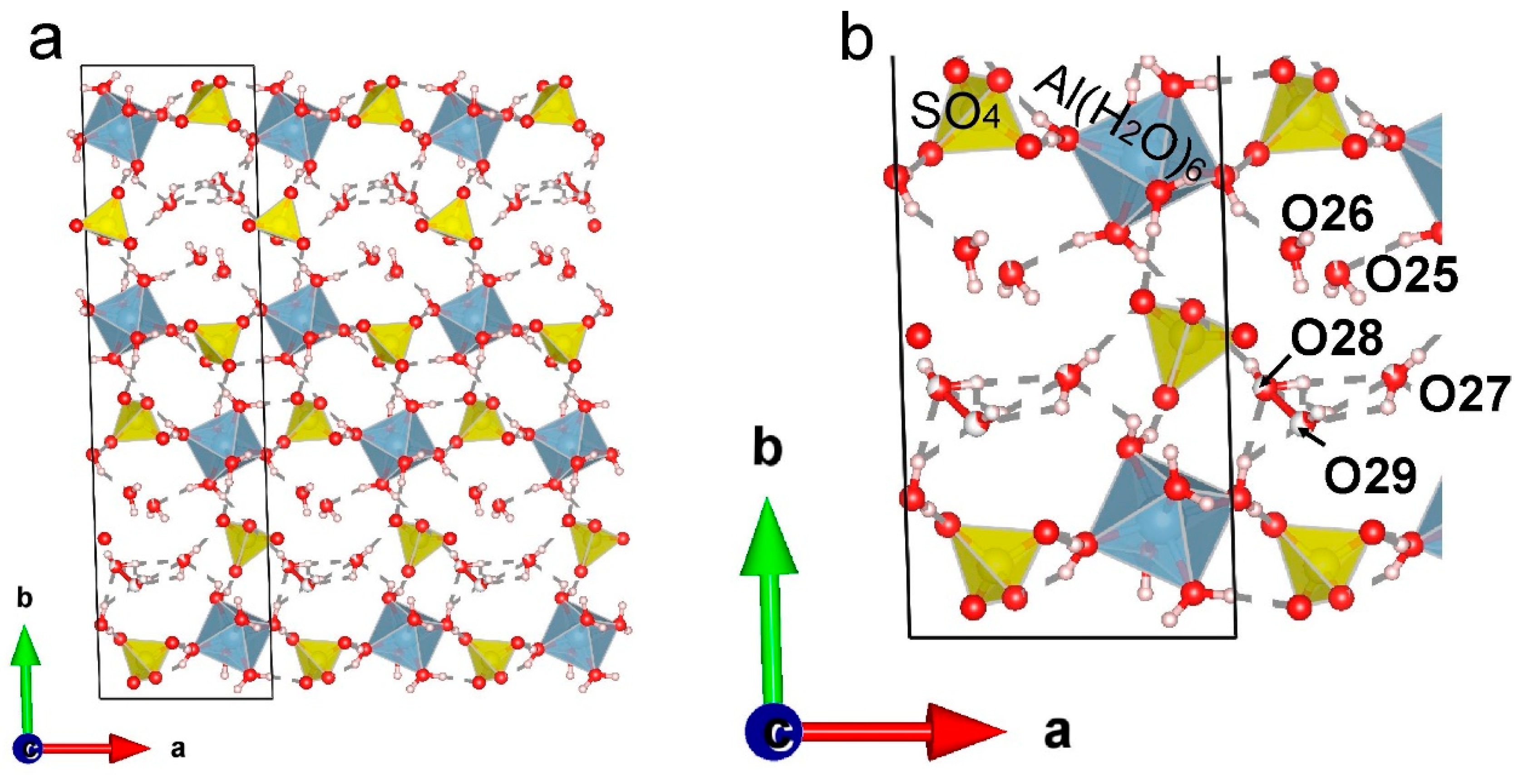
The two Al sites, three S sites and twenty-nine O sites were localized from the difference Fourier maps and refined anisotropically. Site occupancies of Al, S, O are given in
Table 4 and they are all fully occupied with the exception of two oxygen positions (Ow25 and Ow27) of “zeolite” H
2O molecules. The two-component twinning by matrix {-1 0 0 0 1 0 0 0 -1} was applied to the structure refinement. The hydrogen positions were localized from the difference Fourier maps for nine H
2O molecules of Al(H
2O)
6 octahedra or taking into account geometrical assumptions for other three H
2O molecules of Al(H
2O)
6 octahedra and for all four “zeolite” H
2O molecules. In the first case the O‒H distances were softly restrained as 0.9 Å. Some of O
…H and H
…H distances were also softly restrained as 2.4-2.5 Å. All thirty-four H sites were located (
Figure 3a) and refined isotropically with and Ueq refined freely for H atoms of nine out of twelve H
2O molecules of Al(H
2O)
6 octahedra and using a riding model with Ueq values restrained as 1.5 of donor O atom for H atoms of the other three H
2O molecules of Al(H
2O)
6 octahedra and for all “zeolite” H
2O molecules. Additionally, one “zeolite” H
2O molecule is disordered and split into two partially occupied sites: Ow28 and Ow29 (
Figure 3b) located at the distance of 1.56 Å with occupancies 0.65 and 0.35 correspondingly.
