Submitted:
06 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
Introduction
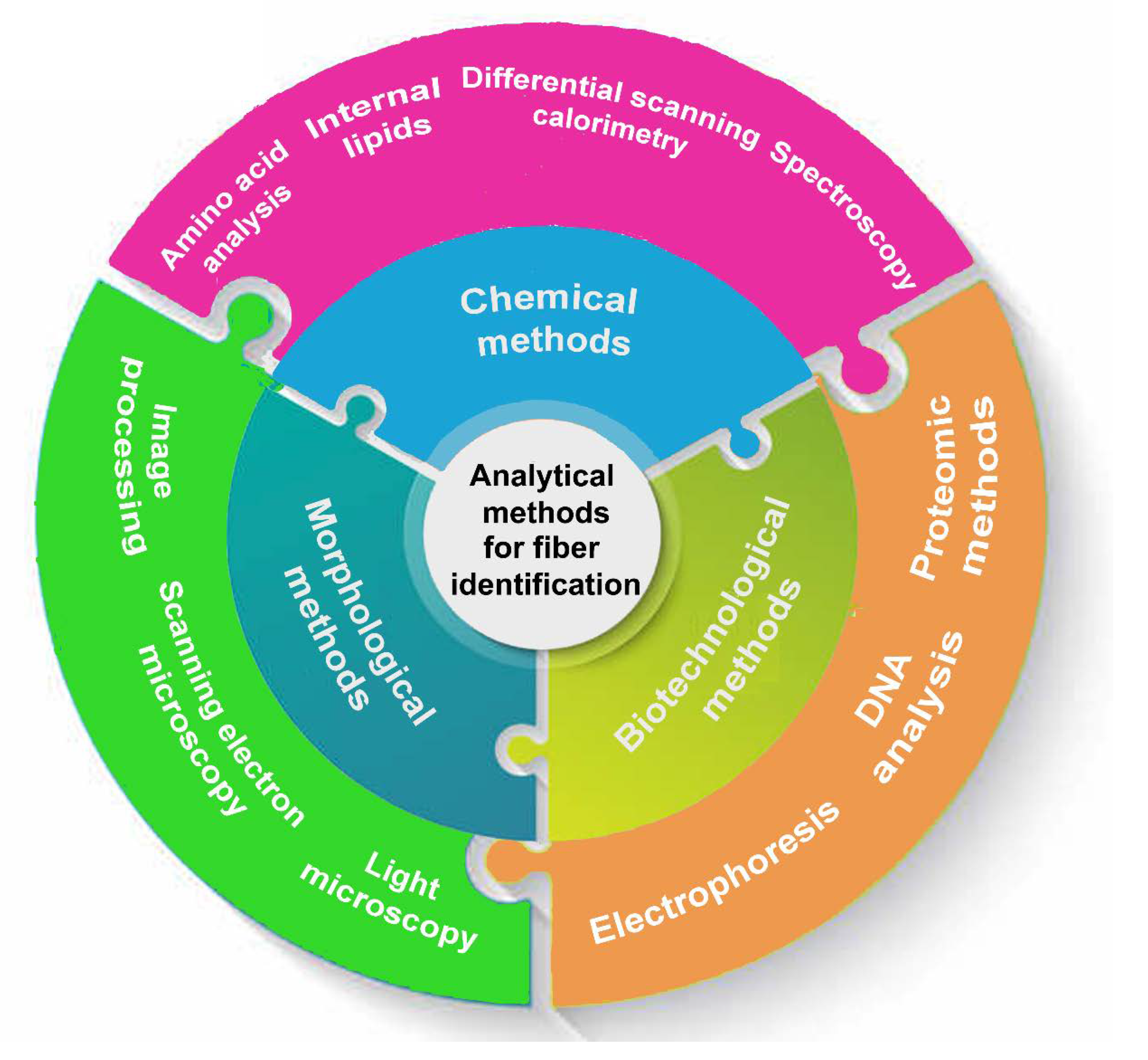
Morphological methods to identify wool and fine animal fibers
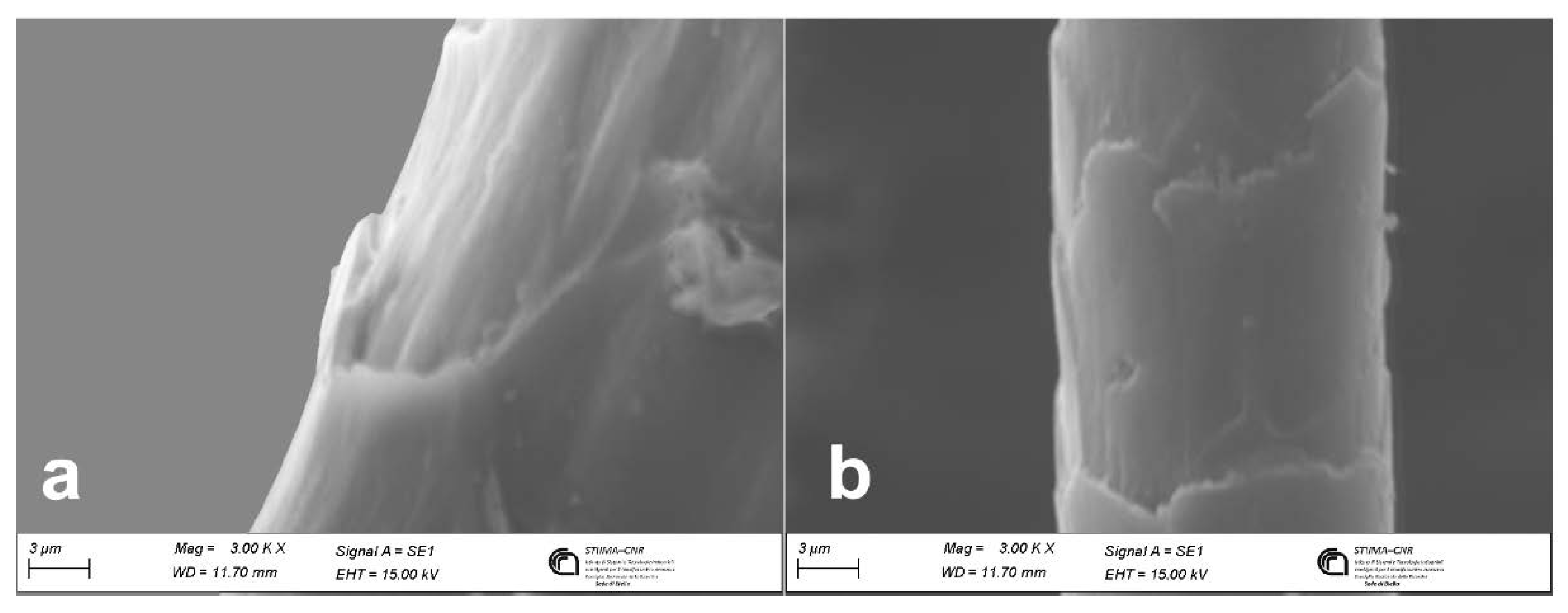
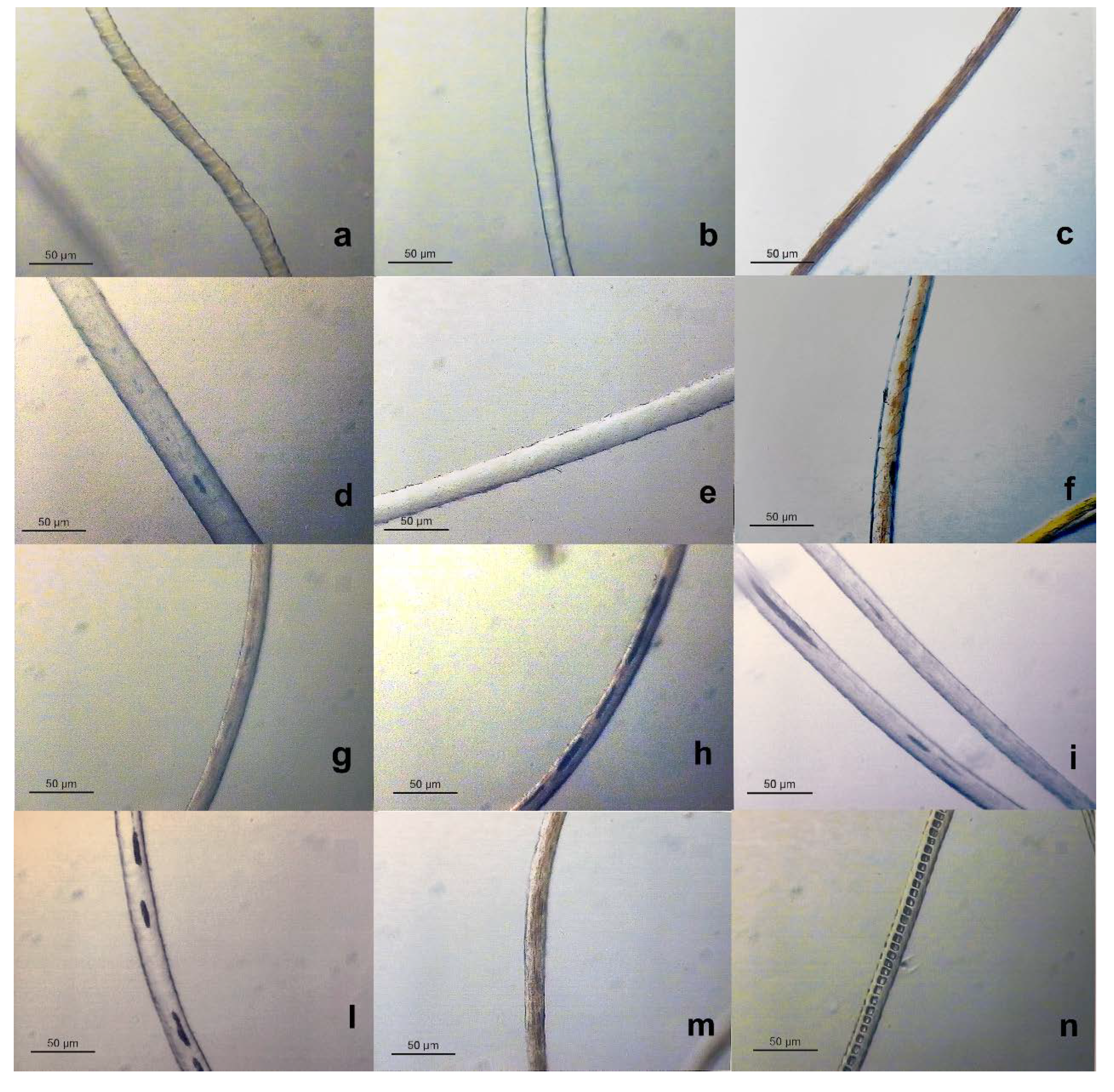
Light and Scanning Electron Microscopy
Image processing
Chemical methods
Biotechnological methods
Proteomic analysis
Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McGregor, B.A. Physical, Chemical, and Tensile Properties of Cashmere, Mohair, Alpaca, and Other Rare Animal Fibers. In Handbook of Properties of Textile and Technical Fibres (Second Edition); Bunsell, A.R., Ed.; Woodhead Publishing, 2018; pp. 105–136. ISBN 978-0-08-101272-7. [Google Scholar]
- Gutierrez, G.; Pablo Gutiérrez, J. Challenges and Opportunities of Genetic Improvement in Alpacas and Llamas in Peru. In Proceedings of the Proceedings of the World Congress on Genetics Applied to Livestock Production, 11.; p. 7622019.
- Madkour, F.A.; Abdelsabour-Khalaf, M. Performance Scanning Electron Microscopic Investigations and Elemental Analysis of Hair of the Different Animal Species for Forensic Identification. Microsc Res Tech 2022, 85, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Sharma, S.; Gopal, S.; Rawat, G.; Singh, R. Rapid and Non-Destructive Differentiation of Shahtoosh from Pashmina/Cashmere Wool Using ATR FT-IR Spectroscopy. Science & Justice 2022, 62. [Google Scholar] [CrossRef]
