1. Introduction
The skin is the largest human organ, covering an area of approximately 1.8 m2, comprising three main layers: the epidermis as the outermost layer, the dermis, and the hypodermis. Skin protects the human body from several external harmful agents, reduces electrolytes loss, regulates evapotranspiration and body temperature, plus it consists of an immune defense barrier against microorganisms [
1]. It is estimated that half of adult population have developed some kind of skin ailment at one point in their lives, with 1/3 manifesting chronic or mild skin diseases. In fact, skin diseases represent a major concern affecting the quality-of-life of both children, teenagers, and adults [
2].
AD, also known as eczema, is a chronic relapsing inflammatory skin disorder, characterized by recurrent eczematous lesions and severe skin itching. This skin inflammatory disease, tends to appear mostly in the first five years of age, thus affecting near 30% of children and teenagers, in comparison to only 2-10% of adults’ population. Even though, it is currently assumed that AD may appear at any age. Besides the mental impact on patients’ life with increasing probability of depression and suicide, plus severe skin itching and pain, there are also many associated expensive costs, including skin cleaning products, appropriated clothes, creams, and ointments. Interestingly, the real pathogenic source of the disease is not yet fully comprehended, but main findings point for the interaction between three major mechanisms, that comprise skin structure defects, changes in the skin microbiome, and impairment of Th2 immune responses [
3,
4,
5,
6].
Nowadays, the clinical treatment for AD relies on topical application of corticosteroids, topical calcineurin inhibitors, antihistamines, antibiotics systemic immunosuppressors, and phototherapy. Despite of the effective treatment arising of such treatments, the derived side-effects of such approaches are an equally challenging reality. Among these undesired therapy outcomes, skin atrophy, striae, telangiectases, rosacea and acne, glaucoma, hyperglycemia, hypertension, are some examples of problems coming from corticosteroids’ topical application [
7,
8,
9]. On the contrary, natural products and derived isolated bioactive compounds are emerging alternatives to these synthetic drugs, given their high efficacy rates while encompassing reduced side-effects, plus the more cost-effective acquisition [
2]. Indeed, medicinal plants comprise the primary healthcare solutions of near 65% of worldwide population, and almost 80% of people inhabiting in developing countries [
1].
Natural products are the source of several, and highly heterogenous, molecules such as multiple phenolic rings-bearing compounds like flavonoids, tannins, and catechins, nitrogen-containing molecules like alkaloids, carotenoids, polysaccharides, and small volatile molecules like those found in essential oil-bearing plants [
2]. Interestingly, their anti-inflammatory properties in the treatment of skin inflammatory-based diseases have been depicted, as it for vitiligo [
10], psoriasis [
11], and more important AD [
9]. Recent research suggest that these molecules exert an antioxidant activity, improving cells’ redox status, that in turn ameliorates the inflammatory response, by suppressing the activity of key regulators in mitogen-activated protein kinases (MAPK), and nuclear factor kappa-light-chain-enhancer of activated B cells NF-κB signaling pathways, which are key molecular inflammatory responses [
8]. To overcome some transdermal delivery problems, augment drug-to-site targeting, and efficacy of such natural ingredients in AD treatment, some nanotechnology-based solutions have been developed in the last years. As successful examples that are herein reviewed , investigators designed quercetin nanostructured lipid carriers [
12], solid lipid nanoparticles loaded with capsaicin, curcurmin and resveratrol [
13], nanoparticles of epigallocatechin-3-gallate [
14,
15], transfersomes loaded with glycyrrhizic acid [
16], innovative phytosomes with
Centella asiatica (L.) Urban extracts [
17] , nanocapsules-based films of pomegranate seed oil [
18], and ethosomes-based cream of tea tree oil [
19], and several other formulations presented throughout this manuscript.
In this review, we focused on natural isolated compounds and plant-based extracts/mixtures and oils, that have been included in the last years into nanotechnology-based formulations for the treatment of AD. Some background information about the disease pathophysiology, and nanotechnology tools available to treat it, are provided for a comprehensive interpretation of the topics here included. Besides that, for each natural product and nanosystem herein revised, some pharmacological activity insights, natural products’ physicochemical features and major natural sources, were summarized. As far as we know, this is the first review that focused on describing, the most recent and innovative, nanotechnology-based formulations loaded with natural products for AD treatment. Therefore, in this manuscript information about nanotechnology-based formulations, loaded with natural isolated compounds are reviewed in section 4, and key information summarized in
Table 1 and
Table 2. Similarly, the extracts, oils, and plant mixtures are presented in section 5, with relevant information on this topic gathered in
Table 3 and
Table 4.
2. Materials and Methods
A comprehensive analysis of upcoming surveys regarding the application of nanotechnology-based formulations into the delivery of natural products for AD treatment, was herein carried out. In this review, an extensive revision of the literature was made regarding a lifespan of ten years, from 2013 to 2023. The search was performed in databases, such as ScienceDirect, Scopus, PubMed, Web of Science and Google Scholar. The following keywords were applied individually and/or in combination: atopic dermatitis, eczema, inflammation, skin, natural products, natural compounds, alkaloids, phenolic compounds, flavonoids, terpenes, polysaccharides, oils, plant extract, drug delivery, nanosystems, formulation, nanotechnology. After screening the literature, 16 natural isolated compounds, and 8 plant-derived extracts/mixtures and oils, have been in-depth investigated for their pharmacological activity on AD, and respective nanoformulation-based drug delivery systems, that have been recently reported.
3. Pathophysiology of AD
Among inflammatory skin disorders, AD is one of the most common ones, affecting between 15 to 30% of children and up to 10% of adults in high-income countries [
3,
4,
5,
6]. Atopic eczema and eczema are other names attributed to this condition, being interchangeable terms [
3]. AD is related with a null mutation in the filaggrin gene which compromises the
stratum corneum, changing the epidermal barrier function. This disfunction leads to an increased exposure to external irritants and allergens [
4]. This condition is characterized by itch and pain during the flares, leading to a huge impact in patient’s life, affecting growth, mental health, work productivity, and leading to other burdens like monetary ones[
3].
Atopy, the tendency to produce an exaggerated immunoglobulin E (IgE) immune response is characteristic of AD, asthma, and allergic rhinitis, thus the individuals that have AD usually have other of these conditions associated [
6]. There is not a direct test to diagnose AD, being confused with other similar skin conditions such as psoriasis and keratosis pilaris, with a complex diagnostic that may take years to be precise. In newborns AD usually manifests first during teething [
4].
A multidisciplinary approach is required to properly manage and treat AD. As children are the most affected, family education on this condition and especially how to prevent flares is important [
3]. Keeping the family history related to AD incidence, is of the most importance, because genetics alongside with the environment are decisive factors in this condition. Treatments aim fundamentally in restoring the skin barrier and control de abnormal immune responses. The first step in prevention is to avoid irritants and allergens [
6]. Using emollients twice a day hugely reduces the probability of flares. Treatment with corticosteroids should be considered only as a last option, especially in children, despite being safe under medical prescription. In severe cases immunosuppressants, as anti- interleukin-4 (IL-4), are a very efficient option that changed how AD is treated, but should be avoided in long term treatments [
6].
4. Isolated natural compounds included in nanotechnology-based formulations for the treatment of AD
4.1. Astaxanthin
4.1.1. Natural source, physicochemical features, and bioactive properties.
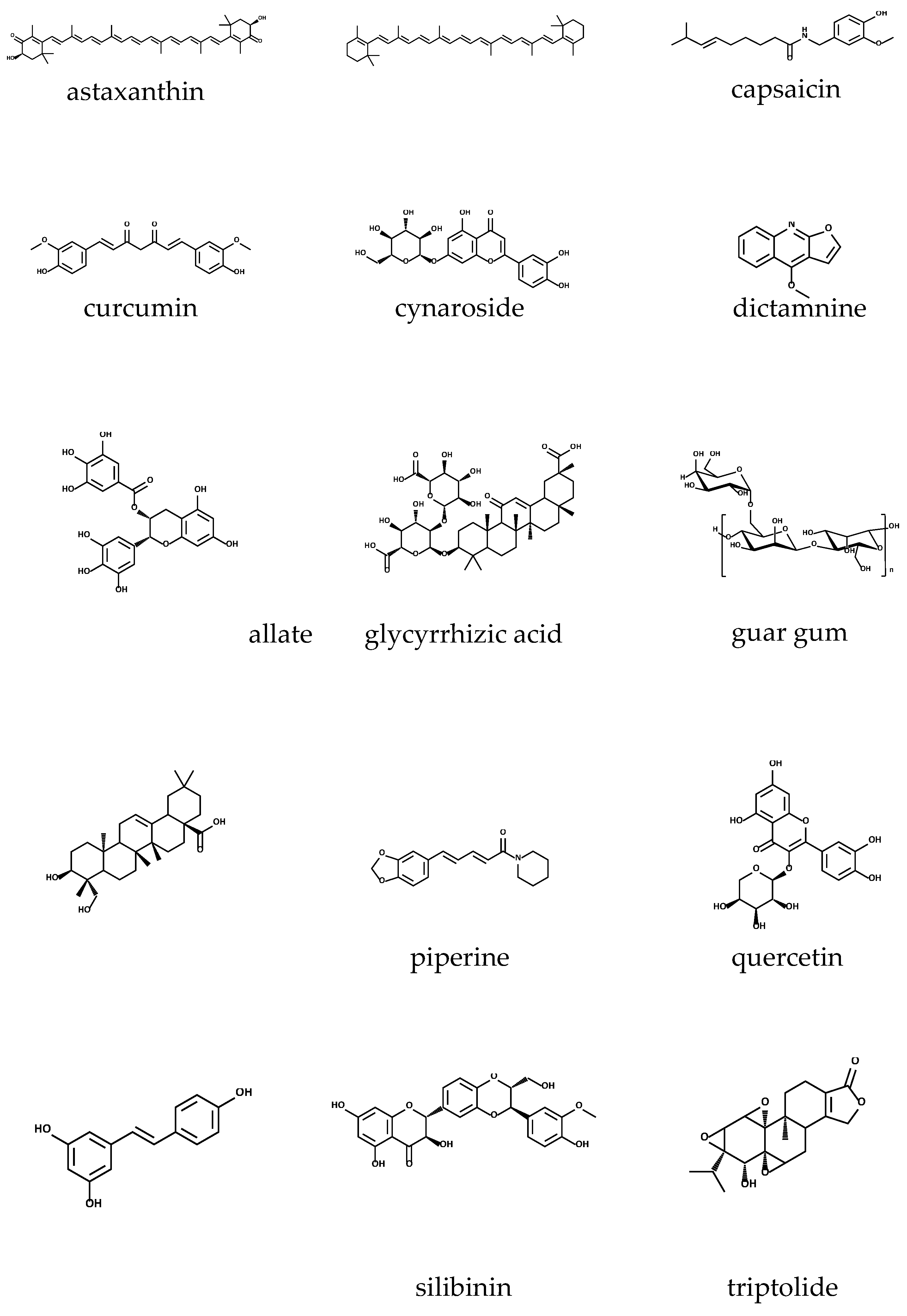
Astaxanthin (C
40H
52O
4) (
Figure 1), with a molar mass=596.84 g/mol, is a xanthophyll carotenoid found in living organisms being present in microalgae, crustaceans, and seafood, but also in yeast, fungi, complex plants, and birds’ feathers. It is a red-coloured lipid-soluble compound that gives marine animals their distinctive red-orange colour and protects from UV radiation. Astaxanthin has a peculiar structure: a non-polar region in the middle, with a series of 13 conjugated double bonds, and two polar regions with two ionone rings with hydroxyl (at 3,3’) and keto (at 4,4’) groups. This justifies its simultaneous hydrophobic and hydrophilic behaviour. It exists in different forms such as optical stereoisomers, geometric isomers, free or esterified forms and complexed with proteins or lipoproteins. The most predominant form in nature is the esterified one [
20,
21,
22,
23].
Astaxanthin displays several biological activities with therapeutic potential and health benefits. It has antioxidant, anti-inflammatory and anti-apoptotic activities which are responsible for the therapeutical use as anti-cancer, as anti-obesity, triglyceride, and cholesterol, as immunomodulator, as anti-diabetic, hepatoprotective and neuroprotector, and with benefits for the human skin [
21].
4.1.2. Drug delivery systems and pharmacological activity
Astaxanthin has proved to be a strong antioxidant that blocks inflammation at the biggening via NF-κB and hinders inflammatory mediators like interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumour necrosis factor-α (TNF-α). It also inhibits cyclooxygenase-1 (COX-1) and nitric oxide (NO) (Fakhri et al., 2018) (Lee et al., 2020). Its anti-dermatitis effect was confirmed also via inhibition of other inflammatory markers: inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and IgE [
23,
24].
Different formulations have been developed to enhance stability and bioavailability of astaxanthin in topical applications which includes nanoemulsions (NEs) [
25], hydrogels/lipogels [
26], liposomes (LIPs) [
22] and nanostructured lipid carriers (NLCs) [
27].
From the former, only the work of Lee et al. [
22] was focused on the evaluation of the developed formulation in AD. Hence, a liposomal formulation containing astaxanthin (L-AST) was prepared where the conjugation with phospholipid structures improved the low water-solubility of the molecule, hence affording to study its effect in the prevention of AD, by skin inflammation’s inhibition. This liposomal astaxanthin was prepared by mixing with phosphatidylcholine in a 1:4 ratio using MicrofluidizerTM, a high-pressure homogenizer. Particle size, evaluated by ELS-Z, was of about 64.5 nm. Results of this study revealed that Signal transducer and activator of transcription 3 (STAT3) and NF-κB were indeed inhibited by L-AST suggesting its anti-AD potential use [
22]. In fact, LIPs are characterized by a double-layered membrane, comparable to the phospholipidic cell membrane, surrounding an aqueous core, and are non-toxic and biodegradable delivery systems. Given their high biocompatibility, LIPs easily merge with the
stratum corneum cells, allowing deep penetration into the epidermal layer [
10]. Once LIPs can incorporate both hydrophobic and lyophobic drugs, they are also characterized to enhance drug solubility, compatibility, and biodegradability, they have been used to deliver drugs into specific affected sites [
28].
4.2. β-carotene
4.2.1. Natural source, physicochemical features, and bioactive properties
β-carotene (
Figure 1) belongs to the carotenoid family and is a vitamin A precursor, an important micronutrient for humans. It can be found in several natural sources, like plants, marine algae, fungi, and bacteria [
29]. Among its isomers (α, β, γ, δ, ε and ζ), β-carotene is the most abundant and effective. β-carotene is known for its antioxidant activity, and immune system stimulation. Its intake is documented as being useful in the prevention of allergic diseases, reducing the risk of AD [
30,
31]. Besides that, it also presents anti-inflammatory properties being used in the treatment of several skin diseases, especially AD [
32].
Structurally, β-carotene has a chemical backbone built by polyene chain with a long conjugated double bond systems that ends with cyclic groups. There are no oxygen atoms in its composition, but its electron-rich conjugated system is responsible for its antioxidant property [
29].
4.2.2. Drug delivery systems and pharmacological activity
Kake and co-workers [
31] have reported that β-carotene blocks inflammation by reducing inflammatory cytokines, factors and MMPs (matrix metalloproteinases) activity, in oxazolone-induced AD-skin mice. Beside this, an increase in filaggrin’s expression was observed concluding that, besides being a potent anti-inflammatory agent, β-carotene also ameliorates the skin’s barrier function [
31]. The same research group studied the oral effect of β-carotene on AD-like skin tissue and observed a significant suppression of TNF-α, IL-1β, Monocyte Chemoattractant Protein-1 (MCP-1), Thymic stromal lymphopoietin (TSLP), IL-6, IL-1β, IL-4, IL-5, and protease activated receptor 2 (Par-2). Also, the expression of filaggrin was elevated. Moreover, β-carotene led to a reduced activity and/or mRNA expression of MMPs, degradation of the extracellular matrix and regulation of chemokines [
33].
Nanofibers (NFs) are nanomaterials that have several applications in the pharmaceutical field, given their properties such as the high surface area-volume ratio [
34]. Moreover, NFs are appreciated in a way that they reduce systemic absorption and the number of required drug administrations, besides the achievement of high production rates [
35]. Among the polymeric NFs used for drug delivery systems, polycaprolactone (PLC) has been most frequently used given its good tissue compatibility and appropriate tensile strength. Semnani and co-workers [
32] developed a PLC NF mat loaded with β -carotene. These new mats were prepared by electrospinning showing NFs with 400-800 nm of diameter, with desirable tensile properties. The in vitro degradability and drug release studies found a very slow degradability rate and gradual release of β-carotene. Results suggested the use of these β-carotene loaded mats for the treatment of skin diseases like AD.
