Submitted:
08 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pathology:
3. Pathogenesis of Bronchopulmonary Dysplasia
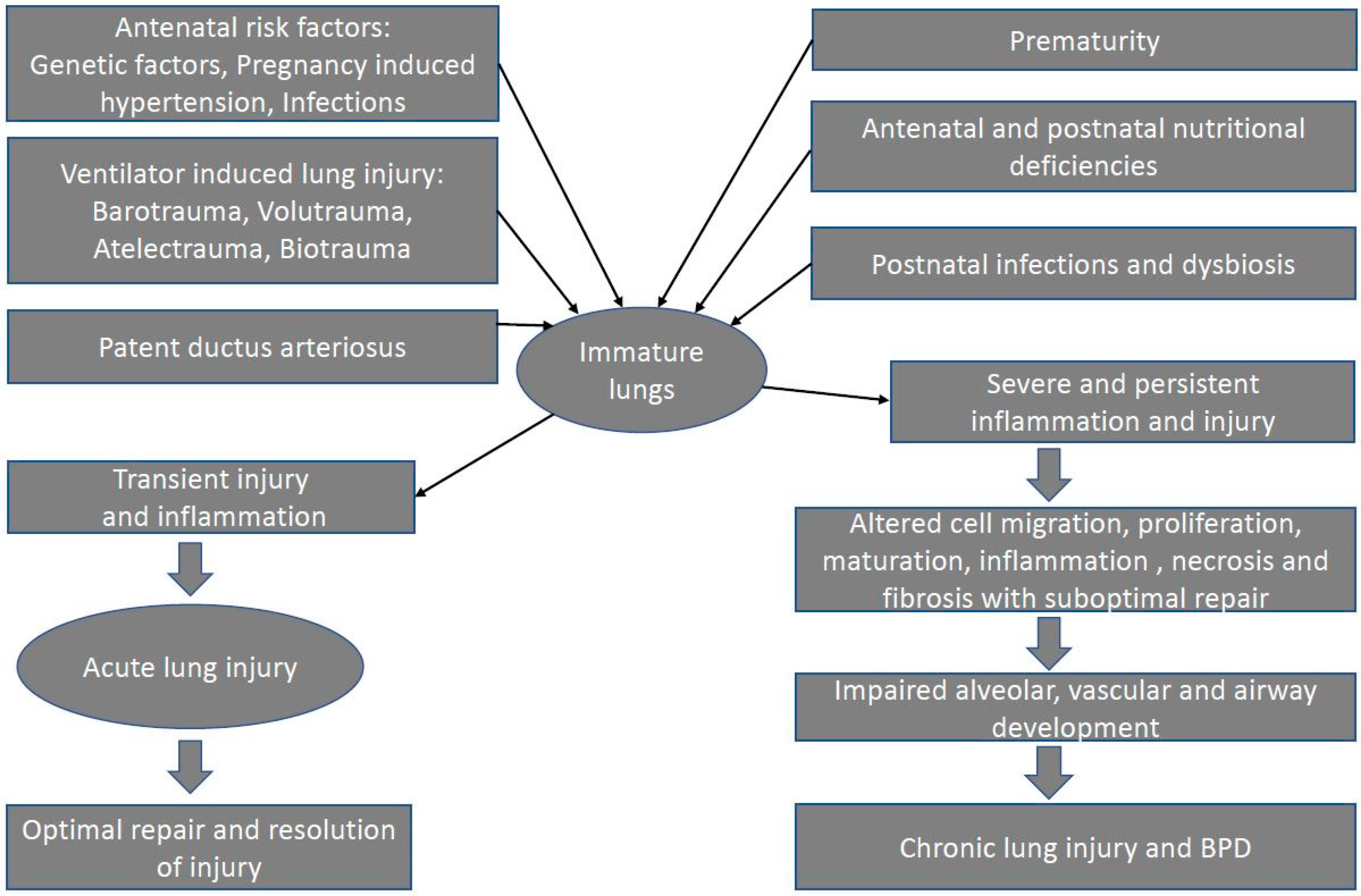
3.1. Prenatal risk factors
3.1.1. Maternal smoking:
3.1.2. Chorioamnionitis:
3.1.3. Pregnancy-induced hypertension (PIH):
3.1.4. Intrauterine Growth Restriction (IUGR):
3.1.5. Genetics:
3.2. Postnatal risk factors
3.2.1. Mechanical ventilation and ventilator-induced lung injury (VILI)
- Barotrauma
- Volutrauma
- Atelectrauma
- Oxygen toxicity
- Biotrauma
- Mechanical power, Stress, and Strain:
- Lung deflation injury
- Pre-existing lung disease
3.2.2. Patent Ductus Arteriosus (PDA)
3.2.3. Sepsis
3.2.4. Dysbiosis
3.2.5. Nutrition
4. Conclusions:
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abman, S.H., E. Bancalari, and A. Jobe, The Evolution of Bronchopulmonary Dysplasia after 50 Years. Am J Respir Crit Care Med, 2017. 195(4): p. 421-424. [CrossRef]
- Northway, W.H., Jr., R.C. Rosan, and D.Y. Porter, Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med, 1967. 276(7): p. 357-68. [CrossRef]
- Jobe, A. and M. Ikegami, Surfactant for the treatment of respiratory distress syndrome. Am Rev Respir Dis, 1987. 136(5): p. 1256-75. [CrossRef]
- Matute-Bello, G., et al., An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol, 2011. 44(5): p. 725-38. [CrossRef]
- Kalikkot Thekkeveedu, R., et al., Ventilation-Induced Lung Injury (VILI) in Neonates: Evidence-Based Concepts and Lung-Protective Strategies. J Clin Med, 2022. 11(3). [CrossRef]
- Tomashefski, J.F., Jr., Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med, 2000. 21(3): p. 435-66. [CrossRef]