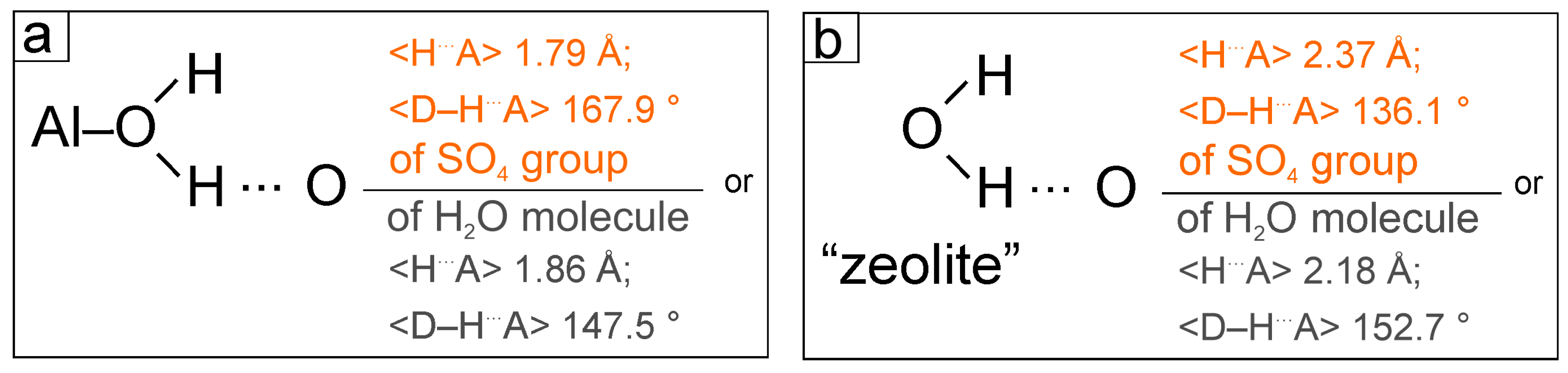
The hydrogen bonding scheme in alunogen is complex. The oxygen atoms labeled O1, O2…O12 are coordinating Al1 and Al2 octahedra being donors (
D) of two-center hydrogen bonds [Jeffrey, 1997]. The
D–H bonds are in the range 0.85-0.90 Å (
Table 6). The oxygen atoms of the sulfate tetrahedra and “zeolite” H
2O molecules act as an acceptor. For the case when the oxygen of the sulfate group is the acceptor (
A), the following H
…A distances are characteristic 1.70–1.92 Å and
D–H
…A angles in the range 147.8–179.5 º (with averaged values as 1.79 Å and 167.9 º, respectively). When oxygen of “zeolite” H
2O molecules is an acceptor, the H
…A distances range from 1.72 to 2.02 Å and
D–H
…A angles are in the range 125.4–163.9 º (with averaged values as 1.86 Å and 147.5 º, respectively).
The oxygen atoms labeled Ow25, Ow26, Ow27 and Ow28 + Ow29 (split) belong to “zeolite” H2O molecules. For this type of H2O molecules, the D–H bonds are ~ 0.85 Å. The oxygen atoms of SO4 tetrahedra and symmetrically independent “zeolite” H2O molecules act as acceptors. In case when O atoms of SO4 are acceptors the H…A distances range from 1.99 to 2.72 Å (with the average value of 2.37 Å) and D–H…A angles are in the range 108.1–174.7 º (with averaged value of 136.1 °). When oxygen of “zeolite” H2O molecules is an acceptor, the H…A distances range from 1.84 to 2.55 Å (average value is 2.18 Å) and D–H…A angles ranging from 120.4 to 176.4 ° (average value is 152.7 °).