4.3. Capsaicin
4.3.1. Natural source, physicochemical features, and bioactive properties
Capsicum annum L., that is a member of the Solanaceae botanical family, and widely known as chili pepper, is the main natural source of capsaicin (C
18H
27NO
3) (
Figure 1). This pungent and lipophilic alkaloid with a molecular weight of 305.40 g/mol, represents more than 90% of all capsaicinoids present in chilli pepper. Besides that, chili pepper is also the source of other capsaicinoid compounds like dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin and homocapsaicin, all of them found in
Capsicum fruits. The pungent property of capsaicinoids arises from the presence of an amide bond, linking the acyl chain with the vanillyl ring. In fact, capsaicin has some similar structural features to piperine, another alkaloid. Such pungency had driven several bioactivities to be scientifically explored, as the nociceptive, anti-inflammatory, anti-carcinogenic, anti-obesity, and antimicrobial activity [
36,
37,
38].
4.3.2. Drug delivery systems and pharmacological activity
The basis of analgesic activity of capsaicin is mainly related for the agonist activity upon transient receptor potential vanilloid 1 (TRPV1) ion channel, which is expressed in nociceptive sensory nerves, namely C and some Aδ fibers, ultimately affecting the capacity of cutaneous sensory nerves to feel pain stimulus. On the other hand, its anti-inflammatory effect is evidenced by its suppressive action upon pro-inflammatory mediators such as COX-2 and iNOS. Despite the recognized nociceptive and anti-inflammatory properties of capsaicin, the poor bioavailability of the compound due its lipophilic nature, and potential skin irritation side-effects, have led some investigators to develop innovative nanosystems for the topical delivery of capsaicin [
39].
Therefore, the report published by Cassano et al. (2022)[
13] and colleagues aimed at incorporating linolenic acid into solid lipid nanoparticles (SLNs) based on curcumin, resveratrol, and capsaicin-derived esters for the treatment of AD. In this study, the results obtained for the curcumin and resveratrol monooleates, regarding the improvement of AD-like symptoms, were comparatively better to those obtained for the particles containing capsaicin oleate, which justifies that such results are discussed in their own sections, elsewhere in this manuscript. Nevertheless, the capsaicin SLNs presented an average size of 277.4
± 12.0 nm, with a polydispersity index (PDI) of 0.192
± 0.095, and the entrapment efficiency (EE) (%) was almost total, reaching 99% score. The advantages of SLNs include their highly targeting power to the affected sites on the skin, an improvement on the drug permeabilization into the dermal layer, controlled released, and decrease in systemic absorption, as well as avoidance of compounds’ degradation through hydrolysis and oxidation phenomena [
13,
40].
On the other hand, some authors have attempted an improved capsaicin topical delivery, disregarding possible pharmacological evidence directly related to AD, by only considering its anti-inflammatory and analgesic potential. From this perspective, Ghiasi et al (2019)[
39] developed an oil-in-water NE through the spontaneous emulsification methodology, aiming at creating an effective carrier for in vivo topical delivery of capsaicin. This NE was included in a cream, and in a gel, and its security and efficiency were compared to the conventional cream containing free capsaicin. According to skin irritation tests, there was no signs of ear edema or erythema, besides rats’ paw edema has decreased under these nanosystems’ treatment, in comparison to the group treated with the conventional cream. Moreover, the analgesic activity of the capsaicin NE-based gel was evidenced, once rats better resisted to the pain inflected by a heat stimulus, plus the fact that it revealed to be a better dosage form for the administration of the drug, improving skin permeability. NEs are isotropic binary systems, composed of two immiscible liquids, forming oil droplets with a particle size varying from 10 nm to 200 nm, dispersed in an aqueous phase, and stabilized by at least one surfactant [
41]. The main advantage of NEs is the achievement of increased solubility of hydrophilic active ingredients, by dispersing them into the oily phase, thereby improving skin permeation [
35]. This characteristic relies on the existence of positive charges that interact with negative charges of
stratum corneum cells, enhancing percutaneous drug absorption [
41].
For instance, Wang et al. (2017) proposed capsaicin loaded NLCs to increase its skin permeabilization, encompassing the analgesic and anti-inflammatory potential of the molecule, while avoiding skin irritation. Similarly, Raza et al., (2014)[
42] also had applied NLCs for the topical delivery of capsaicin, improving the analgesic properties of this alkaloid, and reducing skin irritation signs arising from its pungent property. NLCs share great similarity to SLNs and have been suggested as cutting-edge lipid nanoparticles (NPs) for the treatment of AD, but instead they consist of liquid content and a solid matrix. The application of NLCs presents less chances of drug leakage and increases drug loading, also encompassing drug half-life, controlled release, enhanced drug targeting, and entrapment efficiency [
43].
4.4. Curcumin
4.4.1. Natural source, physicochemical features, and bioactive properties.
The main source of curcumin are the roots of
Curcuma longa L., a plant widely known as turmeric, and that belongs to the same botanical family of ginger (
Zingiber officinale L.), the Zingiberaceae family. Curcumin (C
21H
20O
6) (
Figure 1) is a β-diketone polyphenolic compound with unique structural characteristics, arising from the presence of β-diketo groups, carbon-carbon double bonds, and phenyl rings containing hydroxyl and methyl functional groups. Such structural features, enables this potent antioxidant compound to target inflammatory cytokines, proteins, enzymes, as well transcription factors [
8]. Curcumin, a bright yellow compound, has been traditionally used as a digestive facilitator, for gastrointestinal inflammation, and in skin ailments. Besides that, several in vitro and in vivo assays have attempted on the validation of its antimicrobial, anticancer and anti-inflammatory properties [
44]. Interestingly, it has been also employed to control AD symptoms in some Asian countries [
8,
44].
4.4.2. Drug delivery systems and pharmacological activity
Curcumin proved to be an expression TSLP, through blockade of caspase-1/NF-κB pathway, when tested
in vtiro on the human mast cell line, HMC-1 [
45]. Recently, mice were exposed to aerosolized ovalbumin (OVA), and the effect of curcumin in improving AD-induced symptoms was evaluated [
8]. According to the results of this study, curcumin showed to recover epidermal thickness, and inhibited infiltration of inflammatory cells into the dermal layer. At the molecular level, it was observed that under curcumin treatment, the Th2 promoting cytokines (TSLP/IL-33) and Th2 cytokines (IL-4/IL-5/IL-13/IL-31) have their expression inhibited, as well as the STAT-6 phosphorylation, and GATA-3 expression [
8].
Therefore, Zhu et al. (2022)[
46] and colleagues have designed novel curcumin-loaded zein-silk sericin NPs, for the delivery of this polyphenolic compound, and to enhance its skin penetration into the dermal layer, thereby aiming at reducing AD symptoms on an in vivo model, besides presenting minimal side-effects. Briefly, in this study, NPs were prepared by injecting zein hydroalcoholic solutions into silk sericin protein dispersions, following curcumin encapsulation through a facile antisolvent route. Particles varied from 330 to 400 nm in size, showed a zeta potential (ZP) of -22 to -25 mV, and the PDI varied from 0.29 to 0.49. The formulated nanocarriers (zein-to-silk sericin mass ratios of 1:0.25) showed the best penetrating behaviour (240 µm in depth) into the porcine cortex, including cuticle, epidermis, and dermis, which shows the efficiency of the formulated transdermal delivery system. Moreover, the designed NPs suppressed inflammatory cytokines and chemokines, through the inhibition of the nuclear translocation of NF-kBp65, in comparison to free curcumin, when tested in an in vitro AD cell model (HaCaT cell line).
In another study, gels containing SLNs were loaded with tetrahydrocurcumin, a curcumin-derived metabolite with certain pharmacological therapeutic advantages, besides presenting a greater polarity, over curcumin itself [
40]. The nano-based system was obtained through a modified microemulsion technique, followed by a high-speed homogenization approach that ended with an increased tetrahydrocurcumin loading. Following this methodology, a high drug EE of 83.10%
± 2.29% was achieved, and the particles were 109.2 nm in size. Afterwards, SLNs’ dispersion was included into a Carbopol (2%
w/
v) hydrogel. In the in vivo assays, the anti-inflammatory potential of these nanosystem was evidenced, once it decreased expression levels of TNF-α and IL-6, and following the histopathological analyses, complete healing of AD-like lesions was observed. More important, AD-like symptoms alleviation was significantly different (
p ≤ 0.05) from that produced by the marketed ointment Tacroz® Forte, or even the produced gel bearing free tetrahydrocurcumin. Furthermore, the tetrahydrocurcumin-bearing NPs not only ameliorate skin hydration, as also showed a great transdermal penetration over the skin layers, into the dermis [
40].
Another recent study envisaged the encapsulation of linolenic acid into SLNs, that were able to penetrate deeply into the skin. These SLNs were loaded with curcumin, as well as other natural molecules, like resveratrol and capsaicin [
13]. Firstly, esterification reactions with oleic acid were carried out to produce curcumin and resveratrol monooleate, and capsaicin oleate, following a microemulsion methodology to prepare SLNs. The curcumin monooleate presented an EE of 62%, comparing to 85% and 99% of the capsaicin and resveratrol produced esters, respectively. Focusing on the obtained results for the curcumin-based formulations, they were 493.6
± 183.90 nm in size, and showed a PDI of 263
± 0.043, which for instance indicates homogeneity in the distribution of the particle size. Furthermore, these systems were not cytotoxic when tested on NCTC 2544 and THP-1 monocytes differentiated into M2 macrophages, even increasing it in some cases, which was also observed for the resveratrol SLNs. Regarding the anti-inflammatory potential, the authors observed that the curcumin SLNs significantly suppressed the production of IL-6, both in basal conditions and in the presence of TNF-α, used as a pro-inflammatory stimulus.
The inclusion of curcumin into SLN engrossed gels, has been also attempted but envisaging the treatment of irritant contact dermatitis and skin pigmentation [
47]. On the other hand, a work has been carried out to overcome some curcumin delivery drawbacks, and providing insights that the formulated LIPs may serve as vehicles for a broad dermatological application, including AD [
48]. Therefore, neutral, cationic, and anionic deformable LIPs were formulated. According to the main findings, the cationic deformable LIPs presented the most appreciable properties, namely they enhanced penetration of curcumin through the human skin in full thickness, plus the fact that they provided the most interesting retention of the compound. Moreover, these LIPs showed potent in vitro anti-inflammatory activity, besides the absence of cytotoxicity in human skin fibroblasts, along with evidence of cell proliferation stimulation [
48].
4.5. Cynaroside
4.5.1. Natural source, physicochemical features, and bioactive properties
Cynaroside (luteolin-7-O-glucoside or luteoloside; C
21H
20O
11) (
Figure 1) is a natural product found in
Bidens tripartita L.,
Verbascum lychnitis L.
, Elsholtiza bodinieri Vaniot, and other plants. This glycosyloxyflavone is functionally related to luteolin [
49,
50].
It is known by its diaphoretic, diuretic, antiseptic, anti-inflammatory and anti-allergic activities [
50]. Also, anticancer [
51] and against Hepatitis B [
52] effects are known.
4.5.2. Drug delivery systems and pharmacological activity
Cynaroside exerts its anti-inflammatory effect by inhibiting the expression of IL-4 and IgE [
41]. It also blocks IL-22 and IL-6/STAT3 pathway which contributes to control keratinocytes hyperproliferation [
50,
53]. The anti-inflammatory effect was also evaluated in vitro revealing to decrease the production of NO, and of ROS generation. In vivo testing showed inhibition of edema and a decrease in prostaglandin E2 (PGE
2) of mice [
49].
Szekalska and co-workers [
50] repared novel hydrogels as topical carriers for cynaroside. They used the anionic polymer alginate for its bioadhesive properties. Alginate was mixed with glycerol and propylene glycol, followed by the inclusion of crushed cynaroside that had been formerly obtained from aerial parts of
B. tripartita. Particle size ranged from 22 to 26 μm. The in vivo anti-inflammatory and anti-allergic activities were performed using skin from hairless mice. Results revealed that 5% and 10% of cynaroside hydrogels reduced substantially tissue skin and tissue inflammation and inflammatory infiltrates. Hence, the topical application of cynaroside allows the reduction of the number of T and mast cells and histiocytes, in mice skin with inflammation and AD, which supports the idea that flavonoids, like cynaroside, can hinder the overexpression of cytokines and IgE levels [
54].
To overcome cynaroside’s poor solubility, bioavailability and oral absorption, Qing et al. [
55] prepared biodegradable and biocompatible di-block copolymer micelles loaded with cynaroside creating water-soluble copolymer micelles. These micelles have a hydrophobic core, where the active substance is placed, and a hydrophilic shell. Encapsulation was made using methoxy polyethylene glycol-polycaprolactone (mPEG-PCL), methoxy polyethylene glycol-polylactide-co-glycolide (mPEG-PLGA) and methoxy polyethylene glycol-polylactide (mPEG-PDLLA). The self-assembly method created water-soluble torispherical micelles with an average diameter of 70 nm. The mPEG-PLGA showed the higher loading capacity, while mPEG-PCL had better stability. In vitro drug release showed a 30% cynaroside release from micelles [
55]. Before these micelles, Qing and co-workers [
56] investigated a nanocomposite material made by nanocrystalline cellulose (NCC) to improve cynaroside’s bioavailability. NCC has been used in biomedical fields as drug delivery systems because of its biocompatibility, biodegradability, and low cytotoxicity. These last two systems mentioned are valuable formulations, but they were not tested for AD or another inflammatory-based skin condition.
4.6. Dictamnine
4.6.1. Natural source, physicochemical features, and bioactive properties
Dictamnus dasycarpus Turcz. is a traditional Chinese herb medicine frequently used in China, Japan and Korea, to treat inflammatory-related skin diseases like AD, pruritus and urticaria [
57].
From the root bark of
D. dasycarpus, dictamnine (C
12H
9NO
2) (
Figure 1) is extracted, the main compound which has revealed to possess several bioactivities, such as anti-inflammation, antiangiogenic, anticancer, antifungal, antibacterial and anti-yeast [
58]. Dictamnine is a furoquinoline alkaloid [
59].
4.6.2. Drug delivery systems and pharmacological activity
Dictamnine’s anti-inflammatory mechanism has not been assigned exactly [
58]. However, there are several findings concerning
D. dasycarpus’ extract anti-inflammatory effects. Chang and colleagues [
60] showed that it protected skin cells from oxidation and inflammation by attenuating ROS, TNF-α, IL-1 and IL-6 levels, and by modulating antioxidant enzyme activity, cell signalling pathways, and the expression of NF-κB in keratinocytes. Their results suggested it to be interesting in preventing the inflammatory mechanism in dermatitis. Yang et al. [
61] studied the extract´s effect in contact dermatitis mice, and it also showed to inhibit the production of TNF-α, IFN-γ, and IL-6. These effects led to ameliorate skin lesions by reducing epidermal hyperplasia, hyperkeratosis and spongiotic changes.
Recently, Yang et al. [
62] have studied the anti-inflammatory and anti-pruritic effects of dictamnine in an AD mouse model. Results showed an efficient inhibition of AD-induced chronic itch, epidermal thickness, inflammation, and inflammatory cell infiltration. It was also observed a decrease in the expression of Mas-related G-protein–coupled receptor A3 (MrgprA3) and transient receptor potential channel A1 (TRPA1), the signal pathways used for the development of chronic itch. This data is consistent with dictamnine being interesting for the treatment of chronic itch associated to AD.
Focused on studying the dictamnine’s efficacy and mechanism as an anti-inflammatory in AD, Lin’s group [
58] developed a nanoformulation, the PLGA-nanocarrier-encapsulated dictamnine (Dic-PLGA-NC). The nanoformulated dictamnine revealed a particle size of nearly 186 nm, and a PDI of 0.146. As for the encapsulation efficiency and loading capacity, high-performance liquid chromatography (HPLC) results showed to be 93.7% and 51.8%, respectively. In the mouse model created for studying AD, results showed that these nanocarriers were able to penetrate 300 μm deep reaching dermal tissue allowing a sustained release of dictamnine from PLGA carriers. As for anti-inflammation effects using the new formula, results showed a reduced TSLP, IL-1β and TNF-α expression, and an apparent improvement of skin inflammation was observer in treated mice [
58].
4.7. Epigallocatechin-3-gallate
4.7.1. Natural source, physicochemical features, and bioactive properties
Epigallocatechin-3-gallate (EGCG) (
Figure 1) it is a polyphenol, part of the catechins subclass, and it is mostly found in the leaves of green tea, that is
Camellia sinensis (L.) Kuntze (Theaceae family). Focusing on green tea catechins, EGCG represents more than 50% of those compounds, and about 16.5 % of the water-extractable fraction of tea, reason why a cup of tea may contain about 200
-300 mg of EGCG. Despite the relevance of EGCG, there are other important catechins in tea, such as (-)-epicatechin (EC), (-)-epicatechin-3-gallate (ECG), and (-)-epigallocatechin (EGC), all of them differing in respect to their pharmacodynamics and pharmacokinetic properties, which is intimately related with structural features. For example, it is considered that the existence of hydroxyl groups at the following C positions 3’, 4’ and 5’ in the B ring of the EGCG molecule, in addition to the galloyl moiety esterified at carbon 3 on the C ring, are key points justifying the great antioxidant activity. Besides that, other properties have been attributed to EGCG, such as the anticancer, vasoprotective and anti-inflammatory activities [
63].