- Jobe, A.H. and E. Bancalari, Bronchopulmonary dysplasia. Am J Respir Crit Care Med, 2001. 163(7): p. 1723-9.
- Bhandari, A. and V. Bhandari, Pathogenesis, pathology and pathophysiology of pulmonary sequelae of bronchopulmonary dysplasia in premature infants. Front Biosci, 2003. 8: p. e370-80. [CrossRef]
- Hoo, A.F., et al., Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med, 1998. 158(3): p. 700-5. [CrossRef]
- McEvoy, C.T. and E.R. Spindel, Pulmonary Effects of Maternal Smoking on the Fetus and Child: Effects on Lung Development, Respiratory Morbidities, and Life Long Lung Health. Paediatr Respir Rev, 2017. 21: p. 27-33. [CrossRef]
- Antonucci, R., et al., Intrauterine smoke exposure: a new risk factor for bronchopulmonary dysplasia? J Perinat Med, 2004. 32(3): p. 272-7.
- Morrow, L.A., et al., Antenatal Determinants of Bronchopulmonary Dysplasia and Late Respiratory Disease in Preterm Infants. Am J Respir Crit Care Med, 2017. 196(3): p. 364-374. [CrossRef]
- Gonzalez-Luis, G.E., et al., Tobacco Smoking During Pregnancy Is Associated With Increased Risk of Moderate/Severe Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front Pediatr, 2020. 8: p. 160. [CrossRef]
- Kalikkot Thekkeveedu, R., M.C. Guaman, and B. Shivanna, Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir Med, 2017. 132: p. 170-177. [CrossRef]
- Choi, C.W., Chorioamnionitis: Is a major player in the development of bronchopulmonary dysplasia? Korean J Pediatr, 2017. 60(7): p. 203-207.
- Villamor-Martinez, E., et al., Association of Chorioamnionitis With Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review, Meta-analysis, and Metaregression. JAMA Netw Open, 2019. 2(11): p. e1914611.