An analysis of the distribution of hydrogen bonds shows that a stronger H…A bonds observed for H2O molecules coordinating Al sites. At the same time, the sulfate group is a stronger acceptor resulting in a stronger hydrogen bond between the Al(H2O)6 octahedra and the SO4 tetrahedra. “Zeolite” H2O molecules are characterized by longer (and hence weaker) bonds with acceptors. Moreover, in case of “zeolite” H2O molecule a stronger hydrogen bond is formed with other symmetrically independent “zeolite” H2O molecules rather than SO4 tetrahedra. The formation of stronger hydrogen bonds between “zeolite” H2O molecules rather than its bonding with other structural units, in our opinion, explains the possibility of alunogen dehydration and possible formation of meta-alunogen.
3.1. Structure complexity
Crystal structure complexity of alunogen and related phases is shown in
Table 7. The parameters
IG and
IG,total correspond to the complexity calculated for the structural models per atom and per unit cell. Examination of the values in the
Table 7 shows their division into two groups: (i)
IG ~ 5.0–5.1 bits/atom and
IG,total ~ 333–346 bits/cell and (ii)
IG ~ 6.0–6.1 bits/atom and
IG,total ~ 783–828 bits/cell. Such a difference is due to the fact that earlier structure refinements do not contain H positions. Thus, hydrogen atoms contribute to more than a half of structure complexity of alunogen that is in line with high hydration state of alunogen having ~ 45 wt. % of H
2O (
Table 2).
Table 7 also shows obvious correlation between number of O sites corresponding to “zeolite” H
2O molecules. For example, considering the crystal structures with non-localized H atoms, the complexity per unit cell is 333 bits/cell for selenate alunogen with four O sites corresponding to “zeolite” H
2O molecules and 346 bits/cell for natural alunogens having five fully or partly occupied O sites corresponding to “zeolite” H
2O molecules. The same applies to the structures with localized H sites: the synthetic alunogen with
n (number of “zeolite” H
2O molecules) = 4 has complexity per unit cell as 783 bits/cell, while low-temperature monoclinic modification with
n = 4.8 has 828 bits/cell. In our structure refinement
n = 3.8, but one of “zeolite” H
2O molecules is split into two with partial occupancy that leads to the identical complexities of our sample and low-temperature modification. This is also interesting to note that transformation from triclinic to monoclinic symmetry is not outlined by a change of structure complexity. This is because the structure topology is preserved. We were unable to find a cif-file for dehydrated modification of alunogen. However, we can assume that it should have lower complexity since it has lower content of “zeolite” H
2O molecules.
4. Discussion
In our study alunogen comes from Fe-rich (both ferric and ferrous) environment and found in intimate association with other Fe or Fe-Al hydrated sulfates. At the same time, Fe3+ does not incorporate in the structure of alunogen studied therein. Following ideas of Fang and Robinson [Fang, Robinson, 1976] we conclude that the difference in the ionic size and polarization power should be responsible for that rather than non-availability of Fe3+. We can assume that even if some (limited) Al to Fe3+ substitution occurs in individual alunogen crystals, it should have a negative effect on the crystallinity of the alunogen precluding its structural study. It is interesting to note, that according to our data, limited Al to Fe3+ substitution occurred in other hydrated sulfates from geothermal fields: alums and alunite/jarosite-group minerals where formation of Fe3+- or Al-dominant species in intimate association was observed rather than their full solid solution series [Zhitova et al., 2022] and halotrichite where Al site was free of impurities or contained limited Fe3+ [Zhitova et al., 2023a,b]. If terrestrial geothermal fields are indeed a proxy for Martian conditions [Pirajno, Van Kranendonk, 2005], then there should be not only iron sulfates, already well known for Mars, but also aluminum sulfates, and it can be assumed that alunogen or its modifications maybe widespread (following mineral formation at geothermal fields). The alunogen described by us contains the smallest amount of “zeolite” H2O molecules (3.8 apfu vs 4–5 apfu) among structurally characterized natural and synthetic minerals. Despite the structural study of alunogen, including low- and high-temperature modifications [Kahlenberg et al., 20015, 2017], the question seems to be open on whether alunogen with different content of “zeolite” H2O molecules will behave identically under low-temperature conditions that are modelling Martian environments?