4.7.2. Drug delivery systems and pharmacological activity
EGCG and other tea catechins have been highlighted by their beneficial effects on skin-related conditions. From this perspective, the work of Noh et al., (2008) was pioneer in investigating the anti-inflammatory role of EGCG when topically applied on the skin of an AD mouse model NC/Nga induced by 1% DPE (
Dermatophagoides pteronissinus extract). Findings suggest that total clinical severity score and ear swelling were significantly reduced (
P<0.05) after EGCG treatment, along with a histopathological grading improvement. Noteworthy, the mRNA expression of the cytokines MIF (macrophage migration inhibitory factor), TNF-α, interferon gamma (IFN-γ), IL-2 and IL-12 p40 was significantly diminished by EGCG (
P<0.05) in the AD skin lesions, which was also observed on the immunohistochemistry assays. Moreover, the elevated serum MIF and IgE levels also suffered a significant reduction (
P<0.05). Altogether, these findings point that EGCG suppress MIF and T helper 1 cytokines, thus leading to an improvement in AD skin lesions induced by DPE [
64].
Since catechins like EGCG have been highlighted by their outstanding pharmacological activity on the skin, including wound healing effect, anti-aging properties, anti-acne, anti-psoriatic and more important the effect on AD, several strategies have been attempted for their nanoencapsulation [
65]. Therefore, the work of Drew et al., (2017) showed that gelatin/ EGCG nanoparticles (GE NPs) were efficient in reducing IL-6 and IL-8 inflammatory factors, using an in vitro model of lipopolysaccharide (LPS)-inflamed WS1 dermal fibroblasts, at non-toxic concentrations lower than 10 µg/mL. Furthermore, in vivo assays conducted on nude mice skin also showed that GE NPs present skin absorbance while do not causing adverse effects. In this study, the formulated GE NPs were prepared following a self-assembly mechanism, and NPs showed an average size of 112.5 ± 19.09 nm, a positive ZP 23.2 ± 0.5 mV, and a PDI of 0.3 ± 0.05 [
14].
Recently, Han et al., (2022) have created Polyethylene glycol-PLGA-EGCG nanoparticles (EGCG-NPs) following the double emulsion methodology [
15]. The produced formulation presented an average size of 176.2 nm, the zeta potential was -33.3 mV, and the entrapment efficiency was 86% while PDI was 0.044. In addition to these data, EGCG-NPs showed to be spherical in shape, did not suffer aggregation or adhesion, and presented regular arrangement. For instance, EGCG-NPs provided a significant improvement of AD symptoms and skin lesions, namely a diminishment of skin and ear thickness, dermatitis score, and scratching behaviour, when using an AD in vivo model induced by 2,4-dinitrochlorobenzene (DNCB). In addition to it, the authors tested the EGCG-NPs, that led to an improvement in AD-related oxidative stress, by elevating the activities of antioxidative enzymes such as superoxide dismutase (SOD) and glutathione (GSH), even prior to end of the study. Noteworthy, the expression levels of inflammatory cytokines like Th1 (IFN-g and TNF-α), Th2 (IL-4 and IL-6), and Th17 (IL-17A) were significantly down-regulated when compared to the control group following a time-specific pattern. Consequently, receptor-interacting protein 1 (RIP1), receptor-interacting protein 3 (RIP3), and mixed lineage kinase domain like pseudokinase (MLKL) proteins, also had their overexpression blocked upon topical treatment with EGCG-containing particles, demonstrating that necroptosis is inhibited instead of apoptosis. Similarly, the expression of phosphorylated p38 (p-p38), extracellular signal-regulated kinase 1 (ERK1), and extracelluar signal-regulated kinase 2 (ERK2), were blocked as well. In the end, the authors also showed that AD symptoms alleviation were due to MAPK blockage. These drug delivery system, was a promising strategy in AD therapeutics once it improved the redox status, preserved the balance between Th1 and Th2 inflammatory factors, and targeted necroptosis instead of apoptosis in DNCB-mice [
15].
Similarly, epigallocatechin gallate/L-ascorbic acid–loaded poly-
γ -glutamate microneedles also proved to be a successful approach to alleviate AD-related symptoms, when administered once a week, by topically applying it on the skin of a DNCB-mice model. This report shows that this drug delivery system demonstrated to be successful in reducing dermatitis score along with inhibition of mast cell infiltration, plus reduction on the expression of the levels of IFN-
γ, Th2 cytokine secretion, IgE and histamine [
66].
4.8. Glycyrrhizic acid
4.8.1. Natural source, physicochemical features, and bioactive properties
The roots of
Glycyrrhiza glabra L. (Fabaceae), also commonly known as liquorice, are the source of glycyrrhizic acid (GA) (
Figure 1), that confers to the roots a typical sickly-sweet taste. Also known as glycyrrhizin (C
42H
62O
16), GA is a pentacyclic triterpenoid saponin glycoside, with a molecular weight of 822.92 g/mol, that could be found in the form of two stereoisomers, 18α-glycyrrhetinic acid and 18β-glycyrrhetinic acid, both formed after hydrolytic reactions promoted by intestinal bacteria, or in situ by the action of plant’s glucuronidase enzyme. The hydrophilic part of the molecule is represented by glucuronic acid, while glycyrrhetic acid residue corresponds to the non-polar part. Besides these saponin-like compounds, flavonoids and polysaccharides are other bioactive important molecules. It has been mentioned that GA acts as an antiviral, anti-inflammatory, anti-cancer, anti-microbial, antidiabetic and hepatoprotective compound. In fact, most of the pharmacological activity of liquorice arises from the GA alone [
67,
68].
Either the root’s extract or GA, are well known for their beneficial effect as antioxidant and anti-inflammatory in topical applications. Such effects are assumed to positively influence contact and atopic dermatitis, as also other skin inflammatory ailments, like sunburns or acne vulgaris. Most of these diseases’ present inflammatory signs like pruritus, erythema, or even skin pigmentation [
68].
4.8.2. Drug delivery systems and pharmacological activity
Using a DNCB-mice model of AD, investigators have showed that GA mainly acts by inhibiting the high-mobility group box1 (HMGB1) signalling pathway. In addition to it, this natural molecule also suppressed the expression of the receptor for advanced glycation end products (RAGE), the phosphorylation of NF-κB and the infiltration of mast cells. Given the recognized anti-inflammatory value of
G. glabra and respective major compounds such as GA, 18beta-glycyrrhetinic acid, isoliquiritin and liquiritigenin, these have been also tested concerning their inhibitory effects on inflammatory and allergic reactions like AD [
68,
69].
The thin film hydration method was employed to produce transfersomes (TRAs) loaded with GA, that were further included into a hydrogel, as a vehicle for the GA-transfersomal suspension [
16]. The GA-loaded TRAs presented a particle size varying between 270.40 and 56.94 nm, PDI ranged between 1.00 and 0.13, and the ZP was -4.76 mv. For instance, the GA-trans loaded hydrogel presented a ZP of 36.4 mv. Moreover, the EE showed to be improved by an increasing content of the lipidic fraction of TRAs, thus resulting in an EE ranging between 66.23 ± 0.61 and 93.10 ± 0.3. According to the in vitro drug release study, a drug cumulative pattern was evidenced, reaching a drug release percentage of 89.8% up to 24 h. Meanwhile, ex vivo permeation was only 5.8% up to 24 h, thus indicating that the drug effectively deposits on the skin. Such deposition is required for the management of AD, since the drug should not permeabilize into the skin and suffer systemic absorption, thereby to exert its topical therapeutical effect. Moreover, in comparison to other groups, GA-trans loaded gel led to a significant reduction in erythema signs and scratching behaviour in the in vivo assays [
16]. For instance, ammonium glycyrrhizate, which is a derivative salt of GA, were also included in TRAs, envisaging an improvement on topical administration of this anti-inflammatory compound [
70]. TRAs are innovative ultra-deformable vesicles, consisting of a single-chain surfactant which is the edge activator, a lipidic part, and solvent. These nano-based technology shares highly similarity with LIPs and ethosomes (ETOs). TRAs have edge activators that give the capacity to become ultra deformable and highly elastic, squeezing themselves and penetrating across
stratum corneum, resulting in a higher permeation ability [
7,
16].
18β-glycyrrhetinic acid nanocrystals were prepared by high-pressure homogenization method. Afterwards, GA nanocrystalline suspension presented an average size of 288.6 ± 7.3 nm, a PDI around 0.13 ± 0.10, while the thermal stability and crystallinity decreased, but solubility increased significantly after nanocrystallization. In comparison to Coarse GA hydrogel, and the positive control group represented by the drug indomethacin, the formulated nano-GA hydrogel provided better anti-inflammatory activity, by decreasing the signs of ear edema, levels of pro-inflammatory cytokines, reduced myeloperoxidase activity, as well as reduced infiltration and aggregation of neutrophils. Despite these results are not specially directed to the AD context, the authors point that these nanocrystals may be useful in the treatment of skin diseases in general [
71]. In another study, a modified LIP-like vesicle loaded with GA, had some changes in core ingredients, by including ethanol and glycerol, aiming at an improvement of the stability of the nanosystems, and to promote efficacious penetration of the drug into the skin. This modified formulation, called glycethosomes were prepared by ethanol injection and sonication technique, showed a mean particle size of 94.5 nm, a PDI of 0.216, and 99.8% of EE, when the formulations contained glycerol at 50% and ethanol at 25%. Moreover, at the referred concentrations, glycethosomes showed the smallest particle size and the best stability, besides improving the transdermal effect [
72].
4.9. Guar gum
4.9.1. General considerations
Cyamopsis tetragonoloba (L.) Taub. is a leguminous plant and from the endosperm of its bean seeds is extracted the so called guar gum (
Figure 1) [
73]. This is a water-soluble, non-ionic polysaccharide of high molecular weight (50.000 to 8.000.000 Da) with a viscous and gel-like consistency. Chemically classified as a galactomannan, it contains a straight chain of D-mannose units linked by β(1-4) glycoside linkages, and a single D-galactose unit (2:1 ratio) [
73]. Despite its main current use in cosmetic and food industry as a stabilizing agent, and in pharmaceutical industry in drug microencapsulation, it has also been used for its medicinal properties. Guar gum is effective in lowering postprandial glucose and cholesterol, and there are also reports for its antimicrobial and antiproliferative activity [
73,
74].
4.9.2. Drug delivery systems and pharmacological activity
Ghosh and co-workers prepared guar gum NPs (GN) and explored their therapeutical effect in AD, in vitro and in vivo [
74]. GN were prepared by acid hydrolysis from guar gum dispersed in water, without any surfactant, affording spherical NPs with a size range between 30-80 nm [
75]. The in vitro study showed successful wound healing effect of GN and the in vivo a successful decrease in AD symptoms, like redness and epidermal thickness. It was also registered a decrease of serum IgE levels, total counts for blood cells, skin cells, eosinophils, macrophages, and neutrophils. They concluded that the GN prepared are useful agents as anti-inflammatory, anti-allergic and pro-regenerative being efficient in ameliorating AD [
74].
4.10. Hederagenin
4.10.1. Natural source, physicochemical features, and bioactive properties
Hederagenin (
Figure 1) is a pentacyclic oleane-type triterpenoid acid found in the pericarps’ fruits of
Sapindus saponaria L. (Sapindaceae) and the buds of
Lonicera japonica Thunb. These have been traditionally used for the treatment of skin conditions and the dried buds have also revealed anti-AD effects [
76,
77]. Hederagenin has two hydroxyl groups in the A-ring, a doble bond in the C-ring and a carboxylic group at C-28. There are several reports about the different biological properties of this natural compound, such as anti-inflammatory, antimicrobial, and anticancer [
78].
4.10.2. Drug delivery systems and pharmacological activity
Hederagenin ensures an anti-inflammatory effect by regulation of MLK3 signalling, attenuation of the inflammatory cytokines TNF-α, IL-1 and IL-6, and by decreasing other pro-inflammatory factors like TNF-α and COX-2 [
78,
79].
In this sense, hederagenin was used to coat maghemite (γ- Fe
2O
3) nanoparticles (HM) and studied for its immunomodulatory and anti-inflammatory efficacy in AD [
80]. Results revealed a dose-dependent inhibition of AD related cytokines, IFN-γ, TNF-α, IL-4, IL-6, IL-17 and TSLP. They also showed a reduction in mast cells’ infiltration, lowered epidermal and dermal thickness of mice skin, and relieved lumping lymph nodes. These results reveal the HM synergistic effect (hederagenin and maghemite) acting as anti-inflammatory and immunomodulatory agent, hence with great potential for AD medication. The HM were prepared using the emulsion method, by mixing the maghemite NPs that were first prepared with a solution of hederagenin. The obtained HM were round-shaped NPs with average size of 10.9 nm [
80].
4.11. Piperine
4.11.1. Natural source, physicochemical features, and bioactive properties
Considered to be the major alkaloid (approximately 98%) found in black pepper (
Piper nigrum L.), piperine (C
17H
19NO
3) (
Figure 1) is an alkaloid mainly found in the oleoresins of plants from the genus
Piper (Piperaceae family), with amounts ranging from 2 to 9%, depending on the plant species used for extraction. The socioeconomic value of peppers in general, is due to the flavour and pungency arising from piperine, but also from essential oils found in peppers’ oleoresins. As most alkaloids, piperine is a poorly water-soluble compound and a very weak basis, easily solubilizing itself in the presence of acids or alkalis. According to ancient Chinese and Indian medicine practices, black pepper was used for pain relief, rheumatism, as a circulatory, digestive, and appetite stimulant, plus its use in the treatment of fever. Currently, piperine has been referred to its antioxidant, chemopreventive, anti-cancer, among other pharmacological activities [
81].
4.11.2. Drug delivery systems and pharmacological activity
Interestingly, the immunomodulatory and anti-inflammatory potential of this alkaloid was explored by testing the black pepper fruit extract in allergic contact dermatitis. The oral administration of piperine to mice, showed inhibitory effect upon eosinophils, IgE, and especially Th2 cytokines expression, which points to the potential of piperine in other skin inflammatory ailments [
82]. In another work carried out by the same team, using a trimellitic anhydride (TMA)-induced AD-like mouse model, it was demonstrated that topical application of piperine resulted in the suppression of immune responses regulated by Th2 cytokines, noteworthy including the STAT6/GATA3/IL-4 signalling pathway [
83].
From this perspective, one outbreaking study was developed attempting the topical administration of piperine by including it into ETOs, thus overpassing the solubility and delivery issues, while exploring it as a therapeutic agent for AD [
84]. ETOs are phospholipid-based flexible and elastic vesicles bearing an ethanolic core (20–45% of ethanol), but also containing other key ingredients like phosphatidylcholine, cholesterol, and water. Given the high content of ethanol in these nanocarriers, they have the capacity to easily penetrate the epidermic
stratum corneum, thus promoting a deep and localized drug delivery into the skin [
7,
10,
84,
85]. According to the study of Kumar et al. (2021), piperine-loaded ETOs were prepared by the cold method, and for optimized ethosomal dispersion, the nanocarriers presented an EE of 74.30 ± 3.88%, and a vesicle size of 318.1 nm. Besides, the ZP of the formulated vesicles was 32.6 mV, and they were spherical in shape. Regarding the in vitro cytotoxicity assays, the creams were non-toxic when tested in HaCaT cell lines. Following ex vivo assays, the fabricated ETOs-based creams easily penetrated in the skin, mainly depositing at the epidermal and dermal layers. In comparison to the negative control, the ethosomal and conventional creams containing piperine at 0.1% and 0.125% respectively, both significantly reduced the ear and skin thickness, skin severity, white blood cells, granulocytes, and IgE antibodies levels in the BALB/c mice model. In the end, given the efficiency of the piperine ethosomal cream in reducing in AD markers, comparing to tacrolimus (0.1%) and conventional cream applications, the authors suggested that this formulation has great potential for the management of mild to moderate AD [
84].
Nevertheless, regarding the generality of skin inflammation diseases, a very recent investigation reports the development of piperine-loaded NPs included into hyaluronic acid/sodium alginate-based membranes [
86]. The nanoprecipitation technique was used to produce the polymeric NPs composed of Eudragit S100 and Poloxamer 188, resulting in the obtention of spherical-shaped NPs with a mean diameter size of 122.1 ± 2.0 nm, a PDI of 0.266, and an EE of 76.2 %. Afterwards hyaluronic acid/sodium alginate membranes were produced for the subsequent incorporation of the synthesized NPs. The main results suggest that the produced formulation evidenced a reduction of the mouse ear inflammatory symptoms near 46%, besides the absence of cytotoxic adverse effects on the L929 mouse fibroblasts’ cell line [
86].