- Azémard, C.; Zazzo, A.; Marie, A.; Lepetz, S.; Debaine-Francfort, C.; Idriss, A.; Zirah, S. Animal Fibre Use in the Keriya Valley (Xinjiang, China) during the Bronze and Iron Ages: A Proteomic Approach. J Archaeol Sci 2019, 110, 104996. [Google Scholar] [CrossRef]
- Price, E.; Larrabure, D.; Gonzales, B.; McClure, P.; Espinoza, E. Forensic Identification of the Keratin Fibers of South American Camelids by Ambient Ionization Mass Spectrometry: Vicuña, Alpaca and Guanaco. Rapid Communications in Mass Spectrometry 2020, 34. [Google Scholar] [CrossRef]
- Fei, J.; Liu, M.; Zhang, S.; Chen, X.; Zhang, S. Technical Note: A Protein Analysis-Based Method for Identifying Shahtoosh. Forensic Sci Int 2022, 336, 111341. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.G. Merino Improvement Programs in Australia. Proceedings of a National Symposium, Leura, New South Wales 1987. Supervising Editor, B. J. McGuirk. Australian Wool Corporation, Melbourne. 535 Pages. ISBN 0 642 115311. Genet Res (Camb) 1989, 53, 72–72. [Google Scholar] [CrossRef]
- Mcgregor, B.A.; Butler, K.L. The Effects of Cashmere Attributes on the Efficiency of Dehairing and Dehaired Cashmere Length. Textile Research Journal 2008, 78, 486–496. [Google Scholar] [CrossRef]
- Zoccola, M.; Pozzo, P.D.; Bianchetto Songia, M.; Sanchez Nieto, A. SEM and Light Microscopy Investigation of Depigmented Fine Animal Fibres and Melanin Pigments Extracted from Fibres. In Proceedings of the 5th multinational congress on electron microscopy, Lecce, 20 September 2001; pp. 501–502. [Google Scholar]
- Https://Www.Cashmere.Org/Facts.Php.