In this work we provide modern crystal structure refinement of alunogen including the first localization of thirty-four hydrogen sites. Each of Al and S sites has shown 100 % occupancy. The structure consists of isolated Al(H
2O)
6 octahedra, SO
4 tetrahedra and “zeolite” H
2O molecules that are connected to the three-dimensional network by hydrogen bonds. The hydrogen atoms form two-centre hydrogen bonds of Al(H
2O)
6 octahedra and “zeolite” H
2O molecules. Four sites corresponding to “zeolite” H
2O molecules were localized (including O and H atoms) with one “zeolite” H
2O molecule being disordered and split. Analysis of hydrogen bonding network explains possibility of alunogen dehydration since independent “zeolite” H
2O molecules form stronger hydrogen bonding to each other rather than to other acceptors. The detailed information on hydrogen bonding network should be helpful for band assignments in the vibration spectra of alunogen and its modifications that is in demand in connection with studies of Martian mineralogy by rovers and the development of remote and express methods of terrestrial minerals identification [Košek et al., 2018]. In general, crystal structure of alunogen shows variability only in the content of “zeolite” H
2O molecules outlined by the consistency of the unit-cell volume (
Table 1 and
Table 2) that possibly can be regarded as structure inflexibility towards isomorphic substitution.
Hydrogen sites contribute significantly to the structure complexity of alunogen increasing structure complexity per unit cell from 346 to 828 bits/cell. An increase in structure complexity by a factor of two or more due to hydrogen positions is also characteristic of other hydrated sulfates occurring in association with alunogen [Zhitova et al., 2023a]. This is because the crystal structure of alunogen and other hydrated sulfates from the same association (e.g. halotrichite, alum-group minerals [Zhitova et al., 2019, 2022]) is built of isolated units (octahedra, tetrahedra and H2O molecules) connected by hydrogen bonds. As suggested previously highly hydrated minerals are good candidates for better understanding of ionic species existing in solution [Fang, Robinson, 1976]. In general, low polymerization of structural units is characteristic for the main efflorescent minerals growing from hydrothermal solution, including alunogen. Taking into account the abundance of alunogen at geothermal fields and recent experiments of transformation of alunogen to meta-alunogen at 40-80 °C [Kahlenberg et al., 2015] we consider that possibility of meta-alunogen occurrence at geothermal and/or fumarole fields is high despite questionable mineral status of meta-alunogen.
5. Conclusions
We consider the crystal structure study of alunogen from Verkhne-Koshelevsky geothermal field is an important outcome for characterization of naturally occurring highly hydrated sulfate minerals and their possible modifications. The chemical specificity of alunogen from geothermal field are absence of impurities (including Fe3+ despite Fe2+,3+-rich environment) and lower H2O content in comparison to previously structurally characterized natural and synthetic alunogens. From the structural point of view alunogen studied in this work is isotypic to previously reported structures of alunogen. However, in addition to previous studies we were able to localize hydrogen atoms and characterize complex hydrogen bonding network of the mineral. This data maybe helpful for detailed assignment of band positions in the vibrational spectra and useful for Mars mineralogical missions. Hydrogen atoms increase structure complexity per unit cell more than twice similarly to other highly hydrated sulfates found in association with alunogen. Combining the recently obtained experimental data on the transition of alunogen to meta-alunogen at temperature ~ 40–80 °C and our field temperature measurements at geothermal and fumarole fields, we assume that the findings of meta-alunogen under low-temperature volcano-related conditions are very likely. At the same time, the structural study of alunogen from geothermal fields can be a starting point in the search and characterization of meta-alunogen or its possible structural varieties in nature.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, ESZ and RMS; methodology, RMS; software, ESZ, RMS, AAZ, AAN; validation, ESZ, RMS, AAZ, AAN; formal analysis, ESZ, RMS, AAZ; investigation, ESZ, RMS, AAN; resources, AAN; data curation, RMS, AAZ; writing—original draft preparation, ESZ, RMS, AAZ, AAN; writing—review and editing, ESZ, RMS, AAZ, AAN; visualization, ESZ; supervision, ESZ, AAZ; project administration, ESZ; funding acquisition, ESZ. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 22-77-10036.
Data Availability Statement
The crystallographic information file (cif) has been deposited via the joint Cambridge Crystal Data Centre CCDC/FIZ Karlsruhe deposition service.