4.12. Quercetin
4.12.1. Natural source, physicochemical features, and bioactive properties
Quercetin (
Figure 1) (C
15H
10O
7) is a widespread flavonol found in several daily food products, like fruits (berries, grapes, nuts, and apples), vegetables (onions, tomatoes, and cabbages), in beverages like tea and red wine, besides its presence in well-recognized medicinal plants like
Sambucus nigra L.,
Hypericum perforatum L., and
Ginkgo biloba L. [
87]. Quercetin is a water insoluble molecule, while easily solubilizing itself in alcohol, acetic acid, and lipids. In nature, quercetin is often found bonded to other molecules that may enhance the solubility of the aglycone, namely sugars forming quercetin glycosides, like quercetin-3-
O-glucoside, an important pigment in vegetables and fruits[
2,
88]. Structurally, quercetin bears four active groups, namely a dihydroxy group between the A ring,
O-dihydroxy group B, C ring C2, C3 double bond, and 4-carbonyl [
2,
87]. In addition to it, the presence of several OH group and double bonds confers to this flavonoid a strong antioxidant activity [
87,
88]. Besides that, several other skin-related beneficial effects have been reported, namely wound healing, anti-psoriatic, photoprotective, anti-inflammatory, and skin whitening, thus justifying the critical role of this molecule in cosmetics and pharmaceuticals acting on the skin [
88].
4.12.2. Drug delivery systems and pharmacological activity
Despite numerous studies have been carried out, either in vitro or in vivo as well as some clinical trials, exploring the molecular effects of quercetin, the truth is that the exact antioxidant, anti-allergic and anti-inflammatory mechanisms are not fully uncovered [
89]. As an example, the anti-inflammatory mechanisms of quercetin, along with those from the flavonol galangin, were assessed in vitro in LPS-stimulated RAW264.7 macrophages, and in vivo by using DNCB-mice models of AD. In this investigation, the authors found that NF-κB, ERK1 and 2, and c-Jun N-terminal kinase (JNK) may be potential molecular targets of quercetin, as well as of galangin. In addition to these findings, oral administration of both flavanols to DNCB-mice models of AD also led to decrease in inflammation, once the compounds decreased ear edema, as well the levels of serum IgE [
90].
An in vitro model of AD was used, by stimulating HaCaT keratinocytes with pro-inflammatory factors like IL-4, IL-13 and TNF-α, to induce an in vitro AD model. The anti-inflammatory and antioxidant power of quercetin in AD context was unveiled, when cells’ pre-treatment with quercetin (1.5 µM), had led to a decrease in the expression of IL-1b, IL-6, IL-8, and TSLP, encompassing an improvement of the oxidative cellular defences by an augmentation of the expression of SOD1, SOD2, catalase, glutathione peroxidase, and IL-10. On the other hand, quercetin also evidenced its wound healing potential mainly by the targeting inhibition of MMP1, MMP2 and MMP9, by leading to a decrease in phosphorylation of ERK1 and 2 in MAPK pathway, as well the expression of NF-kB, while the phosphorylation of STAT6 remained unaltered [
91].
In this regard, some efforts to enhance the delivery and bioavailability of the molecule have been carried out. Therefore, the method of emulsion evaporation–solidification at low temperature was employed to develop quercetin-loaded NLCs [
12]. The characterization of these formulations revealed that particles were spherical in shape, presented a particle size of 215.2 nm, ZP was -20.10 ± 1.22 mV, mean EE was 89.95 ± 0.16%, while drug loading was 3.05%. According to the results, in comparison to a quercetin propylene glycol solution, the developed nanosystem increase the amount of drug retention in epidermal and dermal skin layers, while revealing an easy percutaneous permeabilization across the
stratum corneum. On the other hand, the in vivo assays evidenced that these NLCs also improved inflammation symptoms, and enhanced antioxidant effect, thus proving to be an efficient topical delivery system for AD management [
12].
4.13. Resveratrol
4.13.1. Natural source, physicochemical features, and bioactive properties
Resveratrol (
Figure 1) is a stilbene polyphenol, also considered to be a phytoalexin, once it is involved in plant defence against abiotic and biotic hazards, like UV radiation and fungal infections, respectively, which in turn usually leads to an increase in its synthesis in plant tissues [
92,
93]. Resveratrol (C
14H
12O
3) has a molecular weight of 228.247 g/M and a melting point of 254°C, easily dissolving in alcohols like ethanol and acetone, but poorly dissolving in water [
93]. Among plants, UV radiation-mediated reactions may lead to the isomerization of the bioactive form
trans-resveratrol to the
cis isomer, both found in plants’ tissues [
92,
93]. Despite resveratrol had been firstly identified in the roots of white hellebore (
Veratrum grandiflorum O. Loes), vines and red grapes’ skin (
Vitis vinifera L., Vitaceae) are by far the major sources of resveratrol [
93,
94,
95]. In addition, this stilbenoid is also found in several berries like blueberries and cranberries, peanuts, cocoa, and tomatoes. Despite some adverse effects have been reported to resveratrol, its bioactive properties are outstanding, namely the anti-inflammatory, antimicrobial, anti-cancer, anti-aging, cardioprotective, vasorelaxant, phytoestrogenic, and neuroprotective activities, besides being a well-validated antioxidant protector, given its action as a strong radical scavenger. Specifically, the antioxidant power of resveratrol arises from both phenolic rings connected by a double bond [
95,
96]. Such properties, namely the anti-aging and antioxidant, have allowed the pure compound to be included in cosmetics at concentrations rising to 5%, or even in the form of extract or derivative-like compounds [
94]. Despite its natural sources, either chemical or biological synthetic approaches, have been employed for a large-scale obtention of the compound, namely through
Saccharomyces cerevisiae fermentation [
95].
4.13.2. Drug delivery systems and pharmacological activity
The potentiality of resveratrol in AD management was investigated in DNCB-induced NC/Nga mice, and in an in vitro 3D skin model [
97]. In this study a resveratrol-enriched rice obtained through genetic engineering was included. Rice (
Oryza sativa L. var.
japonica) was considered given its many recognized skin-associated benefits, and therefore the synergistic effect with resveratrol, was herein investigated by combining both natural products. The investigation was carried out during five weeks, and it was found that the resveratrol-enriched rice markedly suppressed dermatitis score, scratching behaviour and trans-epidermal water loss. Moreover, serum IL-31 and IgE levels, as well the production of IL-6 in keratinocytes, were suppressed following resveratrol-enriched rice treatment [
97]. In addition, the work carried out by Karuppagounder et al. (2014), evidenced that oral administration of resveratrol (20 mg/kg/day) in NC/Nga mice attenuates DPE-induced AD-like symptoms, by causing the suppression of several inflammatory patterns like HMGB1, RAGE, toll like receptor (TLR)4, NF-κB, phosphatidylinositide 3-kinase (PI3K), ERK1 and 2, COX-2, TNF-α, IL-1β, and IL-2Rα[
98]. In the end, this scientific report suggested that resveratrol potentially targets AD disease by modulating protein expression on the HMGB1 pathway. On the other hand, resveratrol treatment (30 mg/kg/day) for 6 weeks, showed that the stilbene was effective against AD-like inflammation symptoms in BALB/c mice, by targeting epithelial apoptosis through caspase-3, and epithelium-derived cytokines like IL-25, IL-33, and TSLP. In this work, an improvement in epithelial thickness was also observed [
99].
Recently, the encapsulation of linolenic acid into SLNs with resveratrol, curcumin, and capsaicin has been also attempted [
13]. In this study, esterification reactions led to the obtention of resveratrol monooleate, as well as esters for the other bioactive molecules. The EE of resveratrol was 85%, comparatively higher than the obtained for curcumin (62%). The SLN resveratrol-based formulations, were 271.8
± 4.0 nm in size, and showed a PDI of 0.005. The resveratrol nanosystems presented no cytotoxic effects when tested on NCTC 2544 and THP-1 monocytes differentiated into M2 macrophages. In comparison to the SLN without linolenic acid, the ones that contained resveratrol markedly suppressed the production of MCP-1, a key cytokine for the recruitment of monocytes under an inflammation scenario, as also decreased the production of IL-6 under TNF-α stimulus. Besides that, the resveratrol-containing NPs presented the best antioxidant activity in comparison to the other formulations [
13].
The rising interest on resveratrol potentialities have led investigators to incorporate it into different nanosystems for multiple skin applications. For example, SLN containing the seed butter of
Theobroma grandiflorum (Willd. ex Spreng.) K. Schum. as the lipidic core, were designed for the controlled topical delivery of resveratrol as active principle. This formulation presented antioxidant potential, as well permeation and drug accumulation in the upper skin [
100]. Meanwhile, Sun et al. (2014) and colleagues performed a comparative study, by preparing NEs, SLNs, and NLCs through the hot high-pressure homogenization technique. The authors demonstrated that the lipidic ratio and composition of the lipid-based formulations highly influence the resveratrol delivery and retention on the skin. Moreover, evidence was gathered pointing that a high lipid ratio of the formulation may improve resveratrol topical release [
101]. It is worth mentioning that resveratrol SLNs incorporated into a carbopol gel, have been also developed for irritant contact dermatitis [
102].
4.14. Sacran
4.14.1. Natural source, physicochemical features, and bioactive properties
Aphanothece sacrum is a cyanobacterium from which sacran (
Figure 1) is extracted [
54,
103]. This polysaccharide has a high molecular weight (2.35x10
7 g/mol) and presents many carboxylic and sulfuric acid groups. Its sugars are fructose, rhamnose, xylose, arabinose, mannose, glucose, galactose, galactosamine, glucuronic and galacturonic acids. It has a huge water retention affording it to cells [
104].
Sacran has been reported to prevent bacteria’ and virus’ invasion, as well as the potential to prevent lipids’ absorption and improve intestinal microbiota [
105].
4.14.2. Drug delivery systems and pharmacological activity
This sulphated polysaccharide has been used as basic material in hydrogel films for skin application given its safety and moisturizing effect, being documented its use in AD patients. This results from its ability to suppress inflammatory cytokine and reduce chemokine mRNA levels. It is indeed a novel biomaterial useful in improving skin barrier in AD. It has also been reported that it was able to block IL-5, IFN-γ and TNF-α in AD mice model (Fukushima et al., 2016). Ren et al. observed that sacran relieved the symptoms of AD-induced mice, specifically AD score, ear thickness, and IgE release. They also concluded that it inhibits the activation of Th2 cells [
104].
Based on this, Wathoni and co-workers developed sacran hydrogel films and studied, among other properties, their skin hydration efficacy [
103]. These physically crosslinked-sacran hydrogels were prepared by a solvent-casting method and characterized by several techniques. These properties together with results from the following in vivo assays in hairless mice with significant increase of moisture content, allowed to conclude that sacran hydrogels have potential properties as basic biomaterial in AD given their moisturizing and anti-inflammatory effect [
103].
4.15. Silibinin
4.15.1. Natural source, physicochemical features, and bioactive properties
Silybum marianum (L.) Gaertn. (Asteraceae family), commonly known as milk thistle or wild artichoke, is the main botanical source of silymarin. Interestingly, silymarin is not an isolated compound, instead is a complex of other important compounds, like silychristin, silydianin, isosilybin, and silibinin (
Figure 1), the last also known as silybin. The last and most important, silibinin (C
25H
22O
10) is a flavonolignan-type compound formed when the flavononol taxifolin conjugates with coniferyl alcohol, a structural building block mostly found within lignin scaffold. The structure of silibinin bears several hydroxyl groups, that give the molecule a high antioxidant capacity, as well as the capacity to chelate metals, plus the presence of the chromone fragment that enables silibinin to easily react with bases. The main potential of these flavonoid-like compounds is the hepatoprotective effects, which are directly correlated to their high antioxidant activity and membrane stabilizer capacity, that avoids lipid peroxidation phenomena [
106,
107].
4.15.2. Drug delivery systems and pharmacological activity
The rising interest on the silibinin incorporation into topical nanaocarriers is owned to its recognized antioxidant activity, but more important the anti-inflammatory activity on the skin, arising from the capacity of this flavonoid to suppress NF-ĸB factor activation as well other pro-inflammatory genes [
108]. Nevertheless, it is worth to mention that a SLN enriched gel [
109], as well as hydrogels containing pomegranate oil based NCs loaded with silibinin [
108], have been designed as innovative drug delivery systems to also irritant contact dermatitis.
A gellan gum/pullulan bilayer film containing silibinin-loaded nanocapsules (NCs) was developed by Gehrcke et al. (2022) and colleagues [
110]. NCs were produced by the method of interfacial deposition of preformed polymer. These silibinin-loaded NCs (content around 98.9%), presented a diameter size of 115 ± 3 nm, the PDI was under 0.2, and the ZP was near -10 mV. For instance, bilayer films were prepared by the method of two-step solvent casting, using gellan gum as the first polymeric layer, followed by pullulan as the second layer, thus forming a homogeneous bilayer film. According to the results of this study, silibinin was slowly released from the nano-based film, and presented high affinity for the cutaneous tissue, thus remaining retained there. In vivo assays were conducted by testing the nano-based formulation on DNCB-mice models of AD. In comparison either to the silibinin solution alone, the vehicle film itself, or even the ordinary hydrocortisone treatment, the formulation showed to positively influence the inflammatory and oxidative responses, which was transduced in a reduction of the scratching behaviour, and ear edema. Notwithstanding, the gellan gum/pullulan bilayer film itself, without the silibinin-loaded NCs, also showed some ameliorative effects alone on the DNCB-mice model. Altogether, these data highlight the combination of films and silibinin-loaded NCs as a strategy in AD management, by their anti-inflammatory and antioxidant effects, while encompassing skin hydration and protective properties, in just one formulation [
110]. In fact, NCs are vesicular systems consisting of a polymeric membrane surrounding an oily core. Interestingly, once vegetable oils can either function as part of NCs scaffold or as an active ingredient, they have been investigated in this context. NCs’ structural organization favours the encapsulation of lipophilic substances, thus increasing their solubility and therapeutic efficacy, while enhancing drug stability, controlled drug release, and decreased toxicity of active ingredients [
18,
28,
110].
On the other hand, a report attempts on the incorporation of silymarin into a pluronic-lecithin organogel, which was then tested in patients with AD symptoms [
111]. These drug delivery systems have been deserving attention in topical drug administration, once they are formed by a biphasic composition, thus enhancing solubility of hardly solubilizing molecules such as silymarin, plus the fact that these formulations also facilitate the penetration of hydrophilic compounds. The authors attempted to produce several formulations with different ratios of pluronic and lecithin, finding the optimal concentrations of 20 and 3% for each constituent, respectively, thus achieving an optimal silymarin permeation on the ex vivo assays. Furthermore, the designed delivery system improved inflammation symptoms in patients, such as redness and swelling [
111].
4.16. Triptolide
4.16.1. Natural source, physicochemical features, and bioactive properties
Tripterygium wilfordii Hook.f., known as Thunder God Vine or
Lei Gong Teng, belongs to the Celastraceae botanical family, and it comprises the natural source from which triptolide was firstly isolated. Triptolide (C
20H
24O
6) (
Figure 1) it is an abietane-type diterpene, made up of three epoxy groups and an α, β-unsaturated five-membered lactone. This diterpenoid has been mainly investigated by its anti-leukemic, anti-inflammatory, immunosuppressive, and anticancer activities. However, the obtention of triptolide directly from medicinal plants it is unreasonable given the low concentrations in which is found. However, efforts have been made to develop a lab-scale and industrialized manner of synthesize this compound [
112].
4.16.2. Drug delivery systems and pharmacological activity
The anti-inflammatory and immunosuppressive activities of triptolide have been extensively afforded by several authors, however not directly related with skin disorders like AD. Main findings suggest that such activities are fundamentally related to its suppressive action upon the NF-κB signalling pathway, on the IL-17, and IL-6 signals, as well as inhibition of STAT3-activated signalling pathway. Other evidence points that triptolide also inhibits the expression of pro-inflammatory factors [
112].
To overcome some challenges related to percutaneous drug delivery, lipidic nanosystems have been proposed as feasible and effective alternatives. In this context, a study on the inclusion of triptolide into NEs was carried out [
57]. These nanosystems were prepared by the high-energy emulsification method and demonstrated to provide the best topical drug release and maintenance of concentration. Furthermore, triptolide-loaded NEs improved epidermal lipidic components and keratin characteristics at the epidermal
stratum corneum layer, refining not only skin hydration, as also allowing a better drug permeation. Focusing on the triptolide-containing gels tested
in vivo, in moderate to high dosages, lead to an amelioration of AD-like inflammation, and of the mice ears’ erythematous edema. Meanwhile, at the molecular level, these NE-based gels were able to reduce the expression of IFN-γ and IL-4. Once the triptolide-loaded NE gels achieved the best results, they were characterized as sphere-shaped with a two-layer structure, besides presenting a narrow size distribution of 62.1 ± 9.9 nm, and a PDI score of 0.19 ± 0.023 [
57].
Table 1.
Physicochemical Properties of key nanoformulation-based natural isolated compounds for the treatment of AD.
Table 1.