- Wildman, A.B. The Identification of Animal Fibres. Journal of the Forensic Science Society 1961, 1, 115–119. [Google Scholar] [CrossRef]
- Robert R Franck Silk, Mohair, Cashmere and Other Luxury Fibres. In Silk, Mohair, Cashmere and Other Luxury Fibres; Woodhead Publishing Series in Textiles; Franck, R.R., Ed.; Woodhead Publishing, 2001; p. ii. ISBN 978-1-85573-540-8. [Google Scholar]
- Gardetti, M.A.; Muthu, S.S. Environmental Footprints and Eco-Design of Products and Processes Handbook of Sustainable Luxury Textiles and Fashion Volume 2; 2016. [Google Scholar]
- Wheeler, J.C. South American Camelids - Past, Present and Future. Journal of Camelid Science 2012, 5, 1–24. [Google Scholar]
- Frank, E.N.; Hick, M.V.H.; Gauna, C.D.; Lamas, H.E.; Renieri, C.; Antonini, M. Phenotypic and Genetic Description of Fibre Traits in South American Domestic Camelids (Llamas and Alpacas). Small Ruminant Research 2006, 61, 113–129. [Google Scholar] [CrossRef]
- Samanta, K.; Roy, A. Chemical Modification of Indian Yak Fibre for Development of Jute/Yak Fibres Blended Warm Textile; 2019; pp. 151–165. ISBN 978-981-13-7720-4. [Google Scholar]
- Perincek, S.; Bahtiyari, M.İ.; Körlü, A.E.; Duran, K. Ozone Treatment of Angora Rabbit Fiber. J Clean Prod 2008, 16, 1900–1906. [Google Scholar] [CrossRef]
- Zoccola, M.; Aluigi, A.; Patrucco, A.; Tonin, C. Extraction, Processing and Applications of Wool Keratin. In Keratin: Structure, properties and applications; Nova Science Publisher Inc: New York, 2012; pp. 36–62. [Google Scholar]
- Cashmere Clothing Market Overview, Trends, Segmentation, Scenario, and Forecast Analysis to 2029. Available online: https://www.databridgemarketresearch.com/reports/global-cashmere-clothing-market (accessed on 18 April 2023).
- ISO 17751-1:2016 - Textiles. Quantitative analysis of cashmere, wool, other specialty animal fibers and their blends — Part 1: Light microscopy method; 2016. [Google Scholar]
- ISO 17751-2:2016 - Textiles. Quantitative analysis of cashmere, wool, other specialty animal fibers and their blends — Part 2: Scanning electron microscopy method; 2016. [Google Scholar]
- Wortmann, F.J.; Wortmann, G. Scanning Electron Microscopy as a Tool for the Analysis of Wool/ Speciality Fiber Blends; Universidade de Minho: Guimarães, 1991. [Google Scholar]
- Greaves, P.H.; Saville, B.P. Microscopy of Textile Fibres, 1st ed.; Taylor & Francis Group: London, 1995. [Google Scholar]
- Langley, K.D.; Kennedy, T.A. The Identification of Specialty Fibers. Textile Research Journal 1981, 51, 703–709. [Google Scholar] [CrossRef]
- Wortmann, F.-J.; Arns, W. Quantitative Fiber Mixture Analysis by Scanning Electron Microscopy: Part I: Blends of Mohair and Cashmere with Sheep’s Wool. Textile Research Journal 1986, 56, 442–446. [Google Scholar] [CrossRef]
- Wortmann, G.; Wortmann, F.J. Chemical Characterization of Fine Animal Hair. In Proceedings of the 1st International Symposium on Speciality animal fibers; Aachen, October 26 1987; pp. 39–70. [Google Scholar]
- Vineis, C.; Aluigi, A.; Tonin, C. Outstanding Traits and Thermal Behaviour for the Identification of Speciality Animal Fibres. Textile Research Journal 2010, 81, 264–272. [Google Scholar] [CrossRef]
- Langley, K.D. Development of Precision and Bias Statement for Cashmere Quantitative Fiber Analysis. In Proceedings of the Proceedings of the 4th International Cashmere Determination Technique Symposium; China National Cashmere Products Engineering and Technical Centre and China Inner Mongolia Erdos Cashmere Group Corporation: Erodosi, China, 2008; pp. 154–160. [Google Scholar]
- Rippon, J.A.; Evans, D.J. Improving the Properties of Natural Fibres by Chemical Treatments. In Handbook of natural fibres; Woodhead Publishing Series in Textiles: Philadelphia, 2012; Volume 2, pp. 63–140. [Google Scholar]
- Sun, M.; Fei, J.; Cai, J.; Zhao, J. Application of DNA Analysis in Quantifying Cashmere and Wool Binary Blend. In Proceedings of the Key Engineering Materials; Trans Tech Publications Ltd, 2015; Vol. 671, pp. 378–384. [Google Scholar]
- Paolella, S.; Bencivenni, M.; Lambertini, F.; Prandi, B.; Faccini, A.; Tonetti, C.; Vineis, C.; Sforza, S. Identification and Quantification of Different Species in Animal Fibres by LC/ESI-MS Analysis of Keratin-Derived Proteolytic Peptides. J Mass Spectrom 2013, 48, 919–926. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.A.; Quispe Peña, E.C. Cuticle and Cortical Cell Morphology of Alpaca and Other Rare Animal Fibres. Journal of the Textile Institute 2018, 109, 767–774. [Google Scholar] [CrossRef]
- Tian, Z.; Hong, X.; Xu, X.; Ma, H. Identification of Kid Cashmere. Wool Textile Journal 2020, 48, 83–88. [Google Scholar]
- Zhu, Y.; Zhao, L.; Chen, X.; Li, Y.; Wang, J. Identification of Cashmere and Wool Based on LBP and GLCM Texture Feature Selection. J Eng Fiber Fabr 2023, 18, 15589250221146548. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiayi, H.; Li, Y.; Li, W. Image Identification of Cashmere and Wool Fibers Based on the Improved Xception Network. Journal of King Saud University - Computer and Information Sciences 2022, 34, 9301–9310. [Google Scholar] [CrossRef]
- Xuebao, F. Cashmere and Wool Classification Based on Sparse Dictionary Learning. Journal of Textile Research 2022, 43, 28–32. [Google Scholar]
- Zhu, Y.; Zhao, L.; Chen, X.; Li, Y.; Wang, J. Animal Fiber Recognition Based on Feature Fusion of the Maximum Inter-Class Variance. Autex Research Journal 2022, 0. [Google Scholar] [CrossRef]
- Zang, L.; Xin, B.; Deng, N. Identification of Wool and Cashmere Fibers Based on Multiscale Geometric Analysis. Journal of the Textile Institute 2022, 113, 1001–1008. [Google Scholar] [CrossRef]