Acknowledgments
The technical support of St. Petersburg State University Resource Centers “XRD” and “Geomodel” is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beudant, F.S. Alunogène, sulfate d’alumine. In Traité Élémentaire de Minéralogie, 2nd Edition ed; 1832; pp. 488–492. [Google Scholar]
- Košek, F.; Culka, A.; Žáček, V.; Laufek, F.; Škoda, R.; Jehlička, J. Native alunogen: A Raman spectroscopic study of a well-described specimen. J. Mol. Struct. 2018, 1157, 191–200. [Google Scholar] [CrossRef]
- Bariand, P.; Cesbron, F.; Berthelon, J-P. Les sulfates de fer de Saghand près de Yazd (Iran). Mémoire Hors-Série de la Société Géologique de France. 1977, 8, 77–85. [Google Scholar]
- Biagioni, C.; Mauro, D.; Pasero, M. Sulfates from the pyrite ore deposits of the Apuan Alps (Tuscany, Italy). A review. Minerals 2020, 10, 1092. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Siidra, O.I.; Belakovsky, D.I.; Shilovskikh, V.V.; Nuzhdaev, A.A.; Ismagilova, R.M. Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18, a new mineral from the Severo-Kambalny geothermal field, Kamchatka, Russia. Mineral. Mag. 2018, 1057–1077. [Google Scholar] [CrossRef]
- Menchetti, S.; Sabelli, C. Alunogen. Its structure and twinning. Tschermaks Mineral. Petrogr. Mitt. 1974, 21, 164–178. [Google Scholar] [CrossRef]
- Larsen, E.S.; Steiger, G. Dehydration and optical studies of alunogen, nontronite and griffithite. Am. J. Sci. 1928, 15, 1–19. [Google Scholar] [CrossRef]
- Gordon, S.G. Results of the Chilean mineralogical expedition of 1938. Part VII. The crystallography of alunogen, meta-alunogen and pickeringite. Notulae Naturae of The Academy of Natural Sciences of Philadelphia 1942, 101, 1–9. [Google Scholar]
- Forti, P.; Panzica La Manna, M.; Rossi, A. The peculiar mineralogic site of the Alum cave (Vulcano, Sicily). In Proceedings of the 7th International Symposium on Vulcano speleology, Canary Islands, Spain, November 1994; pp. 35–44. [Google Scholar]
- Fang, J.H.; Robinson, P.D. Alunogen, Al2(H2O)12(SO4)3·5H2O: Its atomic arrangement and water content. Am. Min., 1976, 61, 311–317. [Google Scholar]
- Sun, X.; Sun, Y.; Yu, J. Crystal structure of aluminum sulfate hexadecahydrate and its morphology. Cryst. Res. Technol., 2015, 50, 293–298. [Google Scholar] [CrossRef]
- Bibring, J.-P.; Langevin, Y.; Mustard, J.F.; Poulet, F.; Arvidson, R.; Gendrin, A.; Gondet, B.; Mangold, N.; Pinet, P.; Forget, F. Global mineralogical and aqueous Mars history derived from OMEGA/Mars express data. Sci., 2006, 312, 400–404. [Google Scholar] [CrossRef]
- Swayze, G.A.; Ehlmann, B.L.; Milliken, R.E.; Poulet, F.; Wray, J.J.; Rye, R.O.; Clark, R.N.; Desborough, G.A.; Crowley, J.K.; Gondet, B.; Mustard, J.F.; Seelos, K.D.; Murchie, S.L. Discovery of the acid-sulfate mineral alunite in Terra Sirenum, Mars, using MRO CRISM: Possible evidence for acid-saline lacustrine deposits? EoS Transactions of the American Geophysical Union, Fall Meeting Supplement, 2008, 89, Abstract P44A-04. [Google Scholar]
- Kounaves, S.P.; Hecht, M.H.; Kapit, J.; Quinn, R.C.; Catling, D.C.; Clark, B.C.; Ming, D.W.; Gospodinova, K.; Hredzak, P.; McElhoney, K.; Shusterman, J. Soluble sulphate in the martian soil at the Phoenix landing site. Geophys. Res. Lett., 2010, 37, L09201. [Google Scholar] [CrossRef]
- Bish, D.L.; Blake, D.F.; Vaniman, D.T.; Chipera, S.J.; Morris, R.V.; Ming, D.W.; Treiman, A.H.; Sarrazin, P.; Morrison, S.M.; Downs, R.T.; and others and the MSL Science Team. X-ray diffraction results from Mars Science Laboratory: Mineralogy of Rocknest at Gale Crater. Sci., 2013, 341, 1238932. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, V.; Braun, D.E.; Orlova, M. Investigations on alunogen under Mars-relevant temperature conditions: An example for a single-crystal-to-single-crystal phase transition. Am. Min. 2015, 100, 2548–2558. [Google Scholar] [CrossRef]