Physicochemical Properties of key nanoformulation-based natural isolated compounds for the treatment of AD.
| Natural isolated compound |
Nanotechnology-based formulation |
Preparation approach |
EE (%) |
PS (nm) |
ZP (mV) |
PDI |
References |
| Astaxanthin |
LIPs |
Mixing with high pressure homogenizer |
NA |
64.5 ± 2.8 |
NA |
NA |
[22] |
| β-carotene |
NFs |
Electrospinning |
NA |
400-800 |
NA |
NA |
[32] |
| Capsaicin |
SLNs |
Microelmusion method |
99% |
277.4 ± 12.0 |
NA |
0.192 ± 0.095 |
[13] |
| Curcumin |
SLNs |
Microelmusion method |
62% |
493.6 ± 183.90 |
NA |
263 ± 0.043 |
[13] |
| |
SLNs-based gel |
Microelmusification with high-speed homogenization method |
83.10 ± 2.29 |
109,2 |
NA |
NA |
[40] |
| |
Zein-silk sericin NPs |
Antisolvent method |
NA |
330 to 400 |
–22 to –25 |
0.29 to 0.49 |
[46] |
| Cynaroside |
Hydrogels |
Mixing |
NA |
22-26 μm |
NA |
NA |
[50] |
| Dictamnine |
Nanocarrier-encapsulated |
Using U-SiM bioreactor (ultrasound composite streams-impinging mixer) |
93,70% |
186 ± 30 |
NA |
0.146 ± 0.072 |
[58] |
| Epigallocatechin-3-gallate |
Gelatin NPs |
Self-assembly method |
NA |
112.5 ± 19.09 |
+23.2 ± 0.5 |
0.3 ± 0.05 |
[14] |
| |
Polyethylene glycol-Poly lactic-co-glycolic acid -Epigallocatechin-3-gallate nanoparticles (PEG-PLGA-EGCG-NPs) |
Double emulsion method |
86% |
176,2 |
–33.3 |
0,044 |
[15] |
| Glycyrrhizic acid |
TRAs |
Thin film hydration method |
66.23 ± 0.61 to 93.10 ± 0.3 |
56.94 to 270.40 |
–4.76 |
0.13 to 1.00 |
[16] |
| Guar gum |
NPs |
Acid hydrolysis from guar gum dispersed in water |
NA |
30-80 |
−30 ± 5 |
0,259 |
[74,75] |
| Hederagenin |
NPs |
Emulsion method |
NA |
10,9 |
NA |
NA |
[80] |
| Piperine |
ETOs-based cream |
Cold method |
74.30 ± 3.88 |
318,1 |
–32.6 |
NA |
[19] |
| Quercetin |
NLCs |
Emulsion evaporation-solidification method |
89.95 ± 0.16 |
215,2 |
–20.10 ± 1.22 |
NA |
[12] |
| Resveratrol |
SLNs |
Microelmusion method |
85% |
271.8 ± 4.0 |
NA |
0,005 |
[13] |
| Silibinin |
NCs-based bilayer film |
NCs were prepared by interfacial deposition of preformed polymer method. Films were prepared by two-step solvent casting method |
99% |
115 ± 3 |
–10 |
< 0.2 |
[110] |
| Triptolide |
NEs-based gel |
High-energy emulsification method |
85% |
62.1 ± 9.9 |
NA |
0.19 ± 0.023 |
[57] |
Table 2.
Key nanoformulation-based natural isolated compounds and their main pharmacological effects.
Table 2.
Key nanoformulation-based natural isolated compounds and their main pharmacological effects.
| Natural isolated compound |
Major Natural Source |
Nanotechnology-based formulation |
Pharmacological effects |
References |
| Astaxanthin |
Microalgae, crustaceans, seafood, yeast, fungi, complex plants, birds’ feathers |
LIPs |
In vivo: STAT3 and NF-kB inhibition. |
[22] |
| β-carotene |
Plants, marine algae, fungi, and bacteria |
NFs |
In vitro: very slow degradability rate and gradual release of beta-carotene. |
[32] |
| Curcumin |
Curcuma longa L. |
SLNs |
In vitro: ↓ IL-6. No cytotoxic effects for NCTC 2544 and THP-1 monocytes differentiated into M2 macrophages. |
[113] |
| |
|
SLNs-based gel |
In vivo: ↓TNF-α and IL-6. ↑healing lesions and skin hydration. Improved redox status (↑GSH and Catalase; MDA↓). Ex vivo: ↑ In-depth penetration to the dermis. |
[40] |
| |
|
Zein-silk sericin NPs |
Ex vivo: ↑ In-depth penetration and skin permeability. In vitro: ↓ NF-kBp65, inflammatory cytokines and chemokines in HaCaT keratinocytes. |
[46] |
| Cynaroside |
Bidens tripartita L., Verbascum lychnitis L., Elsholtiza bodinieri Vaniot |
Hydrogels |
In vivo: ↓ tissue skin and tissue inflammation and inflammatory infiltrates; ↓ number of T and mast cells and histiocytes; hinder the overexpression of cytokines and IgE levels. |
[50] |
| Dictamnine |
Dictamnus dasycarpus Turcz. |
Nanocarrier-encapsulated |
In vivo: ↓ thymic stromal lymphopoietin (TSLP), IL-1β and TNF-α expression; improvement of skin inflammation. |
[58] |
| Epigallocatechin-3-gallate |
Vitis vinifera L. |
Gelatin NPs |
In vivo: ↑ skin absorbance and no side-effects. In vitro: ↓ IL-6 and IL-8 in LPS-inflamed WS1 dermal fibroblasts. |
[14] |
| |
|
Polyethylene glycol-Poly lactic-co-glycolic acid -Epigallocatechin-3-gallate nanoparticles (PEG-PLGA-EGCG-NPs) |
In vivo: ↓ ear and skin thickness, dermatitis score, and scratching behaviour. Restoration of Redox status (↑ SOD, GSH, and T-AOC).↓ Th1 (IFN-g and TNF-α), Th2 (IL-4 and IL-6), and Th17 (IL-17A) cytokines. Supression of RIP1, RIP3, MLKL, p-p38, ERK1 and ERK2. |
[15] |
| |
|
Epigallocatechin gallate/L-ascorbic acid–loaded poly-γ -glutamate microneedles (EGCG/AA-loaded-γ -PGA MNs) |
In vivo: ↓ dermatitis score, mast cell infiltration, IFN-γ expression, Th2 cytokine secretion, IgE and histamine. |
[66] |
| Glycyrrhizic acid |
Glycyrrhiza glabra L. |
TRAs |
In vivo: ↓ erythema signs and scratching behavior. Ex vivo: ↓ permeation and ↑ skin deposition. |
[16] |
| Guar gum |
Cyamopsis tetragonoloba (L.) Taub. |
NPs |
In vitro: successful wound healing effect; In vivo: ↓ in AD symptoms, like redness and epidermal thickness; ↓ serum IgE levels, total counts for blood cells, skin cells, eosinophils, macrophages, and neutrophils. |
[74] |
| Hederagenin |
Sapindus saponaria L., Lonicera japonica Thunb. |
NPs |
In vivo: Dose-dependent inhibition of IFN-γ, TNF-α, IL-4, IL-6, IL-17 and thymic stromal lymphopoietin (TSLP); ↓ mast cell infiltration, epidermal and dermal thickness of mice skin; relieved lumping lymph nodes |
[80] |
| Piperine |
Piper nigrum L. |
ETOs-based cream |
In vivo: ↓ ear and skin thickness, skin severity, white blood cells, granulocytes, and IgE. Ex vivo: Penetration and deposition. In vitro: no cytotoxic effects in HaCaT keratinocytes. |
[84] |
| Quercetin |
Present in several food products like fruits and vegetables |
NLCs |
In vivo: ↓ Inflammation symptoms. In vitro: ↑ Percutaneous permeabilization and retention at the dermis and epidermis. |
[12] |
| Resveratrol |
Vitis vinifera L. |
SLNs |
In vitro: ↓ IL-6 and MCP-1. No cytotoxic effects for NCTC 2544 and THP-1 monocytes differentiated into M2 macrophages. |
[113] |
| Sacran |
Aphanothece sacrum (Sur.) Okada |
Hydrogel films |
In vivo: ↑ moisture contente. |
[103] |
| Silibinin |
Silybum marianum L. |
NCs-based bilayer film |
In vivo: ↓ oxidative and inflammatory markers, ↓ scratching behaviour and ear edema, ↑ skin hydration. Ex vivo: controlled dug release, ↑ drug retention. In vitro: ↑ antioxidant potential. |
[110] |
| Triptolide |
Tripterygium wilfordii Hook. F. |
NEs-based gel |
In vivo: ↓ ear edema. ↓ IFN-γ and IL-4. Ex vivo: ↑ In-depth penetration and percutaneous delivery. |
[57] |
5. Extracts, oils, and plant mixtures, included in nanotechnology-based formulations for the treatment of AD
5.1. Centella asiatica (L.) Urban extract
5.1.1. General considerations
C. asiatica is particularly rich in triterpenes, namely asiaticoside and madecassoside, and their aglycones, asiatic acid and madecassic acid, respectively. This plant is also characterized by sesquiterpene-rich essential oils, plus other non-volatile compounds like catechins, and the flavonoids kaempferol and quercetin, that are present in plant-derived extracts. Even though, triterpenes are by far the most important compounds in this recognized medicinal plant. In fact, such compounds have been showing, in several in vitro and in vivo approaches, to act on dermatological diseases such as acne, burns, atopic dermatitis, and wounds via NF-
κB, MAPK, and STAT signalling pathways, among others. It is also worth mentioning that
C. asiatica has scientifically proved to positively influence different nervous and cognitive functions, namely on Alzheimer’s and Parkinson’s diseases [
114].
5.1.2. Drug delivery systems and pharmacological activity
The establishment of hydrogen bond interactions between phyto-derived molecules and phospholipids, forms lipidic-based vesicles called phytosomes. The existence of a double layer phospholipid membrane enables this type of drug delivery systems to interact with both polar and non-polar compounds. These characteristics have led their exploration for the delivery of natural compounds with cosmeceutical purposes and for the management of several skin ailments [
115], including AD [
17].
The antioxidant and anti-inflammatory properties of currently marketed
C. asiatica phytosomes, either containing extracts or isolated bioactive compounds, are well-recognized, as for wound healing or other skin ailments. Even though, the effects on AD were only explored by Ho et al., (2018). Therefore, an in vivo phthalic anhydride-induced AD-mice model, and in vitro RAW 264.7 murine macrophages, were used to study the anti-AD potential of commercially available
C. asiatica phytosomes. Regarding the in vivo assays, after AD-like lesions have been inflicted, 20 µL/cm
2 of 0.2% and 0.4% of the obtained phytosomes, were topically applied on the dorsal skin and mice ears’, for a period of four weeks, three times each. According to the histological analysis, the phytosome decreased hyperkeratosis, proliferation of mast cells, and infiltration of inflammatory cells. Moreover, this formulation not only reduced the expression of NO, iNOS, COX-2, TNF-α, IL-1β, and IgE
in vivo, as also reduced the expression of NO, iNOS, and COX-2 in in vitro LPS-stimulated RAW 264.7 macrophage cells. The authors also find that LPS-induced DNA binding activities of NF-κB were affected by phytosome application, thus suggesting that this has link with the discontinuation of IκBα degradation, and consequent decrease in the translocation of p65 and p50 into the nucleus. Overall, the fact that this
C. asiatica phytosome shows its mechanism of action by the inhibition of the NF-κB signaling pathway, its potential in the management of AD was detailed depicted [
17].
5.2. Cortex Moutan and PentaHerbs
5.2.1. General considerations
Cortex Moutan it is a traditional Chinese medicine comprising the root bark of
Paeonia x suffruticosa Andrews (Paeoniaceae family). A wide range of phytochemicals have been identified, including flavonoids, tannins, triterpenoids, despite the predominance and importance of phenolic compounds, and glycosylated monoterpenes. This water insoluble drug presents several pharmacological activities, namely the anti-inflammatory, anti-allergic, and antioxidant effects, which justifies its use in traditional Chinese medical practices for the treatment of AD (He and Xiao, 2017). Nevertheless, Cortex Moutan it is also present in PentaHerbs formula, which consists of a mixture of plant-derived drugs, also including other Chinese traditional medicines, like the bark of
Phellodendron chinensis Schneid. (Rutaceae), the flower of
Lonicera japonica Thunb. (Caprifoliaceae), the aerial parts of
Mentha haplocalyx Briq. (Lamiaceae) and the rhizome of
Atractylodes lancea (Thunb.) DC. (Asteraceae), at the ratio of 2:2:2:1:2 [
116]. Moreover, according to traditional Chinese medical practices, this herbal mixture has also anti-allergic, anti-inflammatory, anti-pruritic and sedative properties, reasons why it is extensively used for the treatment of allergic diseases including AD, asthma, and allergic rhinitis. Similarly, to Cortex Moutan, the anti-inflammatory and anti-allergic potential of PentaHerbs, are suggested to be comparable to corticosteroids’ effects, but without adverse reactions for patients with AD [
117].
5.2.2. Drug delivery systems and pharmacological activity
The effect of PentaHerbs on the release of inflammatory factors from RMPC cells and cytokine production arising from HMC-1 cell line, was investigated to understand how it positively affects AD symptoms [
116]. The investigators studied the whole mixture and the effect of different components, finding that Cortex Moutan and
Herba Menthae significantly reduced histamine release, and prostaglandin D2 synthesis in RPMC cell line. Interestingly, Cortex Moutan was the only component affecting the production of cytokines in HMC-1, while PentaHerbs formula and the remaining four constituents failed it. Overall, PentaHerbs formula can reduce AD-associated inflammation, and its positive effect has been pointed to be improved if the concentration of Cortex Moutan is increased [
116]. In other investigation, using an in vivo oxazolone-induced dermatitis model, it was found a significant reduction (
p < 0.05) of ear swelling, epidermis thickening and eosinophils infiltration in epidermis and dermis, as well as the release of serum IL-12, when the aqueous-based extract of the drug was given to the animals by oral or topical administration. In this study, gallic acid, chlorogenic acid and berberine contents were determined, and the effects of individual compounds was afforded. Therefore, both gallic acid and chlorogenic acid inhibited the release of pro-inflammatory cytokine IL-6, and chemokine CCL7 and CXCL8, when eosinophils-dermal fibroblasts co-cultures were submitted to IL-31- and IL-33 treatments, respectively. On the other hand, in the eosinophil culture and eosinophils-dermal fibroblasts co-culture, the release of IL-6, CXCL8, CCL2, and CCL7 was also significantly (
p < 0.05) suppressed by berberine [
118].
Recently, investigators developed a dual-responsive hydrogel from thermo-responsive polymer PF127, and two chemically synthesized pH-responsive compounds N,N,N-trimethyl chitosan (TMC), and polyethylene glycosylated hyaluronic acid (PEG-HA). In this hydrogel, gallic acid was loaded as the active molecule, once it is a major compound found in Cortex Moutan. In this report, the team showed that the dual-responsive hydrogel (PF127/TMC/PEG-HA) evidenced proper release of gallic acid. Moreover, it was shown that the hydrogel formed by PF127 improved its delivery capacity after adding TMC and PEG-HA [
117]
Afterwards, the same team of investigators, has formulated a dual-responsive hydrogel, using PF127 as the thermo-responsive polymer, while the conjugate made of polysaccharide HA and chitosan oligosaccharide lactate [Chito(oligo)] represents the functional core that is responsive to pH. The polysaccharide-based conjugate was synthesized following carbodiimide chemistry techniques, while the NPs of the conjugate [HA-Ala-chito(oligo)] were produced by the ultra-sonication methodology. Considering this investigation, gallic acid was selected once again, to be the main bioactive compound into the PF127/HA-Ala-Chito(oligo) formulation. According to the characterization of the synthesized hydrogel, this showed to be highly porous and presented an optimal dispersion of the micellar structures, after modification with the nano-conjugate. Such modification resulted in an improvement on the gallic acid delivery behaviour. Moreover, the formulation also had its rheological properties improved, as well as mechanical stability, and pH-responsiveness, after the nanoconjugate has been included into the system. The evaluation of the cytotoxicity on HaCaT keratinocytes of the PF127 based formulations, presented a cell viability higher than 80.0 %, considering drug concentrations ranging between 0.0 and 20.0 μg/mL. From this perspective, the authors further suggest that future research would be necessary to find more harmless biomaterials to successfully use Cortex Moutan in the treatment of AD, through textile-based transdermal therapy [
117].
5.3. Eupatorium japonicum Thunb. extract
5.3.1. General considerations
E. japonicum it is a plant species that belongs to the botanical family Asteraceae. In several oriental countries like Vietnam, Japan, Korea and China, the leaves are used for the treatment of several gastrointestinal ailments, like nausea, vomiting, indigestion, and diarrhoea [
119] Besides that, both leaves and stems are known to be used as analgesic, diuretic, antimicrobial and vermifuge.
E. japonicum produces an essential oil mainly represented by thymol, and its extracts also have pyrrolizidine alkaloids, namely indicine, amabiline, viridiflorine, echinatine, and rinderine, which are known by their hepatotoxicity and anticancer effects [
120]. Moreover, the extracts of this plant are also recognized by their anti-inflammatory potential along with cytotoxic effects, which were attributed to the presence of sesquiterpene lactones [
119].