- Dessardo, N.S., et al., chorioamnionitis and chronic lung disease of prematurity: a path analysis of causality. Am J Perinatol, 2012. 29(2): p. 133-40. [CrossRef]
- Ballard, A.R., et al., chorioamnionitis and subsequent bronchopulmonary dysplasia in very-low-birth weight infants: a 25-year cohort. J Perinatol, 2016. 36(12): p. 1045-1048. [CrossRef]
- Collins, J.J., et al., inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol, 2010. 299(6): p. L852-60. [CrossRef]
- Viscardi, R., et al., Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr Dev Pathol, 2006. 9(2): p. 143-51. [CrossRef]
- Schelonka, R.L., et al., Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J, 2005. 24(12): p. 1033-9. [CrossRef]
- Burton, G.J., D.S. Charnock-Jones, and E. Jauniaux, Regulation of vascular growth and function in the human placenta. Reproduction, 2009. 138(6): p. 895-902. [CrossRef]
- Shin, S.H., et al., The Association of Pregnancy-induced Hypertension with Bronchopulmonary Dysplasia - A Retrospective Study Based on the Korean Neonatal Network database. Sci Rep, 2020. 10(1): p. 5600. [CrossRef]
- Razak, A., et al., Pregnancy-induced hypertension and neonatal outcomes: a systematic review and meta-analysis. J Perinatol, 2018. 38(1): p. 46-53. [CrossRef]
- Pierro, M., et al., association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: a systematic review, meta-analysis and meta-regression. Thorax, 2022. 77(3): p. 268-275. [CrossRef]
- Sehgal, A., et al., Preterm growth restriction and bronchopulmonary dysplasia: the vascular hypothesis and related physiology. J Physiol, 2019. 597(4): p. 1209-1220. [CrossRef]
- Groene, S.G., et al., Respiratory distress syndrome and bronchopulmonary dysplasia after fetal growth restriction: Lessons from a natural experiment in identical twins. EClinicalMedicine, 2021. 32: p. 100725. [CrossRef]
- Le Cras, T.D., et al., treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol, 2002. 283(3): p. L555-62. [CrossRef]
- Rozance, P.J., et al., Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am J Physiol Lung Cell Mol Physiol, 2011. 301(6): p. L860-71. [CrossRef]
- Bhandari, V., et al., Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics, 2006. 117(6): p. 1901-6. [CrossRef]
- Lavoie, P.M., C. Pham, and K.L. Jang, Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics, 2008. 122(3): p. 479-85. [CrossRef]
- Yu, K.H., et al., The genetic predisposition to bronchopulmonary dysplasia. Curr Opin Pediatr, 2016. 28(3): p. 318-23. [CrossRef]
- Mailaparambil, B., et al., Genetic and epidemiological risk factors in the development of bronchopulmonary dysplasia. Dis Markers, 2010. 29(1): p. 1-9.
- Floros, J., et al., IL-18R1 and IL-18RAP SNPs may be associated with bronchopulmonary dysplasia in African-American infants. Pediatr Res, 2012. 71(1): p. 107-14. [CrossRef]
- Hadchouel, A., et al., Matrix metalloproteinase gene polymorphisms and bronchopulmonary dysplasia: identification of MMP16 as a new player in lung development. PLoS One, 2008. 3(9): p. e3188. [CrossRef]
- Ambalavanan, N., et al., Integrated genomic analyses in bronchopulmonary dysplasia. J Pediatr, 2015. 166(3): p. 531-7 e13. [CrossRef]
- Dreyfuss, D., et al., Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis, 1985. 132(4): p. 880-4. [CrossRef]
- Webb, H.H. and D.F. Tierney, Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis, 1974. 110(5): p. 556-65. [CrossRef]
- Kolobow, T., et al., Severe impairment in lung function induced by high peak airway pressure during mechanical ventilation. An experimental study. Am Rev Respir Dis, 1987. 135(2): p. 312-5. [CrossRef]
- Petersen, G.W. and H. Baier, Incidence of pulmonary barotrauma in a medical ICU. Crit Care Med, 1983. 11(2): p. 67-9. [CrossRef]
- Slutsky, A.S., Lung injury caused by mechanical ventilation. Chest, 1999. 116(1 Suppl): p. 9s-15s. [CrossRef]
- Wada, K., A.H. Jobe, and M. Ikegami, Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J Appl Physiol (1985), 1997. 83(4): p. 1054-61. [CrossRef]
- Bjorklund, L.J., et al., Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res, 1997. 42(3): p. 348-55. [CrossRef]
- Frank, J.A., et al., Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med, 2002. 165(2): p. 242-9. [CrossRef]
- Brower, R.G., et al., ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med, 2000. 342(18): p. 1301-8. [CrossRef]
- Lista, G., et al., impact of targeted-volume ventilation on lung inflammatory response in preterm infants with respiratory distress syndrome (RDS). Pediatr Pulmonol, 2004. 37(6): p. 510-4. [CrossRef]
- Dreyfuss, D., et al., High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis, 1988. 137(5): p. 1159-64. [CrossRef]
- Hernandez, L.A., et al., Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J Appl Physiol (1985), 1989. 66(5): p. 2364-8. [CrossRef]
- Carlton, D.P., et al., Lung overexpansion increases pulmonary microvascular protein permeability in young lambs. J Appl Physiol (1985), 1990. 69(2): p. 577-83. [CrossRef]
- Klingenberg, C., et al., Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev, 2017. 10: p. Cd003666.