- Kahlenberg, V.; Braun, D.E.; Krüger, H.; Schmidmair, D.; Orlova, M. Temperature-and moisture-dependent studies on alunogen and the crystal structure of meta-alunogen determined from laboratory powder diffraction data. Phys. Chem. Miner., 2017, 44, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V. Crystal chemistry of selenates with mineral-like structures. I. (Al(H2O)6)2(SeO4)3(H2O)4—the selenate analog of alunogen. Zapiski RMO, 2006, 135, 106–113. (in Russian). [Google Scholar]
- Zhitova, E.S.; Siidra, O.I.; Belakovsky, D.I.; Shilovskikh, V.V.; Nuzhdaev, A.A.; Ismagilova, R.M. Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18, a new mineral from the Severo-Kambalny geothermal field, Kamchatka, Russia. Mineral. Mag., 2018, 82, 1057–1077. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Sheveleva, R.M.; Zolotarev, A.A.; Krivovichev, S.V.; Shilovskikh, V.V.; Nuzhdaev, A.A.; Nazarova, M.A. The crystal structure of magnesian halotrichite, (Fe,Mg)Al2(SO4)4·22H2O: hydrogen bonding, geometrical parameters and structural complexity. J. Geosci. 2023, accepted. [Google Scholar] [CrossRef]
- Sheveleva, R.M.; Nazarova, M.A.; Nuzhdaev, A.A.; Zhegunov, P.S.; Zhitova, E.S. Distribution and chemical composition of halotrichite from the geothermal fields of Kamchatka. Bulletin of Kamchatka Regional Association Educational-Scientific Center. Earth Sciences. accepted (in Russian). 2023. [Google Scholar] [CrossRef]
- Vakin, E.A.; Dekusar, Z.B.; Serezhnikov, A.I.; Spichenkova, M.V. The hydrothermal occurrences of the Koshelevskii volcanic massif. Hydrothermal Systems and Thermal Fields in Kamchatka, Sugrobov, V.M.; Eds.; Vladivostok: DVNTs AN SSSR, 1976. (in Russian) [Google Scholar]
- Kalacheva, E.G.; Rychagov, S.N.; Koroleva, G.P.; Nuzhdaev, A.A. ; The Geochemistry of Steam Hydrothermal Occurrences in the Koshelev Volcanic Massif, Southern Kamchatka. J. Volcanol. Seismol., 2016, 3, 41–56. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Garavelli, A.; Jakobsson, S.P.; Jonasson, K.; Katerinopoulos, A.; Kyriakopoulos, K.; Acquafredda, P. Fumarolic minerals: An overview of active European volcanoes. In Updates in Volcanology, from Volcano Modelling to Volcano Geology; Nemeth, K., Ed.; InTech: Rijeka, Croatia, 2016; pp. 267–322. [Google Scholar]
- Kruszewski, Ł. Supergene sulphate minerals from the burning coal mining dumps in the Upper Silesian Coal Basin, South Poland. Int. J. Coal Geol., 2013, 105, 91–109. [Google Scholar] [CrossRef]
- CrysAlisPro Software System, version 1. 171.38.46; Rigaku Oxford Diffraction: Oxford, UK, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Krivovichev, S. Topological complexity of crystal structures: quantitative approach. Acta Crystallogr. A, 2012, 68, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V. Structural complexity of minerals: information storage and processing in the mineral world. Mineral.Mag., 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals and mineral parageneses: Information and its evolution in the mineral world. In Highlights in mineralogical crystallography; Armbruster, T., Ed.; Danisi. R.M. Eds.; Walter de Gruyter GmbH: Berlin, Germany, 2016; Volume 1, pp. 31–73. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Crystal Growth & Design, 2014, 14, 3576–3586. [Google Scholar]
- Jeffrey, G.A.; Jeffrey, G.A. An introduction to hydrogen bonding, Vol. 12; Oxford university press: New York, USA, 1997; pp. 1–228. [Google Scholar]
- Pirajno, F.; Van Kranendonk, M.J. Review of hydrothermal processes and systems on Earth and implications for Martian analogues. Australian Journal of Earth Sciences, 2005, 52, 329–351. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Sergeeva, A.V.; Nuzhdaev, A.A.; Krzhizhanovskaya, M.G.; Chubarov, V.M. Tschermigite from thermal fields of Southern Kamchatka: High-temperature transformation and peculiarities of IR-spectrum. Zapiski RMO, 2019, 148, 100–116. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).