5.3.2. Drug delivery systems and pharmacological activity
An inflammation-induced human keratinocyte model was used to evaluate the efficacy of gold nanoparticles (AuNPs) loaded with
E. japonicum flavonoids [
121]. Metal-based NPs such as silver NPs (AgNPs) and AuNPs, usually lead for an improvement in bioactive performance, an effective entrapment of the drug, and increased delivery capacity, plus high target to affected sites, while encompassing a reduced systemic permeation [
85]. In fact, AuNPs are suitable nanossystems given their skin and follicular drug delivery, diagnostic and therapeutic application, reason why some attempts on their use to treat AD has been made [
121].
Briefly, AuNPs were obtained by a reaction between distilled water containing HAuCl
4•3H
2O, and the plant-derived extract. AuNPs’ characterization showed that they were efficiently synthetized without any impurities, showing a crystalline structure, as well they presented a particle size ranging between 31.0 and 149.1 nm with predominant circular, spherical, and polygonal-shaped morphologies.
E. japonicum extract presented as major compounds melilotoside, rutin, hyperoside, nictoflorin, cymaroside, and rhamnetin. According to this recent study, the application of these AuNPs revealed to be less toxic to the HaCaT cell line, in comparison to the
E. japonicum extract alone. Furthermore, these NPs suppressed the production of inflammatory cytokines, and the production of ROS. These effects were found to be linked with the suppression of both MAPK and NF-κB signalling pathways, thus exposing a possible anti-inflammatory mechanism of action for this extract and formulation, and its potential in AD management [
121].
5.4. Houttuynia cordata Thunb. Extract
5.4.1. General considerations
H. cordata is a perennial herb distributed from Nepal throughout Asia to China and Japan, often used as medicinal plant for the treatment of inflammatory diseases such as AD, but also
Herpes simplex and nasal polyps. There are also reports attesting its aqueous extract’s antioxidant and anticancer activities [
122,
123]. The aerial parts contain various types of compounds with twenty of them already isolated: harmala alkaloids, phenolic acids, chlorogenic acid derivatives, phenolic glycosides, phenylpropanoids derivatives and flavonoids [
123].
5.4.2. Drug delivery systems and pharmacological activity
To afford good skin permeation of
H. cordata extracts and enhance their anti-AD activity, Kwon & Kim developed cubosomal and liposomal suspensions. They were prepared using a sonication and film hydration method, respectively. The mean diameters were 231.7 and 273.3 nm, for cubosomes (CUB) and LIPs, respectively, and the size distribution varied from 73 to 90 nm and 216 to 300 nm for CUB and 100 to 130 nm and 330 to 470 nm for LIPs. These were analysed using ZetaPlus analyser. In vitro skin permeations were investigated in hairless mouse skin. It was observed that both lipid carriers, especially the CUB-based suspension, enhanced skin permeation of the extract with decrease of IgE production and IL-4 expression and stimulation of IFN-γ expression. Therefore, they concluded that CUB loaded with
H. cordata extract had inhibitory effect on the development of AD-like skin lesion and was efficacious for the treatment of AD [
122,
124]. Given their capacity to avoid enzymatic degradation, CUB are nanosystems that are usually developed to encapsulate peptide- and protein-derivative drugs. They are produced by promoting emulsification of a lipidic fraction with cubic geometry in water containing NPs, in liquid state and with crystalline features [
41].
5.5. Linseed oil
5.5.1. General Considerations
Among more than two hundred species included in the genus
Linum L., the plant
Linum usitatissimum L. is the oldest one. Known as flax or linseed, it has a high nutritional value: omega-3 fatty acid, such as α-linolenic acid and short chain polyunsaturated fatty acids (PUFA), soluble and insoluble fibres, phytoestrogen-related lignans, proteins, and different antioxidants. It has paved its way in international food supply as a functional food. From its dried ripe seeds, a very interesting oil (linseed oil) (LSO) is extracted, comprising the following fatty acids: stearic, palmitic, linoleic, oleic, and linolenic [
125]. Hence, flaxseed has been spotted in diet and disease research given the health benefits linked to some of its bioactive compounds: α-linolenic acid (almost 60%) and lignan secoisolariciresinol diglycoside (SDG) [
126].
Besides the edible uses of LSO, it also has several beneficial properties such as anti-inflammatory, antioxidant and analgesic, being used for arthritis, cancer, keratoconjunctivitis and for several skin complaints. In fact, this plant has been used topically to treat skin diseases like eczema for many years given its “mucilage”, a substance that soothes and softens the skin [
125,
127].
5.5.2. Drug delivery systems and pharmacological activity
Linseed oil helps controlling inflammation via eicosapentaenoic acid (EPA) that results from the conversion of its main omega-3 fatty acid, α-linolenic acid. EPA works as a competitive inhibitor of the conversion of arachidonic acid into prostaglandin E(2) (PGE2) and leukotriene B(4) (LTB4). It was also reported its potent capacity to inhibit histamine and bradykinin. This makes it a potent anti-inflammatory agent [
127,
128,
129] EPA has been identified as an important compound in AD. The human metabolism can transform the linolenic acid present in LSO into EPA [
130]. So, in order assess LSO application as an alternative in AD therapy, new and more effective drug-delivery systems were developed for LSO, namely emulsions.
Microemulsions have been used as an important technique to increase skin drug permeation, lowering skin irritation with higher drug-loading volume. The direct topical use of linseed oil is however limited by the low permeation by the stratum corneum. To overcome this, Baboota and co-workers designed a topical submicron size microemulsion of linseed. Carbopol 971 was used to improve the microemulsion’s viscosity since this is an oil/water type of emulsion. The particle size and zeta potential analysed revealed an average size of 186 nm with a good size distribution. In vitro skin permeation studies revealed that this microemulsion afforded an enhancement of linseed’s permeation, hence creating a therapeutic approach for inflammatory-based skin diseases [
129].
Kildaci and colleagues [
125] developed new NE formulations containing LSO and investigate their potential
in vitro. NEs, which are delivery systems often used in dermatology given their capacity to improve drug release and skin penetration [
131], included LSO using the ultrasonic emulsification method. The LSO-NEs prepared were then analysed, among other features, attending the average droplet size, polydispersity index and zeta potential. In vitro release assays were also made. Molecular docking analysis was made to determine the binding connections that were most likely to be established between the bioactive compounds of LSO (α-linolenic acid, oleic acid, and linoleic acid) and Human Leukocyte Antigens (HLAs), important players on the immune system activation for AD. The NEs developed had an acceptable droplet size (99.0 nm), PDI (0.14) and ZP (-8.79 mV). Molecular docking analysis showed that α-linolenic acid is the best docked ligand. It was also observed an appropriate skin permeability with LSO being released in 78.4 to 100% at the end of 24 and 48 h, respectively. Kildaci’s results showed that the new LSO-NEs afford a topical skin route for effective AD treatment [
125].
5.6. Pomegranate seed oil
5.6.1. General considerations
Pomegranate seeds oil (PSO) is a vegetable oil obtained from the seeds of
Punica granatum L. (Lythraceae family) [
18]
. Once it is a vegetable oil, PSO comprises a mixture of other individual molecules with recognized value, reason why it has been studied by their health beneficial effects, namely on chronic diseases, such as cancer, osteoporosis, fatty liver disease, and diabetes, plus the antimicrobial, anti-inflammatory, and immunomodulatory properties [
132]. Pomegranate seeds correspond to near 10% of the total fruit weight and they have plenty of carbohydrates like pectin and fibers, besides the presence of vitamins E, C and K, minerals, as well as phenolic and flavonoid compounds. In addition, pomegranate seeds also contain triterpenoids, and phytosterols such as 17-α-estradiol and estriol. Among the fatty acids fraction, there are saturated (ranging from 30 to 35%), monounsaturated (varying between 35 and 37%), di-unsaturated (amounts ranging from 25 to 39%) and poly-unsaturated (1 to 10%), the last mainly represented by punicic acid, the major compound found in PSO [
133].
5.6.2. Drug delivery systems and pharmacological activity
The authors of the following study focused their interest on PSO, one because it has previously showed interesting pharmacological evidence as an anti-inflammatory and antioxidant vegetable oil, which are key properties for AD management, and another because it may function as a main contributor on the construction of the NCs scaffold. Therefore, Cervi et al., (2021) designed pullulan films loaded with PSO NCs, following the solvent casting method to prepare the pullulan films, and the interfacial precipitation of preformed polymer methodology to produce the NCs[
18]. Meanwhile, to have a comparative nanosystem, NEs of PSO were also prepared by the spontaneous emulsification method. In the in vivo assays using DNCB-mice, both free PSO and pullulan films containing PSO NCs, alleviated AD-like lesions. Even though, the biochemical analyses suggest that pullulan films loaded with PSO-loaded NCs, was the only formulation able to promote alleviation of inflammatory and redox status parameters in the AD-like lesions of DNCB-mice model. An in vitro safety test revealed that these formulations are secure once they do not provoke skin irritation. Focusing on the characterization of these PSO-containing NCs, these formulations demonstrated an adequate size, with a mean diameter of 181 ± 6 nm, a PDI under 0.2, while the obtained ZP was around + 43.13 ± 0.7 mV. For instance, the fabricated pullulan films were characterized as hydrophilic and flexible. In this investigation, NCs were incorporated in polymeric films, because such nanosystems require an embedding matrix that enhance their consistency and dosage form to be topically applied. Among the advantages of such stabilizer films, they are known to provoke minor skin irritation side effects and reduced sticky sensation during topical application, besides the fact they remain for longer periods of time at the skin affected sites and are suitable for the inclusion of hydrophilic solutions. Interestingly, pullulan is also a natural-derived polymer, belonging to the carbohydrates group, that it is obtained from the fermentation of a fungus [
18]. It is worth mentioning that PSO has been equally used to produce a hydrogel loaded with silibinin, but targeting irritant contact dermatitis instead [
108].
5.7. Rhus verniciflua Stokes extract
5.7.1. General considerations
Rhus verniciflua Stokes is an Asian tree, native from in China and Indian subcontinent. There are reports attesting its benefit in health by improving circulatory problems, blood homeostasis being used as cathartic, diaphoretic and anti-rheumatic [
134]. Its extract has revealed important bioactivities such as, antibacterial, anti-inflammatory, anti-allergic, neuroprotection and anti-osteoporotic. Also, its oral intake has proved to be protective on AD. The aqueous extract of
R. verniciflua’s timber is composed essentially by fustin, gallic acid, fisetin, resorcinol, garbanzol, butein, and sulfuretin [
135].
5.7.2. Drug delivery systems and pharmacological activity
In fact, Jiang and Sun conducted an in vivo study using sulfuretin and concluded that it suppressed the immune response in Th2 cells ameliorating AD symptoms by targeting GATA3 pathway in those cells[
136]. Jeong and coworkers [
135] prepared a topical film of pullulan hydrogel matrix loaded with
R. verniciflua extract (RVE) and tested their efficacy
in vivo, in AD-like models. Films were prepared by mixture of pullulan and RVE with an average of inclusion of 0.95% (0.26 mg/film) and results in mice showed a decrease in mast cells lesions which suggests the efficacy against AD.
5.8. Tea tree oil
5.8.1. General considerations
Tea tree oil consists of the essential oil obtained by distillation of the leaves of
Melaleuca alternifolia (Maiden & Betche) Cheel, which is part of the family Myrtaceae, the same as that of eucalyptus. Its essential oil is mainly characterized by the presence of oxygenated monoterpene hydrocarbons, as well as monocyclic and bicyclic monoterpenes, from which terpinen-4-ol is the dominant one. Besides that, several other terpenes are found, such
as γ-terpinene, α-terpinene, 1,8-cineole, p-cymene, terpinolene, α-terpineol, α-pinene, sabinene, aromadendrene, ledene, δ-cadinene, limonene, globulol, and viridiflorol [
137].
5.8.2. Drug delivery systems and pharmacological activity
Terpenoids like terpinen-4-ol, 𝛼-terpineol, and 1,8-cineole have been suggested to significantly decrease the level of pro-inflammatory factors, like TNF-𝛼, IL-1𝛽, IL-8, and IL-10. From this perspective, the potential of this essential oil in the treatment of AD has been explored, by loading it into ETOs [
84]. Phosphatidylcholine at 2% and 3% (w/v), and ethanol at 20%, 30% and 40% (w/v), were used to formulate ETOs containing tea tree oil. Optimized ETOs were characterized has having an EE of 76.19 ± 3.26%, a vesicle size of 333.6 nm, and a ZP of –35.3 mV. Afterwards, optimized ETOs were included in a base cream formulated by phase inversion method. In comparison to the conventional cream, the ETO-based formulation presented a better ex vivo permeation and subsequent deposition at the epidermal and dermal layers, besides showing to be not toxic to keratinocytes in vitro (HaCaT cell line). In addition, according to in vivo assays, inflammatory parameters showed a reduction regarding the severity of clinical score in a BALB/c mice model, as well as a decrease in the infiltration of white blood cells, eosinophils, and IgE antibodies. Besides that, this ETO-based cream may avoid oxidative degradation, and improve drug stability and permeation across skin layers. The authors further argued that the easy applicability of the method used to produce this ETO-based formulation, may turn it into an up-scale technique [
84].
Table 3.
Physicochemical properties of key nanoformulation-based extracts and oils for the treatment of AD.
Table 3.
Physicochemical properties of key nanoformulation-based extracts and oils for the treatment of AD.
| Extract/oil/mixture |
Nanotechnology-based formulation |
Preparation approach |
EE (%) |
PS (nm) |
ZP (mV) |
PDI |
References |
|
Houttuynia cordata Thunb. |
CUBs |
Sonication |
NA |
231,7 |
NA |
NA |
[122] |
| |
LIPs suspensions |
Film hydration method |
NA |
273,3 |
NA |
NA |
[122] |
| Linseed oil |
Microemulsion |
NA |
NA |
186 |
NA |
NA |
[129] |
| |
NEs |
Ultrasonication method |
NA |
99.02 ± 1.06 |
−8.79 ± 0.034 |
0.14 ± 0.020 |
[125] |
| Pomegranate seed oil |
NCs-based film |
Solvent casting method to prepare the pullulan films, and the interfacial precipitation of preformed polymer methodology to produce NCs |
NA |
181 ± 6 |
+43.13 ± 0.7 |
<0.2 |
[18] |
|
Rhus verniciflua Stokes |
Hydrogel |
Mixture stirred for complete solubilization at RT and then cast onto glass plates of 4 mm thickness |
0,95% |
NA |
NA |
NA |
[135] |
| Tea tree oil |
ETOs-based cream |
ETOs were obtained by mixing of reagents and subsequent sonication. Creams were obtained by phase inversion method. |
76.19 ± 3.26 |
333,6 |
–35.3 |
NA |
[19] |
Table 4.
Key nanoformulation-based plants’ mixtures, oils and extracts, and their main pharmacological effects.
Table 4.
Key nanoformulation-based plants’ mixtures, oils and extracts, and their main pharmacological effects.
| Extract/oil/mixture |
Major compounds |
Nanotechnology-based formulation |
Pharmacological effects |
References |
|
Centella asiatica (L.) Urban |
Triterpenes, namely asiaticoside and madecassoside, and their aglycones |
Phytosome |
In vivo: ↓ hyperkeratosis, proliferation of mast cells, and infiltration of inflammatory cells. ↓ expression of iNOS, COX-2, NF-κB, TNF-α, IL-1β, and IgE. In vitro: ↓ NO, iNOS, and COX-2 in LPS-stimulated RAW 264.7 macrophage. ↓ LPS-induced DNA binding activities of NF-κB. |
[17] |
| Cortex Moutan |
Plants’ mixture. Gallic acid |
PF127/HA-Ala-Chito(oligo)-based hydrogel |
In vitro: cell viability of > 80.0 % in HaCaT keratinocytes, considering concentrations ranging between 0.0 and 20.0 μg/mL. |
[117] |
|
Eupatorium japonicum Thunb. |
Flavonoids, namely melilotoside, rutin, hyperoside, nictoflorin, cymaroside, and rhamnetin |
AuNPs |
In vitro: suppression of MAPK and nuclear factor-κB signalling pathways. ↓ RANTES, TARC, CTACK, IL-6, and IL-8. ↓ production of ROS. |
[121] |
|
Houttuynia cordata Thunb. |
Harmala alkaloids, phenolic acids, chlorogenic acid derivatives, phenolic glycosides, phenylpropanoids derivatives and flavonoids |
CUBs and LIPs suspensions |
In vivo: ↑ skin permeation of the extract; ↓ IgE production and IL-4 expression; ↑ IFN-γ expression |
[122,123] |
| Linseed oil |
Omega-3 fatty acid, such as α-linolenic acid and short chain polyunsaturated fatty acids (PUFA) |
Microemulsion |
In vitro: ↑ linseed’s permeation |
[129] |
| |
Omega-3 fatty acid, such as α-linolenic acid and short chain polyunsaturated fatty acids (PUFA) |
NE |
In vitro: adequate linseed’s permeation |
[125] |
| Pomegranate seed oil |
Complex mixture rich in punicic acid |
NCs-based film |
In vivo: ↓ AD-like skin injury, ↑ oxidative defenses, ↓ hypernocipetive behaviour. In vitro: absence of irritation. |
[18] |
|
Rhus verniciflua Stokes |
Fustin, gallic acid, fisetin, resorcinol, garbanzol, butein and sulfuretin |
Hydrogel |
In vivo: ↓ mast cells lesions |
[135] |
| Tea tree oil |
Terpinen-4-ol is the major monoterpene in this essential oil |
ETOs-based cream |
In vivo: ↓ severity of clinical score, infiltration of white blood cells, eosinophils, and IgE antibodies. Ex vivo: ↑ Drug permeation and retention. In vitro: absence of cytotoxic effects in HaCaT keratinocytes. |
[19] |
6. Conclusions
Natural products have proved their beneficial effects and advantages in the treatment of several skin diseases, especially when they met nanotechnology-based formualtions. Therefore, in this manuscript, upcoming studies on the development novel nano-based systems regarding the delivery of natural products, including sixteen isolated compounds, four plants’ extracts, one plant mixture, and three plants’ oils, were herein reviewed, regarding the treatment of AD. However, available data on clinical effectiveness of such nanosystems loaded with natural ingredients are sparse or even inexistent in most cases, once most studies remain in a preclinical research stage, and are fundamentally based in a single-animal model. Therefore, with this review we expect to reinforce new investigations in natural products and drug delivery development, but also to inspire clinicians to evolve additional and robust clinical trials that may attest how reliable are these approaches, prompting future application in clinical practice. In this sense, we believe this review constitutes a paramount scientific basis to pave new avenues into the management of AD through natural-based healthcare solutions.