- Xing, W.; Liu, Y.; Xin, B.; Zang, L.; Deng, N. The Application of Deep and Transfer Learning for Identifying Cashmere and Wool Fibers. Journal of Natural Fibers 2022, 19, 88–104. [Google Scholar] [CrossRef]
- Zhu, Y.; Duan, J.; Li, Y.; Wu, T. Image Classification Method of Cashmere and Wool Based on the Multi-Feature Selection and Random Forest Method. Textile Research Journal 2021, 92, 1012–1025. [Google Scholar] [CrossRef]
- Zang, L.; Xin, B.; Deng, N. Identification of Overlapped Wool/Cashmere Fibers Based on Multi-Focus Image Fusion and Convolutional Neural Network. Journal of Natural Fibers 2021, 19, 6715–6726. [Google Scholar] [CrossRef]
- Yaolin, Z.; Wanwan, M.; Yunhong, L.; Tong, W. Identification of Cashmere and Wool Based on Matched Filter and Multi-Feature Fusion. Wool Textile Journal 2021, 49, 64–69. [Google Scholar]
- Sun, C. Image Classification of Cashmere and Wool Fiber Based on LC-KSVD. In Proceedings of the Journal of Physics: Conference Series, 28 June 2021; IOP Publishing Ltd; Vol. 1948. [Google Scholar]
- Luo, J.; Lu, K.; Chen, Y.; Zhang, B. Automatic Identification of Cashmere and Wool Fibers Based on Microscopic Visual Features and Residual Network Model. Micron 2021, 143. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, J.; Wu, T.; Ren, X. Identification Method of Cashmere and Wool Based on Texture Features of GLCM and Gabor. J Eng Fiber Fabr 2021, 16. [Google Scholar] [CrossRef]
- Xing, W.; Liu, Y.; Deng, N.; Xin, B.; Wang, W.; Chen, Y. Automatic Identification of Cashmere and Wool Fibers Based on the Morphological Features Analysis. Micron 2020, 128. [Google Scholar] [CrossRef]
- Yildiz, K. Identification of Wool and Mohair Fibres with Texture Feature Extraction and Deep Learning. IET Image Process 2020, 14, 348–353. [Google Scholar] [CrossRef]
- Lu, K.; Luo, J.; Zhong, Y.; Chai, X. Identification of Wool and Cashmere SEM Images Based on SURF Features. J Eng Fiber Fabr 2019, 14. [Google Scholar] [CrossRef]
- Xing, W.; Deng, N.; Xin, B.; Wang, Y.; Chen, Y.; Zhang, Z. An Image-Based Method for the Automatic Recognition of Cashmere and Wool Fibers. Measurement 2019, 141, 102–112. [Google Scholar] [CrossRef]
- Xing, W.; Deng, N.; Xin, B.; Chen, Y.; Zhang, Z. Investigation of a Novel Automatic Micro Image-Based Method for the Recognition of Animal Fibers Based on Wavelet and Markov Random Field. Micron 2019, 119, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Deng, N.; Xin, B.; YU, C. Identification of Wool and Cashmere Based on Multi-Feature Fusionimage Analysis Technology. Journal of Textile Research 2019, 15, 146–152. [Google Scholar] [CrossRef]
- Xing, W.; Deng, N.; Xin, B.; Liu, Y.; Chen, Y.; Zhang, Z. Identification of Extremely Similar Animal Fibers Based on Matched Filter and HOG-SVM. IEEE Access 2019, 7, 98603–98617. [Google Scholar] [CrossRef]
- Wang, H. Combining the Hough Transform with MLP for Identifying Cashmere and Wool Fibers. In Proceedings of the ACM International Conference Proceeding Series; Association for Computing Machinery, 2019; Vol. Part F148262, pp. 124–128. [Google Scholar]
- Wang, F.; Jin, X.; Luo, W. Intelligent Cashmere/Wool Classification with Convolutional Neural Network. In Proceedings of the Advances in Intelligent Systems and Computing; Springer Verlag, 2019; Vol. 849, pp. 17–25. [Google Scholar]
- Xing, W.; Xin, B.; Deng, N.; Chen, Y.; Zhang, Z. A Novel Digital Analysis Method for Measuring and Identifying of Wool and Cashmere Fibers. Measurement (Lond) 2019, 132, 11–21. [Google Scholar] [CrossRef]
- Lu, K.; Zhong, Y.; Li, D.; Chai, X.; Xie, H.; Yu, Z.; Naveed, T. Cashmere/Wool Identification Based on Bag-of-Words and Spatial Pyramid Match. Textile Research Journal 2018, 88, 2435–2444. [Google Scholar] [CrossRef]
- Wang, F.; Jin, X. The Application of Mixed-Level Model in Convolutional Neural Networks for Cashmere and Wool Identification. International Journal of Clothing Science and Technology 2018, 30, 710–725. [Google Scholar] [CrossRef]
- Lu, K.; Chai, X.; Xie, H.; Yu, Z.; Zhong, Y. Identification of Cashmere/Wool Based on Pairwise Rotation Invariant Co-Occurrence Local Binary Pattern. In Proceedings of the Proceedings - 2017 International Conference on Computational Science and Computational Intelligence, CSCI 2017, 4 December 2018; Institute of Electrical and Electronics Engineers Inc.; pp. 463–467. [Google Scholar]
- Zhong, Y.; Lu, K.; Tian, J.; Zhu, H. Wool/Cashmere Identification Based on Projection Curves. Textile Research Journal 2017, 87, 1730–1741. [Google Scholar] [CrossRef]
- Yuan, S.; Lu, K.; Zhong, Y. Identification of Wool and Cashmere Based on Texture Analysis. In Proceedings of the Key Engineering Materials; Trans Tech Publications Ltd, 2015; Vol. 671, pp. 385–390. [Google Scholar]
- Chen, H.; Liu, Y.X. Research on Feature Extraction and Optimization of Cashmere and Wool Fiber Based on Digital Image. In Proceedings of the Applied Mechanics and Materials; Trans Tech Publications Ltd, 2014; Vol. 667, pp. 260–263. [Google Scholar]
- Liu, Y.; Liu, Y.; Shang, S.; Yuan, Y.; Yang, W. Research on the Scale Density Extraction Technology of Cashmere and Wool. In Proceedings of the Applied Mechanics and Materials; 2012; Vol. 217–219, pp. 947–950. [Google Scholar]
- Yang, Y.; Ji, Y. Study on Classification Technology of Wool and Cashmere Based on GA-SVM. In Proceedings of the Advanced Materials Research; 2011; Vol. 332–334, pp. 1198–1201. [Google Scholar]
- Shi, X.-J.; Yu, W.-D. Intelligent Animal Fibre Classification with Artificial Neural Networks’. Int. J. Modelling, Identification and Control 2011, 12, 107–112. [Google Scholar] [CrossRef]
- Shang The Research on Identification of Wool or Cashmere Fibre Based on Digital Image. In Proceedings of the Proceedings of the Ninth International Conference on Machine Learning and Cybernetics; IEEE, 2010.