- Beitler, J.R., A. Malhotra, and B.T. Thompson, Ventilator-induced Lung Injury. Clin Chest Med, 2016. 37(4): p. 633-646.
- Taskar, V., et al., Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med, 1997. 155(1): p. 313-20. [CrossRef]
- Mead, J., T. Takishima, and D. Leith, Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol, 1970. 28(5): p. 596-608. [CrossRef]
- Clark, R.H., et al., Lung injury in neonates: causes, strategies for prevention, and long-term consequences. J Pediatr, 2001. 139(4): p. 478-86. [CrossRef]
- Froese, A.B., et al., Optimizing alveolar expansion prolongs the effectiveness of exogenous surfactant therapy in the adult rabbit. Am Rev Respir Dis, 1993. 148(3): p. 569-77. [CrossRef]
- Sandhar, B.K., et al., Effects of positive end-expiratory pressure on hyaline membrane formation in a rabbit model of the neonatal respiratory distress syndrome. Intensive Care Med, 1988. 14(5): p. 538-46. [CrossRef]
- Muscedere, J.G., et al., Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med, 1994. 149(5): p. 1327-34. [CrossRef]
- Bhandari, V., Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med, 2010. 15(4): p. 223-9. [CrossRef]
- Frank, L., Antioxidants, nutrition, and bronchopulmonary dysplasia. Clin Perinatol, 1992. 19(3): p. 541-62. [CrossRef]
- Berkelhamer, S.K., et al., Developmental differences in hyperoxia-induced oxidative stress and cellular responses in the murine lung. Free Radic Biol Med, 2013. 61: p. 51-60. [CrossRef]
- Irwin, D., et al., Neonatal lung side population cells demonstrate endothelial potential and are altered in response to hyperoxia-induced lung simplification. Am J Physiol Lung Cell Mol Physiol, 2007. 293(4): p. L941-51. [CrossRef]
- Kulkarni, A.C., P. Kuppusamy, and N. Parinandi, Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid Redox Signal, 2007. 9(10): p. 1717-30. [CrossRef]
- Kapadia, V.S., et al., Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics, 2013. 132(6): p. e1488-96. [CrossRef]
- Saugstad, O.D. and D. Aune, Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology, 2014. 105(1): p. 55-63. [CrossRef]
- Vogel, E.R., et al., Perinatal oxygen in the developing lung. Can J Physiol Pharmacol, 2015. 93(2): p. 119-27. [CrossRef]
- Ranieri, V.M., et al., effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. Jama, 1999. 282(1): p. 54-61. [CrossRef]
- Tremblay, L., et al., Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest, 1997. 99(5): p. 944-52. [CrossRef]
- Chiumello, D., G. Pristine, and A.S. Slutsky, Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med, 1999. 160(1): p. 109-16. [CrossRef]
- Curley, G.F., et al., Biotrauma and Ventilator-Induced Lung Injury: Clinical Implications. Chest, 2016. 150(5): p. 1109-1117.
- Vasques, F., et al., Determinants and Prevention of Ventilator-Induced Lung Injury. Crit Care Clin, 2018. 34(3): p. 343-356. [CrossRef]
- Cressoni, M., et al., Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology, 2016. 124(5): p. 1100-8. [CrossRef]
- Chiumello, D., et al., Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med, 2008. 178(4): p. 346-55. [CrossRef]
- Marini, J.J., P.R.M. Rocco, and L. Gattinoni, Static and Dynamic Contributors to Ventilator-induced Lung Injury in Clinical Practice. Pressure, Energy, and Power. Am J Respir Crit Care Med, 2020. 201(7): p. 767-774. [CrossRef]
- Cressoni, M., et al., Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med, 2014. 189(2): p. 149-58. [CrossRef]
- Protti, A., et al., Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med, 2013. 41(4): p. 1046-55.