Funding
This research was funded by Foundation for Science and Technology (FCT, Portugal), through the following projects: UIDB/04539/2020, UIDP/04539/2020, and LA/P/0058/2020 (CIBB) Strategic Projects. M.M. is supported by Foundation for Science and Technology (FCT) with a PhD grant (Reference: SFRH/BD/146441/2019).
References
- Marques, M.P.; Mendonça, L.; Neves, B.G.; Varela, C.; Oliveira, P.; Cabral, C. Exploring Iberian Peninsula Lamiaceae as Potential Therapeutic Approaches in Wound Healing. Pharmaceuticals 2023, 16, 347. [Google Scholar] [CrossRef]
- Zaid, N.A.M.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Rani, N.N.I.M.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, ume 16, 23–66. [Google Scholar] [CrossRef]
- Archer, C.B. Atopic dermatitis. Medicine 2021, 49, 370–373. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Song, A.; Lee, S.E.; Kim, J.H. Immunopathology and Immunotherapy of Inflammatory Skin Diseases. Immune Netw. 2022, 22, e7. [Google Scholar] [CrossRef]
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Gama, M.; Peixoto, D.; Sousa-Oliveira, I.; Ferreira-Faria, I.; Zeinali, M.; Abbaspour-Ravasjani, S.; Mascarenhas-Melo, F.; Hamishehkar, H.; Veiga, F. Nanocarrier-based dermopharmaceutical formulations for the topical management of atopic dermatitis. Int. J. Pharm. 2022, 618, 121656. [Google Scholar] [CrossRef]
- Sharma, S.; Naura, A.S. Potential of phytochemicals as immune-regulatory compounds in atopic diseases: A review. Biochem. Pharmacol. 2020, 173, 113790. [Google Scholar] [CrossRef]
- Wu, S.; Pang, Y.; He, Y.; Zhang, X.; Peng, L.; Guo, J.; Zeng, J. A comprehensive review of natural products against atopic dermatitis: Flavonoids, alkaloids, terpenes, glycosides and other compounds. Biomed. Pharmacother. 2021, 140, 111741. [Google Scholar] [CrossRef]
- Qadir, A.; Ullah, S.N.M.N.; Jahan, S.; Ali, A.; Khan, N. Drug delivery of natural products through nano-carriers for effective vitiligo therapy: A compendia review. J. Cosmet. Dermatol. 2022, 21, 5386–5404. [Google Scholar] [CrossRef]
- Xie, J.; Huang, S.; Huang, H.; Deng, X.; Yue, P.; Lin, J.; Yang, M.; Han, L.; Zhang, D.-K. Advances in the Application of Natural Products and the Novel Drug Delivery Systems for Psoriasis. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Chen-Yu, G.; Chun-Fen, Y.; Qi-Lu, L.; Qi, T.; Yan-Wei, X.; Wei-Na, L.; Guang-Xi, Z. Development of a Quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012, 430, 292–298. [Google Scholar] [CrossRef]
- Cassano, R.; Serini, S.; Curcio, F.; Trombino, S.; Calviello, G. Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics 2022, 14, 1593. [Google Scholar] [CrossRef]
- Drew, V.J.; Huang, H.-Y.; Tsai, Z.-H.; Tsai, H.-H.; Tseng, C.-L. Preparation of gelatin/epigallocatechin gallate self-assembly nanoparticles for transdermal drug delivery. J. Polym. Res. 2017, 24, 188. [Google Scholar] [CrossRef]
- Han, M.; Wang, X.; Wang, J.; Lang, D.; Xia, X.; Jia, Y.; Chen, Y. Ameliorative effects of epigallocatechin-3-gallate nanoparticles on 2,4-dinitrochlorobenzene induced atopic dermatitis: A potential mechanism of inflammation-related necroptosis. Front. Nutr. 2022, 9, 953646. [Google Scholar] [CrossRef]
- Chauhan, S.; Gulati, N.; Nagaich, U. Fabrication and evaluation of ultra deformable vesicles for atopic dermatitis as topical delivery. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 266–277. [Google Scholar] [CrossRef]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp. Dermatol. 2017, 27, 378–385. [Google Scholar] [CrossRef]
- Cervi, V.F.; Saccol, C.P.; Sari, M.H.M.; Martins, C.C.; da Rosa, L.S.; Ilha, B.D.; Soares, F.Z.; Luchese, C.; Wilhelm, E.A.; Cruz, L. Pullulan film incorporated with nanocapsules improves pomegranate seed oil anti-inflammatory and antioxidant effects in the treatment of atopic dermatitis in mice. Int. J. Pharm. 2021, 609, 121144. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, D.K.; Ashawat, M.S. Development of Phospholipids Vesicular Nanocarrier for Topical Delivery of Tea Tree Oil in Management of Atopic Dermatitis Using BALB/c Mice Model. Eur. J. Lipid Sci. Technol. 2021, 123, 2100002. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A Potential Therapeutic Agent in Cardiovascular Disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeon, S.H.; Ham, H.J.; Lee, H.P.; Song, M.J.; Hong, J.T. Improved Anti-Inflammatory Effects of Liposomal Astaxanthin on a Phthalic Anhydride-Induced Atopic Dermatitis Model. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Alugoju, P.; Swamy, V.K.D.K.; Anthikapalli, N.V.A.; Tencomnao, T. Health benefits of astaxanthin against age-related diseases of multiple organs: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 63, 10709–10774. [Google Scholar] [CrossRef]
- Ho, P.J.; Sung, J.J.; Cheon, K.K.; Tae, H.J. Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis. Phytomedicine 2018, 43, 110–119. [Google Scholar] [CrossRef]
- Hong, L.; Zhou, C.-L.; Chen, F.-P.; Han, D.; Wang, C.-Y.; Li, J.-X.; Chi, Z.; Liu, C.-G. Development of a carboxymethyl chitosan functionalized nanoemulsion formulation for increasing aqueous solubility, stability and skin permeability of astaxanthin using low-energy method. J. Microencapsul. 2017, 34, 707–721. [Google Scholar] [CrossRef]
- Eren, B.; Tanrıverdi, S.T.; Köse, F.A.; Özer. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2018, 18, 242–250. [Google Scholar] [CrossRef]
- Geng, Q.; Zhao, Y.; Wang, L.; Xu, L.; Chen, X.; Han, J. Development and Evaluation of Astaxanthin as Nanostructure Lipid Carriers in Topical Delivery. Aaps Pharmscitech 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Hemrajani, C.; Negi, P.; Parashar, A.; Gupta, G.; Jha, N.K.; Singh, S.K.; Chellappan, D.K.; Dua, K. Overcoming drug delivery barriers and challenges in topical therapy of atopic dermatitis: A nanotechnological perspective. Biomed. Pharmacother. 2022, 147, 112633. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.-T.; Teoh, M.-L.; Phang, S.-M.; Convey, P.; Yap, W.-H.; Goh, B.-H.; Beardall, J. Microalgae as Potential Anti-Inflammatory Natural Product Against Human Inflammatory Skin Diseases. Front. Pharmacol. 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Rühl, R.; Taner, C.; Schweigert, F.J.; Wahn, U.; Grüber, C. Serum carotenoids and atopy among children of different ethnic origin living in Germany. Pediatr. Allergy Immunol. 2010, 21, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Kake, T.; Imai, M.; Takahashi, N. Effects of β-carotene on oxazolone-induced atopic dermatitis in hairless mice. Exp. Dermatol. 2019, 28, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Semnani, D.; Nasari, M.; Fakhrali, A. PCL nanofibers loaded with beta-carotene: a novel treatment for eczema. Polym. Bull. 2017, 75, 2015–2026. [Google Scholar] [CrossRef]
- Takahashi, N.; Kake, T.; Hasegawa, S.; Imai, M. Effects of Post-administration of β-Carotene on Diet-induced Atopic Dermatitis in Hairless Mice. J. Oleo Sci. 2019, 68, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Garip, I.C.; Gulseren, G.; Tekinay, A.B.; Guler, M.O. Bioactive Supramolecular Peptide Nanofibers for Regenerative Medicine. Adv. Heal. Mater. 2014, 3, 1357–1376. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Mascarenhas-Melo, F.; Coimbra, S.C.; Pawar, K.D.; Peixoto, D.; Chá-Chá, R.; Araujo, A.R.; Cabral, C.; Pinto, S.; Veiga, F. Nanotechnology-based formulations toward the improved topical delivery of anti-acne active ingredients. Expert Opin. Drug Deliv. 2021, 18, 1435–1454. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, F.; Sharif, H.M.; Mallick, M.A.; Zahoor, F.; Abdulmalik, A.; Baig, W.; Shujaat, N.; Gul, S.; Bibi, G.; Ramzan, R.; et al. CAPSAICIN: ITS BIOLOGICAL ACTIVITIES AND IN SILICO TARGET FISHING. 2017, 74, 321–329.
- Gwinn, K.D. Bioactive Natural Products in Plant Disease Control, 1st ed.; Elsevier B.V: Amsterdam, The Netherlands, 2018; Volume 56, ISBN 9780444640581. [Google Scholar]
- Melliou, E.; Chinou, I. Chemistry and Bioactivities of Royal Jelly, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, ISBN 9780444634306. [Google Scholar]
- Ghiasi, Z.; Esmaeli, F.; Aghajani, M.; Ghazi-Khansari, M.; Faramarzi, M.A.; Amani, A. Enhancing analgesic and anti-inflammatory effects of capsaicin when loaded into olive oil nanoemulsion: An in vivo study. Int. J. Pharm. 2019, 559, 341–347. [Google Scholar] [CrossRef]
- Saini, K.; Modgill, N.; Singh, K.K.; Kakkar, V. Tetrahydrocurcumin Lipid Nanoparticle Based Gel Promotes Penetration into Deeper Skin Layers and Alleviates Atopic Dermatitis in 2,4-Dinitrochlorobenzene (DNCB) Mouse Model. Nanomaterials 2022, 12, 636. [Google Scholar] [CrossRef]
- Souto, E.B.; Dias-Ferreira, J.; Oliveira, J.; Sanchez-Lopez, E.; Lopez-Machado, A.; Espina, M.; Garcia, M.L.; Souto, S.B.; Martins-Gomes, C.; Silva, A.M. Trends in Atopic Dermatitis—From Standard Pharmacotherapy to Novel Drug Delivery Systems. Int. J. Mol. Sci. 2019, 20, 5659. [Google Scholar] [CrossRef]
- Raza, K.; Shareef, M.A.; Singal, P.; Sharma, G.; Negi, P.; Katare, O.P. Lipid-based capsaicin-loaded nano-colloidal biocompatible topical carriers with enhanced analgesic potential and decreased dermal irritation. J. Liposome Res. 2014, 24, 290–296. [Google Scholar] [CrossRef]
- Ghosalkar, S.; Singh, P.; Ravikumar, P. Emerging topical drug delivery approaches for the treatment of Atopic dermatitis. J. Cosmet. Dermatol. 2021, 21, 536–549. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.-D.; Jeong, H.-J.; Kim, H.-M. Down-regulation of thymic stromal lymphopoietin by curcumin. Pharmacol. Rep. 2013, 65, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, C.; Luo, F.; Yin, S.; Yang, X. Topical application of zein-silk sericin nanoparticles loaded with curcumin for improved therapy of dermatitis. Mater. Today Chem. 2022, 24, 100802. [Google Scholar] [CrossRef]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P.; Vanić. ; Škalko-Basnet, N. Liposomes augment biological benefits of curcumin for multitargeted skin therapy. Eur. J. Pharm. Biopharm. 2019, 144, 154–164. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, M.; Zhang, T.; Wu, J.; Wang, J.; Liu, K.; Zhan, N. Antioxidant and Anti-inflammatory Activities of Cynaroside from Elsholtiza bodinieri. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Tomczykowa, M.; Winnicka, K.; Kasacka, I.; Tomczyk, M. In vivo anti-inflammatory and anti-allergic activities of cynaroside evaluated by using hydrogel formulations. Biomed. Pharmacother. 2019, 121, 109681. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.A.; Ignacimuthu, S.; Michael, G.P.; Al Numair, K.S. Cancer Chemopreventive Potential of Luteolin-7-O-Glucoside Isolated From Ophiorrhiza mungos Linn. Nutr. Cancer 2011, 63, 130–138. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, L.-M.; Liu, X.-Q.; Li, B.; Wang, Q.; Dong, J.-X. Anti-HBV active flavone glucosides from Euphorbia humifusa Willd. Fitoterapia 2010, 81, 799–802. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344–e2344. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Torres, T.; Lima, S.A.C.; Reis, S. Hydrogels: A Promising Vehicle for the Topical Management of Atopic Dermatitis. Adv. Ther. 2021, 4, 2100028. [Google Scholar] [CrossRef]
- Qing, W.; Wang, Y.; Li, H.; Ma, F.; Zhu, J.; Liu, X. Preparation and Characterization of Copolymer Micelles for the Solubilization and In Vitro Release of Luteolin and Luteoloside. Aaps Pharmscitech 2016, 18, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Qing, W.; Wang, Y.; Wang, Y.; Zhao, D.; Liu, X.; Zhu, J. The modified nanocrystalline cellulose for hydrophobic drug delivery. Appl. Surf. Sci. 2016, 366, 404–409. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Yang, D.; Tang, X.; Liu, J. Development of triptolide-nanoemulsion gels for percutaneous administration: physicochemical, transport, pharmacokinetic and pharmacodynamic characteristics. J. Nanobiotechnol. 2017, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Hsieh, Y.-T.; Chan, L.Y.; Yang, T.-Y.; Maeda, T.; Chang, T.-M.; Huang, H.-C. Dictamnine delivered by PLGA nanocarriers ameliorated inflammation in an oxazolone-induced dermatitis mouse model. J. Control. Release 2020, 329, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Gao Chemical Constituents of Plants from the Genus Dictamnus. Chem. Biodivers. 2011, 8, 1234–1244. [CrossRef]
- Chang, T.-M.; Yang, T.-Y.; Niu, Y.-L.; Huang, H.-C. The Extract of D. dasycarpus Ameliorates Oxazolone-Induced Skin Damage in Mice by Anti-Inflammatory and Antioxidant Mechanisms. Antioxidants 2018, 7, 77. [Google Scholar] [CrossRef]
- Yang Decoction of Dictamnus Dasycarpus Turcz. Root Bark Ameliorates Skin Lesions and Inhibits Inflammatory Reactions in Mice with Contact Dermatitis. Pharmacogn. Mag. 2017, 13.
- Yang, N.; Shao, H.; Deng, J.; Yang, Y.; Tang, Z.; Wu, G.; Liu, Y. Dictamnine ameliorates chronic itch in DNFB-induced atopic dermatitis mice via inhibiting MrgprA3. Biochem. Pharmacol. 2023, 208, 115368. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol ( - ) -Epigallocatechin-3-Gallate ( EGCG ) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. 2023.