- Zang, J.; Palmer, S.; Wang, X. Identification of Animal Fibers with Wavelet Texture Analysis. In Proceedings of the Proceedings of the World Congress on Engineering; 2010; pp. 742–747. [Google Scholar]
- Kun, Q.; Li, H.; Haijian, C.; Kejing, Y.; Shen, W.; Qian, K.; Cao, H.; Yu, K.; Shen, W. Measuring the Blend Ratio of Wool/Cashmere Yarns Based on Image Processing Technology. FIBRES & TEXTILES in Eastern Europe 2010, 18, 35–38. [Google Scholar]
- Shi, X.J.; Yu, W.D. Identification of Animal Fiber Based on Scale Shape. In Proceedings of the Proceedings - 1st International Congress on Image and Signal Processing, CISP 2008; 2008; Vol. 3, pp. 573–577. [Google Scholar]
- Kong, L.; She, F.; Nahavandi, S.; Kouzani, A.Z. Feature Extraction for Animal Fiber Identification. In Proceedings of the Second International Conference on Image and Graphics, vol. 4875; International Society for Optics and Photonics, 2002; pp. 699–704. [Google Scholar]
- She, F.H.; Chow, S.; Wang, B.; Kong, L.X. Identification and Classification of Animal Fibres Using Artificial Neural Networks. Journal of Textile Engineering 2001, 47, 35–38. [Google Scholar] [CrossRef]
- Robson, D. Animal Fiber Analysis Using Imaging Techniques Part II: Addition of Scale Height Data. Textile Research Journal 2000, 70, 116–120. [Google Scholar] [CrossRef]
- Robson, D. Animal Fiber Analysis Using Imaging Techniques Part I: Scale Pattern Data. Textile Research Journal 1997, 67, 747–752. [Google Scholar] [CrossRef]
- Kong, L.; She, F.; Nahavandi, S.; Kouzani, A. Fuzzy Pattern Recognition and Classification of Animal Fibers. In Proceedings of the Proceedings Joint 9th IFSA World Congress and 20th NAFIPS International Conference; 2001; pp. 1050–1055. [Google Scholar]
- Wool Market - Growth, Trends, and Forecasts (2023 - 2028). Available online: https://www.mordorintelligence.com/industry-reports/wool-market (accessed on 2 May 2023).
- Rippel, O.; Gülçelik, S.; Rahimi, K.; Kurniadi, J.; Herrmann, A.; Merhof, D. Animal Fiber Identification under the Open Set Condition. In Proceedings of the IEEE International Instrumentation and Measurement Technology Conference; 2021. [Google Scholar]
- Anceschi, A.; Zoccola, M.; Mossotti, R.; Bhavsar, P.; Fontana, G.D.; Patrucco, A. Colorimetric Quantification of Virgin and Recycled Cashmere Fibers: Equilibrium, Kinetic, and Thermodynamic Studies. Journal of Natural Fibers 2022, 19, 11064–11077. [Google Scholar] [CrossRef]
- Solazzo, C. Characterizing Historical Textiles and Clothing with Proteomics. Conservar Patrimonio 2019, 97–114. [Google Scholar] [CrossRef]
- Maclaren, J.A.; Milligan, B. Wool Science. The Chemical Reactivity of the Wool Fibre; Science Press: Marrickville, 1981. [Google Scholar]
- Villarroel, V. Wool handbook; Von Bergen, W., Ed.; Interscience: New York, 1963; Vol 1, pp. 411–413. [Google Scholar]
- Satlow, G.; Cieplik, M. von S.; Fichtner, G. Textiltechnik. 1965, 16, 143–145. [Google Scholar]
- Ferrero, F.; Mossotti, R.; Innocenti, R.; Coppa, F.; Periolatto, M. Enzyme-Aided Wool Dyeing: Influence of Internal Lipids. Fibers and Polymers 2015, 16, 363–369. [Google Scholar] [CrossRef]
- Logan, R.I.; Rivett, D.E.; Tucker, D.J.; Hudson, A.H.F. Analysis of the Intercellular and Membrane Lipids of Wool and Other Animal Fibers. Textile Research Journal 1989, 59, 109–113. [Google Scholar] [CrossRef]
- Tucker, D.J.; Hudson, A.H.F.; Rivett, D.E.; Logan, R.I. The Chemistry of Speciality Animal Fibres. Schriftenreihe des Deutschen Wollforschungsinstitutes 1990, 106. [Google Scholar]