- Katira, B.H., et al., Abrupt Deflation after Sustained Inflation Causes Lung Injury. Am J Respir Crit Care Med, 2018. 198(9): p. 1165-1176. [CrossRef]
- Varsila, E., et al., closure of patent ductus arteriosus decreases pulmonary myeloperoxidase in premature infants with respiratory distress syndrome. Biol Neonate, 1995. 67(3): p. 167-71. [CrossRef]
- Slaughter, J.L., et al., Comparative Effectiveness of Nonsteroidal Anti-inflammatory Drug Treatment vs No Treatment for Patent Ductus Arteriosus in Preterm Infants. JAMA Pediatr, 2017. 171(3): p. e164354. [CrossRef]
- McCurnin, D., et al., Ibuprofen-induced patent ductus arteriosus closure: physiologic, histologic, and biochemical effects on the premature lung. Pediatrics, 2008. 121(5): p. 945-56. [CrossRef]
- Brooks, J.M., et al., Is surgical ligation of patent ductus arteriosus necessary? The Western Australian experience of conservative management. Arch Dis Child Fetal Neonatal Ed, 2005. 90(3): p. F235-9. [CrossRef]
- Madan, J.C., et al., Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics, 2009. 123(2): p. 674-81. [CrossRef]
- Sellmer, A., et al., morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed, 2013. 98(6): p. F505-10. [CrossRef]
- Clyman, R.I., et al., Prolonged Tracheal Intubation and the Association Between Patent Ductus Arteriosus and Bronchopulmonary Dysplasia: A Secondary Analysis of the PDA-TOLERATE trial. J Pediatr, 2021. 229: p. 283-288 e2. [CrossRef]
- Mirza, H., et al., duration of significant patent ductus arteriosus and bronchopulmonary dysplasia in extremely preterm infants. J Perinatol, 2019. 39(12): p. 1648-1655. [CrossRef]
- Hundscheid, T., et al., Expectant Management or Early Ibuprofen for Patent Ductus Arteriosus. N Engl J Med, 2023. 388(11): p. 980-990. [CrossRef]
- Huang, X., et al., Decreased plasma levels of PDGF-BB, VEGF-A, and HIF-2alpha in preterm infants after ibuprofen treatment. Front Pediatr, 2022. 10: p. 919879.
- Ambalavanan, N., et al., Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics, 2009. 123(4): p. 1132-41. [CrossRef]
- Franco, M.L., et al., LPS-induced lung injury in neonatal rats: changes in gelatinase activities and consequences on lung growth. Am J Physiol Lung Cell Mol Physiol, 2002. 282(3): p. L491-500. [CrossRef]
- Choi, C.W., et al., Protective effect of chorioamnionitis on the development of bronchopulmonary dysplasia triggered by postnatal systemic inflammation in neonatal rats. Pediatr Res, 2016. 79(2): p. 287-94. [CrossRef]
- Shrestha, A.K., et al., Consequences of early postnatal lipopolysaccharide exposure on developing lungs in mice. Am J Physiol Lung Cell Mol Physiol, 2019. 316(1): p. L229-L244. [CrossRef]
- Oh, W., et al., association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr, 2005. 147(6): p. 786-90. [CrossRef]
- Lahra, M.M., P.J. Beeby, and H.E. Jeffery, Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics, 2009. 123(5): p. 1314-9. [CrossRef]
- Zemanick, E.T., et al., Assessment of airway microbiota and inflammation in cystic fibrosis using multiple sampling methods. Ann Am Thorac Soc, 2015. 12(2): p. 221-9. [CrossRef]
- Pammi, M., et al., Airway Microbiome and Development of Bronchopulmonary Dysplasia in Preterm Infants: A Systematic Review. J Pediatr, 2019. 204: p. 126-133 e2. [CrossRef]
- Taft, D.H., et al., Center Variation in Intestinal Microbiota Prior to Late-Onset Sepsis in Preterm Infants. PLoS One, 2015. 10(6): p. e0130604. [CrossRef]
- Wagner, B.D., et al., Airway Microbial Community Turnover Differs by BPD Severity in Ventilated Preterm Infants. PLoS One, 2017. 12(1): p. e0170120. [CrossRef]
- Lohmann, P., et al., The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res, 2014. 76(3): p. 294-301. [CrossRef]
- Imamura, T., et al., The Microbiome of the Lower Respiratory Tract in Premature Infants with and without Severe Bronchopulmonary Dysplasia. Am J Perinatol, 2017. 34(1): p. 80-87. [CrossRef]
- Surana, N.K. and D.L. Kasper, Deciphering the tete-a-tete between the microbiota and the immune system. J Clin Invest, 2014. 124(10): p. 4197-203.