- Noh, S.U.; Cho, E.A.; Kim, H.O.; Park, Y.M. Epigallocatechin-3-gallate improves Dermatophagoides pteronissinus extract-induced atopic dermatitis-like skin lesions in NC/Nga mice by suppressing macrophage migration inhibitory factor. Int. Immunopharmacol. 2008, 8, 1172–1182. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Lin, C.-H.; Yang, S.-C.; Alalaiwe, A.; Fang, J.-Y. Nanoencapsulation of Tea Catechins for Enhancing Skin Absorption and Therapeutic Efficacy. Aaps Pharmscitech 2022, 23, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Wu, Y.-W.; Hung, J.-I.; Chen, M.-C. Epigallocatechin gallate/L-ascorbic acid–loaded poly-γ-glutamate microneedles with antioxidant, anti-inflammatory, and immunomodulatory effects for the treatment of atopic dermatitis. Acta Biomater. 2021, 130, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.H.M.D.; de Araújo, D.R. Exploring the Pharmacological Potential of Glycyrrhizic Acid: From Therapeutic Applications to Trends in Nanomedicine. Futur. Pharmacol. 2022, 2, 1–15. [Google Scholar] [CrossRef]
- Kowalska, A.; Kalinowska-Lis, U. 18β-Glycyrrhetinic acid: its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Peng, G.; Han, X. Glycyrrhizin ameliorates atopic dermatitis-like symptoms through inhibition of HMGB1. Int. Immunopharmacol. 2018, 60, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Cristiano, M.C.; Cilurzo, F.; Locatelli, M.; Iannotta, D.; Di Marzio, L.; Celia, C.; Paolino, D. Ammonium glycyrrhizate skin delivery from ultradeformable liposomes: A novel use as an anti-inflammatory agent in topical drug delivery. Colloids Surfaces B: Biointerfaces 2020, 193, 111152. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Kong, S.; Ouyang, Q.; Tao, J.; Lu, S.; Huang, Y.; Li, S.; Luo, H. Use of 18β-glycyrrhetinic acid nanocrystals to enhance anti-inflammatory activity by improving topical delivery. Colloids Surfaces B: Biointerfaces 2021, 205, 111791. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, R.; Liu, C.; Yang, C. Improved stability and skin penetration through glycethosomes loaded with glycyrrhetinic acid. Int. J. Cosmet. Sci. 2022, 44, 249–261. [Google Scholar] [CrossRef]
- Rashmirekha Sahoo,Patricia Jayshree Samuel Jacob, S. S. Biomedical Applications of Green Biopolymer Guar Gum. J. Pharm. Biomed. Sci. (J Pharm Biomed Sci. 2013, 35, 1783–1787.
- Ghosh, N.; Mitra, S.; Banerjee, E.R. Therapeutic effects of topically-administered guar gum nanoparticles in oxazolone-induced atopic dermatitis in mice. Biomed. Res. Ther. 2018, 5, 2305–2325. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Abdullah, F.; Mukherjee, A. Fabrication and fluorescent labeling of guar gum nanoparticles in a surfactant free aqueous environment. Mater. Sci. Eng. C 2015, 46, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, D.; Demuner, A.J.; Barbosa, L.C.; Csuk, R.; Heller, L. Hederagenin as a triterpene template for the development of new antitumor compounds. Eur. J. Med. Chem. 2015, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.H.; Oh, T.-W.; Nguyen, U.T.; Choi, M.-J.; Yang, I.-J.; Shin, H.-M. A Natural Compound Mixture Containing Arctigenin, Hederagenin, and Baicalein Alleviates Atopic Dermatitis in Mice by Regulating HPA Axis and Immune Activity. Evidence-Based Complement. Altern. Med. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, D.; Barbosa, L.C.; Demuner, A.J.; Nain-Perez, A.; Ferreira, S.R.; Fujiwara, R.T.; de Almeida, R.M.; Heller, L.; Csuk, R. Leishmanicidal and cytotoxic activity of hederagenin-bistriazolyl derivatives. Eur. J. Med. Chem. 2017, 140, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, J.; Yang, B.; Ma, S.; Zhang, C.; Zhao, G. Therapeutic Effect of Tetrapanax papyriferus and Hederagenin on Chronic Neuropathic Pain of Chronic Constriction Injury of Sciatic Nerve Rats Based on KEGG Pathway Prediction and Experimental Verification. Evidence-Based Complement. Altern. Med. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; Ratih, K.; Kim, G.-J.; Lee, Y.-R.; Shin, J.-S.; Chung, K.-H.; Choi, E.-J.; Kim, E.-K.; An, J.H. Immunomodulatory and anti-inflammatory efficacy of hederagenin-coated maghemite (γ-Fe2O3) nanoparticles in an atopic dermatitis model. Colloids Surfaces B: Biointerfaces 2021, 210, 112244. [Google Scholar] [CrossRef] [PubMed]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2016, 16, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Choi, D.W.; Jung, C.H.; Kim, Y.-J.; Jung, S.Y.; Shon, D.-H. Piper nigrum Fruit Extract Prevents TMA-Induced Allergic Contact Dermatitis by Regulating Th2 Cytokine Production. J. Agric. Sci. 2015, 7. [Google Scholar] [CrossRef]
- Choi, D.W.; Jung, S.Y.; Shon, D.-H.; Shin, H.S. Piperine Ameliorates Trimellitic Anhydride-Induced Atopic Dermatitis-Like Symptoms by Suppressing Th2-Mediated Immune Responses via Inhibition of STAT6 Phosphorylation. Molecules 2020, 25, 2186. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, D.K.; Ashawat, M.S. Topical creams of piperine loaded lipid nanocarriers for management of atopic dermatitis: development, characterization, and in vivo investigation using BALB/c mice model. J. Liposome Res. 2021, 32, 62–73. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Mascarenhas-Melo, F.; Coimbra, S.C.; Pawar, K.D.; Peixoto, D.; Chá-Chá, R.; Araujo, A.R.; Cabral, C.; Pinto, S.; Veiga, F. Nanotechnology-based formulations toward the improved topical delivery of anti-acne active ingredients. Expert Opin. Drug Deliv. 2021, 18, 1435–1454. [Google Scholar] [CrossRef] [PubMed]
- Politi, F.A.S.; Carvalho, S.G.; Rodero, C.F.; dos Santos, K.P.; Meneguin, A.B.; Sorrechia, R.; Chiavacci, L.A.; Chorilli, M. Piperine-loaded nanoparticles incorporated into hyaluronic acid/sodium alginate-based membranes for the treatment of inflammatory skin diseases. Int. J. Biol. Macromol. 2023, 227, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, K.; Kadian, V.; Puri, V.; Bhardwaj, B.Y.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New insights into quercetin nanoformulations for topical delivery. Phytomedicine Plus 2022, 2, 100257. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2017, 41, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy, Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharopov, F.; Tumer, T.B.; Ozleyen, A.; Rodríguez-Pérez, C.; Ezzat, S.M.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I.; et al. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.-Q. Role of Resveratrol in Regulating Cutaneous Functions. Evidence-Based Complement. Altern. Med. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J. Cosmet. Laser Ther. 2018, 21, 84–90. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Zaid, N.A.M.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Rani, N.N.I.M.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, ume 16, 23–66. [Google Scholar] [CrossRef]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Resveratrol attenuates HMGB1 signaling and inflammation in house dust mite-induced atopic dermatitis in mice. Int. Immunopharmacol. 2014, 23, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Sozmen, S.C.; Karaman, M.; Micili, S.C.; Isik, S.; Ayyildiz, Z.A.; Bagriyanik, A.; Uzuner, N.; Karaman, O. Resveratrol ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis-like lesions through effects on the epithelium. PeerJ 2016, 4, e1889. [Google Scholar] [CrossRef] [PubMed]
- Soldati, P.P.; Polonini, H.C.; Paes, C.Q.; Restrepob, J.A.; Creczynksi-Pasa, T.B.; Chaves, M.G.; Brandão, M.A.; Pittella, F.; Raposo, N.R. Controlled release of resveratrol from lipid nanoparticles improves antioxidant effect. IFAC-PapersOnLine 2018, 51, 16–21. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, G.; Ni, S.; Xia, Q. Lipid based nanocarriers with different lipid compositions for topical delivery of resveratrol: comparative analysis of characteristics and performance. J. Drug Deliv. Sci. Technol. 2014, 24, 591–600. [Google Scholar] [CrossRef]
- Shrotriya, S.N.; Ranpise, N.S.; Vidhate, B.V. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Deliv. Transl. Res. 2016, 7, 37–52. [Google Scholar] [CrossRef]
- Wathoni Physically Crosslinked-Sacran Hydrogel Films for Wound Dressing Application. Int. J. Biol. Macromol. 2016, 89, 465–470. [CrossRef]
- Ren, S.; Gao, Y.; Wang, L.; Qiu, C.; Yang, L.; Li, L.; Xiao, Y.; Xiao, N.; Liao, L.; Zuo, Z.; et al. Sacran polysaccharide improves atopic dermatitis through inhibiting Th2 type immune response. Life Sci. 2021, 288, 120205. [Google Scholar] [CrossRef]
- Goto Sacran, a Sulfated Polysaccharide, Suppresses the Absorption of Lipids and Modulates the Intestinal Flora in Non-Alcoholic Steatohepatitis Model Rats. Life Sci. 2021, 268.
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [PubMed]
- Rigon, C.; Marchiori, M.C.L.; Jardim, F.d.S.; Pegoraro, N.S.; Chaves, P.d.S.; Velho, M.C.; Beck, R.C.R.; Ourique, A.F.; Sari, M.H.M.; de Oliveira, S.M.; et al. Hydrogel containing silibinin nanocapsules presents effective anti-inflammatory action in a model of irritant contact dermatitis in mice. Eur. J. Pharm. Sci. 2019, 137, 104969. [Google Scholar] [CrossRef]
- Shrotriya, S.N.; Vidhate, B.V.; Shukla, M.S. Formulation and development of Silybin loaded solid lipid nanoparticle enriched gel for irritant contact dermatitis. J. Drug Deliv. Sci. Technol. 2017, 41, 164–173. [Google Scholar] [CrossRef]
- Gehrcke, M.; Martins, C.C.; Brum, T.d.B.; da Rosa, L.S.; Luchese, C.; Wilhelm, E.A.; Soares, F.Z.M.; Cruz, L. Novel Pullulan/Gellan Gum Bilayer Film as a Vehicle for Silibinin-Loaded Nanocapsules in the Topical Treatment of Atopic Dermatitis. Pharmaceutics 2022, 14, 2352. [Google Scholar] [CrossRef]
- Hussein, A.K.; Ammawi, T.E.; Mady, F.M.; Elkader, H.A.; Essa, H. Formulation and clinical evaluation of silymarin pluronic-lecithin organogels for treatment of atopic dermatitis. Drug Des. Dev. Ther. 2016, 10, 1101–1110. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Liu, X.; Wu, X.; Huang, L.; Gao, W. Triptolide: pharmacological spectrum, biosynthesis, chemical synthesis and derivatives. Theranostics 2021, 11, 7199–7221. [Google Scholar] [CrossRef]
- Cassano, R.; Serini, S.; Curcio, F.; Trombino, S.; Calviello, G. Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics 2022, 14, 1593. [Google Scholar] [CrossRef]
- Park, K.S. Pharmacological Effects of Centella asiatica on Skin Diseases: Evidence and Possible Mechanisms. Evidence-Based Complement. Altern. Med. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Susilawati, Y.; Chaerunisa, A.Y.; Purwaningsih, H. Phytosome drug delivery system for natural cosmeceutical compounds: Whitening agent and skin antioxidant agent. J. Adv. Pharm. Technol. Res. 2021, 12, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.L.; Hon, K.L.E.; Leung, P.C.; Sam, S.W.; Fung, K.P.; Lee, M.Y.H.; Lau, H.Y.A. Traditional Chinese medicine for atopic eczema: PentaHerbs formula suppresses inflammatory mediators release from mast cells. J. Ethnopharmacol. 2008, 120, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.C.-L.; Wat, E.; Kan, C.-W.; Leung, P.-C.; Wang, W. Drug delivery system of dual-responsive PF127 hydrogel with polysaccharide-based nano-conjugate for textile-based transdermal therapy. Carbohydr. Polym. 2020, 236, 116074. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.S.M.; Jiao, D.; Chan, B.C.L.; Hon, K.-L.; Leung, P.C.; Lau, C.B.S.; Wong, E.C.W.; Cheng, L.; Chan, C.K.M.; Lam, C.W.K.; et al. Anti-Inflammatory Activities of Pentaherbs Formula, Berberine, Gallic Acid and Chlorogenic Acid in Atopic Dermatitis-Like Skin Inflammation. Molecules 2016, 21, 519. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.G.; Do, T.T.; Nguyen, T.N.; Dong, N.P.; Vu, M.T. Chemical Constituents of Eupatorium japonicum and Anti-Inflammatory, Cytotoxic, and Apoptotic Activities of Eupatoriopicrin on Cancer Stem Cells. Evidence-Based Complement. Altern. Med. 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-I.; Jeon, Y.-J.; Lee, S.; Lee, Y.G.; Kim, J.B.; Kwon, H.C.; Kim, S.H.; Kim, I.; Lee, K.; Han, Y.S. Apoptotic and Anti-Inflammatory Effects of Eupatorium japonicum Thunb. in Rheumatoid Arthritis Fibroblast-Like Synoviocytes. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Moon, S.-K.; Kim, J.-K.; Kim, W.J.; Kim, Y.-J.; Kim, H. Structural properties and anti-dermatitis effects of flavonoids-loaded gold nanoparticles prepared by Eupatorium japonicum. Front. Pharmacol. 2022, 13, 1055378. [Google Scholar] [CrossRef] [PubMed]
- Kwon & Kim In Vitro Skin Permeation and Anti-Atopic Efficacy of Lipid Na- Nocarriers Containing Water Soluble Extracts of Houttuynia Cordata, Drug Dev. Ind. Pharm 2014, 40, 1350–1357.
- Ahn, J.; Kim, J. Chemical constituents from Houttuynia cordata. Planta Medica 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Damiani, G.; Eggenhöffner, R.; Pigatto, P.D.M.; Bragazzi, N.L. Nanotechnology meets atopic dermatitis: Current solutions, challenges and future prospects. Insights and implications from a systematic review of the literature. Bioact. Mater. 2019, 4, 380–386. [Google Scholar] [CrossRef]
- Kildaci, I.; Budama-Kilinc, Y.; Kecel-Gunduz, S.; Altuntas, E. Linseed Oil Nanoemulsions for treatment of Atopic Dermatitis disease: Formulation, characterization, in vitro and in silico evaluations. J. Drug Deliv. Sci. Technol. 2021, 64, 102652. [Google Scholar] [CrossRef]
- Toure & Xueming Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. FOOD Sci. FOOD Saf. 2010, 9.
- Hashempur Effect of Linum Usitatissimum, L. (Linseed) Oil on Mild and Moderate Carpal Tunnel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial DARU. J. Pharm. Sci. 2014, 22. [Google Scholar]
- James, M.J.; Gibson, R.A.; Cleland, L.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000, 71, 343s–348s. [Google Scholar] [CrossRef] [PubMed]
- Baboota Submicron Size Formulation of Linseed Oil Containing Omega-3 Fatty Acid for Topical Delivery. J. Dispers. Sci. Technol. 2012, 33, 1259–1266. [CrossRef]
- Takic, M.; Pokimica, B.; Petrovic-Oggiano, G.; Popovic, T. Effects of Dietary α-Linolenic Acid Treatment and the Efficiency of Its Conversion to Eicosapentaenoic and Docosahexaenoic Acids in Obesity and Related Diseases. Molecules 2022, 27, 4471. [Google Scholar] [CrossRef]
- Thakur Nanoemulsions: A Review on Various Pharmaceutical Application. Glob. J. Pharmacol. 2012, 6, 222–225. [CrossRef]
- Shaban, N.Z.; Mohammed, A.S.; Abu-Serie, M.M.; Maher, A.M.; Habashy, N.H. Inhibition of oxidative stress, IL-13, and WNT/β-catenin in ovalbumin-sensitized rats by a novel organogel of Punica granatum seed oil saponifiable fraction. Biomed. Pharmacother. 2022, 154, 113667. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Talaat, I.M.; Elrashidy, F.H.; Hegazy, A.Y.; Sultan, A.S. Therapeutic role of Punica granatum (pomegranate) seed oil extract on bone turnover and resorption induced in ovariectomized rats. J. Nutr. Heal. Aging 2017, 21, 1299–1306. [Google Scholar] [CrossRef]
- Park DK, Lee YG, P.H.-J. Extract of Rhus Verniciflua Bark Sup- Presses 2,4-Dinitrofluorobenzene-Induced Allergic Contact Dermati- Tis. Evid Based Complement Altern. Med 2013.
- Jeong, J.H.; Back, S.K.; An, J.H.; Lee, N.; Kim, D.; Na, C.S.; Jeong, Y.; Han, S.Y. Topical film prepared with Rhus verniciflua extract-loaded pullulan hydrogel for atopic dermatitis treatment. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2019, 107, 2325–2334. [Google Scholar] [CrossRef]
- Jiang, P.; Sun, H. Sulfuretin alleviates atopic dermatitis-like symptoms in mice via suppressing Th2 cell activity. Immunol. Res. 2018, 66, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.S.; Long, X.; Su, X.-Z.; Lu, F. Melaleuca alternifolia (tea tree) oil and its monoterpene constituents in treating protozoan and helminthic infections. Biomed. Pharmacother. 2020, 130, 110624. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).