- Korner, A. Lipids in the Analysis of Fine Animal Fibres. In Proceedings of the 1st International Symposium on Speciality Animal Fibres, Aachen; 1988; pp. 104–127. [Google Scholar]
- Zoccola, M.; Aluigi, A.; Tonin, C. Characterisation of Keratin Biomass from Butchery and Wool Industry Wastes. J Mol Struct 2009, 938, 35–40. [Google Scholar] [CrossRef]
- Wortmann, F.J.; Deutz, H.D. Characterizing Keratins Using High-pressure Differential Scanning Calorimetry (HPDSC). J Appl Polym Sci 1993, 48, 137–150. [Google Scholar] [CrossRef]
- Tonetti, C.; Varesano, A.; Vineis, C.; Mazzuchetti, G. Differential Scanning Calorimetry for the Identification of Animal Hair Fibres. J Therm Anal Calorim 2015, 119, 1445–1451. [Google Scholar] [CrossRef]
- Notayi, M.; Hunter, L.; Engelbrecht, J.A.; Botha, A.F.; Minnaar, E.G.; Lee, M.E.; Erasmus, R. The Application of Raman Spectroscopic Ratiometric Analysis for Distinguishing between Wool and Mohair. Journal of Natural Fibers 2022, 19, 11536–11546. [Google Scholar] [CrossRef]
- Prakash Sharma, C.; Sharma, S.; Singh Rawat, G.; Singh, R. Rapid and Non-Destructive Differentiation of Shahtoosh from Pashmina/Cashmere Wool Using ATR FT-IR Spectroscopy. Science & Justice 2022, 62, 349–357. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, H.; Song, C.; Li, X.; Hu, A.; Yu, S.; Ren, Z. A Novel Drying-Free Identification Method of Cashmere Textiles by NIR Spectroscopy Combined with an Adaptive Representation Learning Classification Method. Microchemical Journal 2019, 149. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, L.; Ding, Q.; Wang, R. Textile Fiber Identification Using Near-Infrared Spectroscopy and Pattern Recognition. Autex Research Journal 2019, 19, 201–209. [Google Scholar] [CrossRef]
- Chen, H.; Lin, Z.; Tan, C. Classification of Different Animal Fibers by near Infrared Spectroscopy and Chemometric Models. Microchemical Journal 2019, 144, 489–494. [Google Scholar] [CrossRef]
- McGregor, B.A.; Liu, X.; Wang, X.G. Comparisons of the Fourier Transform Infrared Spectra of Cashmere, Guard Hair, Wool and Other Animal Fibres. Journal of the Textile Institute 2018, 109, 813–822. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, R.; Wu, X.; Xu, B. Fiber-Content Measurement of Wool–Cashmere Blends Using Near-Infrared Spectroscopy. Appl Spectrosc 2017, 71, 2367–2376. [Google Scholar] [CrossRef]
- Wang, C.; Wu, X.; Ding, X. Non-Destructive Identification of Wool Blended Fabrics with near Infrared Spectroscopy Based on Support Vector Machine. Wool Textile Journal 2016, 44, 1–5. [Google Scholar]
- Mao, M.-H.; Li, W.-S. Determination of Cashmere and Wool Contents in Textile by near Infrared Spectroscopy. Wool Textile Journal 2014, 42, 41–43. [Google Scholar]
- Zoccola, M.; Lu, N.; Mossotti, R.; Innocenti, R.; Montarsolo, A. Identification of Wool, Cashmere, Yak, and Angora Rabbit Fibers and Quantitative Determination of Wool and Cashmere in Blend: A near Infrared Spectroscopy Study. Fibers and Polymers 2013, 14, 1283–1289. [Google Scholar] [CrossRef]
- Yuan, H.; Chang, R.-X.; Tian, L.-L.; Song, C.-F.; Yuan, X.-Q.; Li, X.-Y. Study of Nondestructive and Fast Identification of Fabric Fibers Using Near Infrared Spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi 2010, 30, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Yu, C.; Zhao, G.-L. Study on Identification of Cashmere and Wool Using near Infrared Spectroscopy. Journal of Beijing Institute of Clothing Technology (Natural Science Edition) 2010, 30, 29–34. [Google Scholar]