- Gray, J., et al., Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci Transl Med, 2017. 9(376). [CrossRef]
- Novitsky, A., et al., Prolonged early antibiotic use and bronchopulmonary dysplasia in very low birth weight infants. Am J Perinatol, 2015. 32(1): p. 43-8. [CrossRef]
- Dickson, R.P., et al., Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol, 2016. 1(10): p. 16113. [CrossRef]
- Budden, K.F., et al., Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol, 2017. 15(1): p. 55-63. [CrossRef]
- Fischader, G., et al., Release of MCP-1 and IL-8 from lung epithelial cells exposed to volatile organic compounds. Toxicol In Vitro, 2008. 22(2): p. 359-66. [CrossRef]
- Yoon, H.I., et al., exposure to volatile organic compounds and loss of pulmonary function in the elderly. Eur Respir J, 2010. 36(6): p. 1270-6. [CrossRef]
- Romijn, M., et al., Exhaled Volatile Organic Compounds for Early Prediction of Bronchopulmonary Dysplasia in Infants Born Preterm. J Pediatr, 2023: p. 113368. [CrossRef]
- Davidson, S., et al., Energy intake, growth, and development in ventilated very-low-birth-weight infants with and without bronchopulmonary dysplasia. Am J Dis Child, 1990. 144(5): p. 553-9. [CrossRef]
- Underwood, M.A., et al., Malnutrition, poor postnatal growth, intestinal dysbiosis and the developing lung. J Perinatol, 2021. 41(8): p. 1797-1810. [CrossRef]
- Milanesi, B.G., et al., Assessment of early nutritional intake in preterm infants with bronchopulmonary dysplasia: a cohort study. Eur J Pediatr, 2021. 180(5): p. 1423-1430. [CrossRef]
- Stephens, B.E., et al., Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J Perinatol, 2008. 28(2): p. 123-8. [CrossRef]
- Massaro, D. and G.D. Massaro, Retinoids, alveolus formation, and alveolar deficiency: clinical implications. Am J Respir Cell Mol Biol, 2003. 28(3): p. 271-4.
- Shenai, J.P., F. Chytil, and M.T. Stahlman, Liver vitamin A reserves of very low birth weight neonates. Pediatr Res, 1985. 19(9): p. 892-3. [CrossRef]
- Araki, S., et al., Vitamin A to prevent bronchopulmonary dysplasia in extremely low birth weight infants: a systematic review and meta-analysis. PLoS One, 2018. 13(11): p. e0207730. [CrossRef]
- Rakshasbhuvankar, A.A., et al., Enteral Vitamin A for Reducing Severity of Bronchopulmonary Dysplasia: A Randomized Trial. Pediatrics, 2021. 147(1). [CrossRef]
- Lykkedegn, S., et al., The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am J Physiol Lung Cell Mol Physiol, 2015. 308(7): p. L587-602. [CrossRef]
- Mandell, E., et al., Vitamin D treatment improves survival and infant lung structure after intra-amniotic endotoxin exposure in rats: potential role for the prevention of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol, 2014. 306(5): p. L420-8. [CrossRef]
- Ge, H., et al., Effects of early vitamin D supplementation on the prevention of bronchopulmonary dysplasia in preterm infants. Pediatr Pulmonol, 2022. 57(4): p. 1015-1021. [CrossRef]
- Aristizabal, N., et al., Safety and Efficacy of Early Vitamin D Supplementation in Critically Ill Extremely Preterm Infants: An Ancillary Study of a Randomized Trial. J Acad Nutr Diet, 2023. 123(1): p. 87-94. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