- Ciukzak, W. Principles of Near- Infrared Spectroscopy. In Handbook of Near-Infrared Analysis; Burns, D.A., Ciurczak, E.W., Eds.; Marcel Dekker: New York, 1992; pp. 7–11. [Google Scholar]
- Zoccola, M.; Mossotti, R.; Innocenti, R.; Loria, D.I.; Rosso, S.; Zanetti, R. Near Infrared Spectroscopy as a Tool for the Determination of Eumelanin in Human Hair. Pigment Cell Res 2004, 17 4, 379–385. [Google Scholar] [CrossRef]
- Zoccola, M.; Mossotti, R.; Montarsolo, A.; Patrucco, A.; Innocenti, R. Near Infrared Spectroscopy in the Textile Industry. In Infrared Spectroscopy: Theory, Developments and Applications; Cozzolino, D., Ed.; Nova Science Publishers, 2014; pp. 491–516. ISBN 978-1-62948-523-2. [Google Scholar]
- Wu, G.; He, Y. Identification of Varieties of Cashmere by Vis/NIR Spectroscopy Technology Based on PCA-SVM. In Proceedings of the 7th World Congress on Intelligent Control and Automation; 2008; pp. 1548–1552. [Google Scholar]
- Anceschi, A.; Zoccola, M.; Mossotti, R.; Bhavsar, P.; Dalla Fontana, G.; Patrucco, A. Identification and Quantitative Determination of Virgin and Recycled Cashmere: A Near-Infrared Spectroscopy Study. ACS Sustain Chem Eng 2022, 10, 738–745. [Google Scholar] [CrossRef]
- Rajabinejad, H.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Rovero, G.; Tonin, C. Physicochemical Properties of Keratin Extracted from Wool by Various Methods. Textile Research Journal 2018, 88, 2415–2424. [Google Scholar] [CrossRef]
- Marshall, R.; Zahn, H.; Blankenburg, G. Possible Identification of Specialty Fibers by Electrophoresis. Textile Research Journal 1984, 54, 126–128. [Google Scholar] [CrossRef]
- Tucker, D.; Hudson, A.; Laudani, A.; Marshall, R.; Rivett, D. Variation in Goat Fibre Protein. Aust J Agric Res 1989, 40, 675–683. [Google Scholar] [CrossRef]
- Kalbé, J.; Kuropka, R.; Meyer-Stork, L.; Souther, S.L.; Höcher, H.; Bernt, H; Riesner, D.; Henco, K. Isolation and Characterization of Hight Molecular Weigt DNA from Hair Shafts of Wool and Fine Animal Hairs Fibres. In Proceedings of the 1st International Symposium on Speciality animal fibers221, Aachen, 26 October 1987; pp. 221–227. [Google Scholar]
- Hamlyn, P.F.; Bayliffe, A.; McCarty, B.J.; Nelson, G. Molecular Speciation of Animal Fibres. Journal of the Society of Dyers and Colourist 1998, 114, 78–80. [Google Scholar] [CrossRef]
- Kerkhoff, K.; Cescutti, G.; Kruse, L.; Müssig, J. Development of a DNA-Analytical Method for the Identification of Animal Hair Fibers in Textiles. Textile Research Journal 2009, 79, 69–75. [Google Scholar] [CrossRef]
- Wang, M.; Ren, L.; Kong, P.; et al. Qualitative Analyzing Method Based on Real-Time Fluorescence Quantitative PCR for Identifying Rabbit Hair. Wool Textile Journal 2015, 55–58. [Google Scholar]
- Geng, Q.R. A Duplex Polymerase Chain Reaction Assay for the Identification of Goat Cashmere and Sheep Wool. Mitochondrial DNA A DNA Mapp Seq Anal 2016, 27, 1808–1811. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.Q. Species-Specific PCR for the Identification of Goat Cashmere and Sheep Wool. Mol Cell Probes 2015, 29, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, W.; Zhou, H.; Fei, J.; Yang, J.; lu, W.; Zhang, S.; ye, S.; Wang, X. A Real-Time PCR Method for Quantifying Mixed Cashmere and Wool Based on Hair Mitochondrial DNA. Textile Research Journal 2014, 84, 1612–1621. [Google Scholar] [CrossRef]
- Fei, J.; Yang, J.; Zhou, H.; Tang, M.; Lu, W.; Yan, A.; Hou, Y.; Zhang, S. A Novel Method for Identifying Shahtoosh. J Forensic Sci 2014, 59, 723–728. [Google Scholar] [CrossRef]
- Fei, J.; Lu, W.M.; Duan, J.Y.; Yang, J.; Zhou, Z.P.; Yu, J. Study on Precision of a DNA Analysis Method for Quantifying Cashmere/Wool Mixture. In Proceedings of the Advanced Materials Research; Trans Tech Publications Ltd, 2013; Vol. 821–822, pp. 243–247. [Google Scholar]
- Geng, R.Q.; Yuan, C.; Chen, Y.L. Identification of Goat Cashmere and Sheep Wool by PCR-RFLP Analysis of Mitochondrial 12S RRNA Gene. Mitochondrial DNA 2012, 23, 466–470. [Google Scholar] [CrossRef]
- Ji, W.; Bai, L.; Ji, M.; Yang, X. A Method for Quantifying Mixed Goat Cashmere and Sheep Wool. Forensic Sci Int 2011, 208, 139–142. [Google Scholar] [CrossRef]
- Nelson, G.; Hamlyn, P.F.; Holden, L. A Species-Specific DNA Probe for Goat Fiber Identification. Textile Research Journal 1992, 62, 590–595. [Google Scholar] [CrossRef]
- ISO 18074 Textiles. Identification of some animal fibers by DNA analysis method —Cashmere, wool, yak and their blends.
- Azémard, C.; Dufour, E.; Zazzo, A.; Wheeler, J.C.; Goepfert, N.; Marie, A.; Zirah, S. Untangling the Fibre Ball: Proteomic Characterization of South American Camelid Hair Fibres by Untargeted Multivariate Analysis and Molecular Networking. J Proteomics 2021, 231. [Google Scholar] [CrossRef] [PubMed]
- Vineis, C.; Tonetti, C.; Sanchez Ramirez, D.O.; Carletto, R.A.; Varesano, A. Validation of UPLC/ESI-MS Method Used for the Identification and Quantification of Wool, Cashmere and Yak Fibres. Journal of the Textile Institute 2017, 108, 2180–2183. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, T.; Choi, H.M. Qualitative Identification of Cashmere and Yak Fibers by Protein Fingerprint Analysis Using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Ind Eng Chem Res 2013, 52, 5563–5571. [Google Scholar] [CrossRef]
- Hollemeyer, K.; Altmeyer, W.; Heinzle, E. Identification and Quantification of Feathers, down, and Hair of Avian and Mammalian Origin Using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Anal Chem 2002, 74 23, 5960–5968. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Wang, J.; Yang, Y.; Miao, C.; Guo, Y.; Zhang, Z.; Cao, Q.; Shui, W. Combining Untargeted and Targeted Proteomic Strategies for Discrimination and Quantification of Cashmere Fibers. PLoS One 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Yang, Y.; Li, S.; Guo, Y.; Shui, W.; Cao, Q. Discrimination and Quantification of Homologous Keratins from Goat and Sheep with Dual Protease Digestion and PRM Assays. J Proteomics 2018, 186, 38–46. [Google Scholar] [CrossRef]
- Ohashi, S.; Demura, Y.; Sano, M. Identification of Cashmere Fiber by Using SDS-PAGE and Maldi-Tof Mass Spectrometry. Journal of Fiber Science and Technology 2012, 68, 276–281. [Google Scholar] [CrossRef]
- Ohashi, S.; Demura, Y.; Sano, M.; Yoshioka, Y. Quantitative Analysis of Cashmere and Other Animal Hair Fibers in Textiles Using MALDI-TOF Mass Spectrometry. Journal of Fiber Science and Technology 2014, 114–120. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, J.; Zhou, Y.; Wang, Y. Study of the Principle of Time of Flight Mass Spectrometry for the Identification of Cashmere and Wool Fiber Protein. Wool Textile Journal 2016, 44, 21–24. [Google Scholar]
- Izuchi, Y.; Tokuhara, M.; Takashima, T.; Kuramoto, K. Peptide Profiling Using Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight Mass Spectrometry for Identification of Animal Fibers. Mass Spectrometry 2013, 2, A0023–A0023. [Google Scholar] [CrossRef]
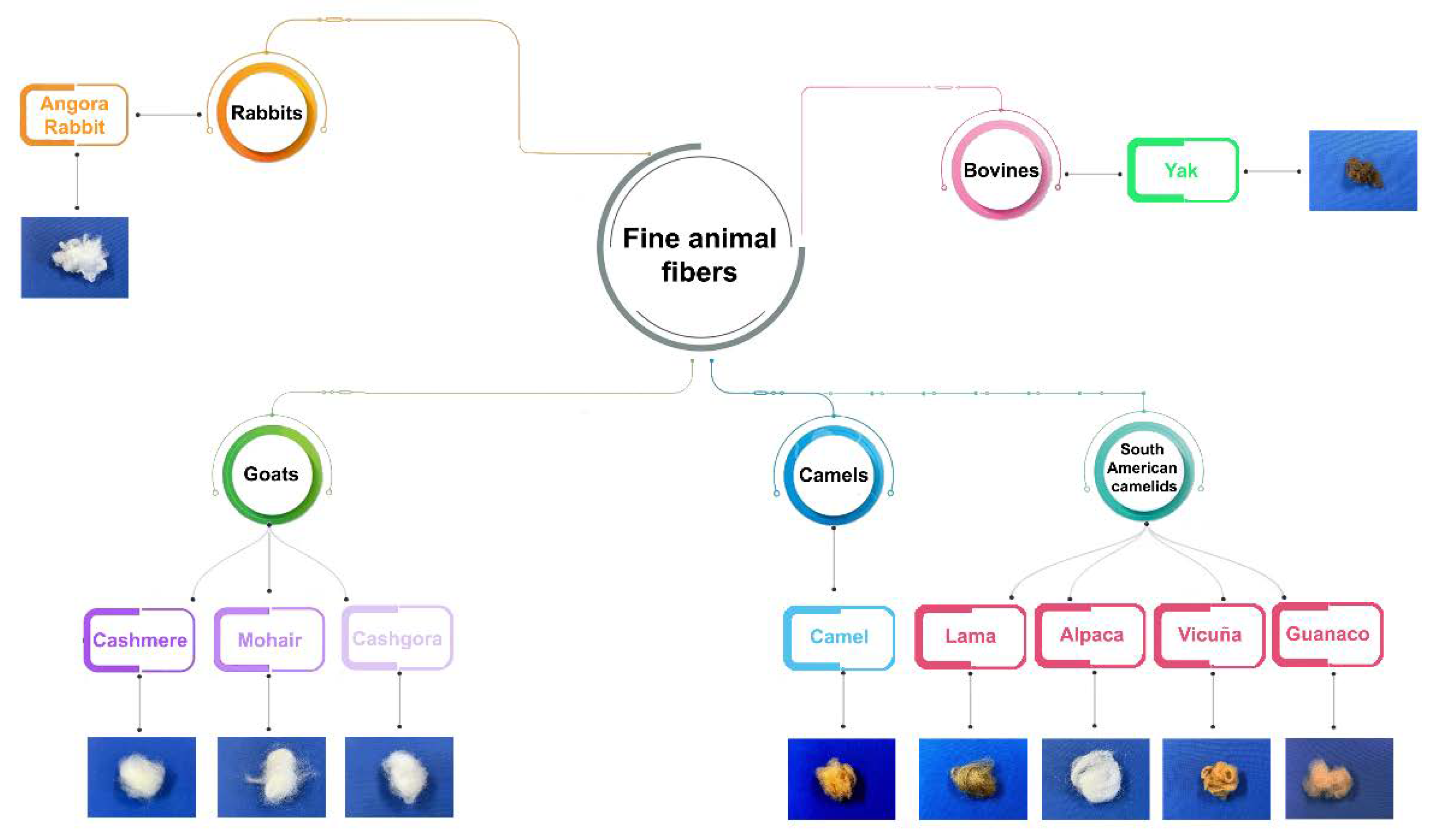
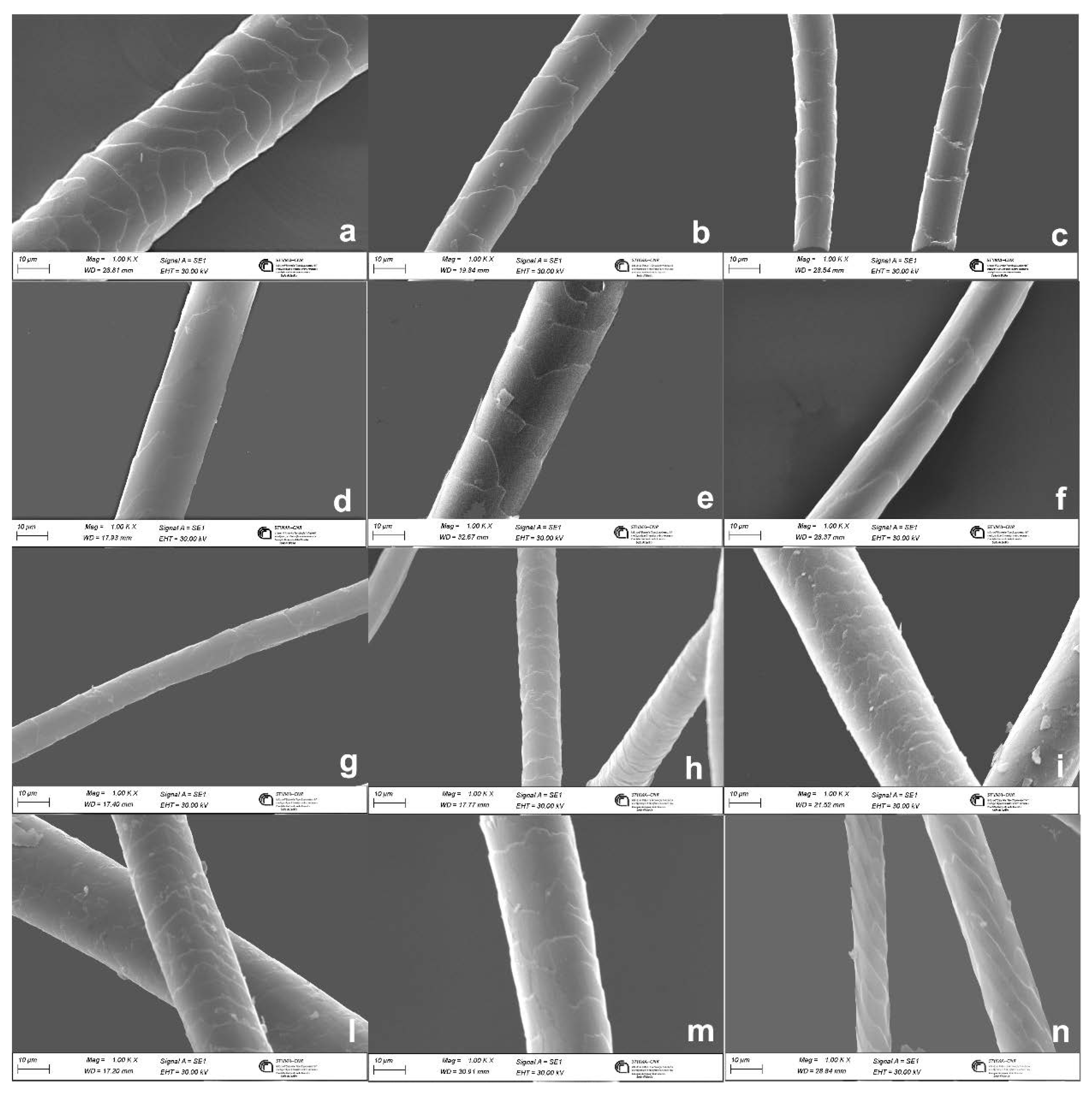

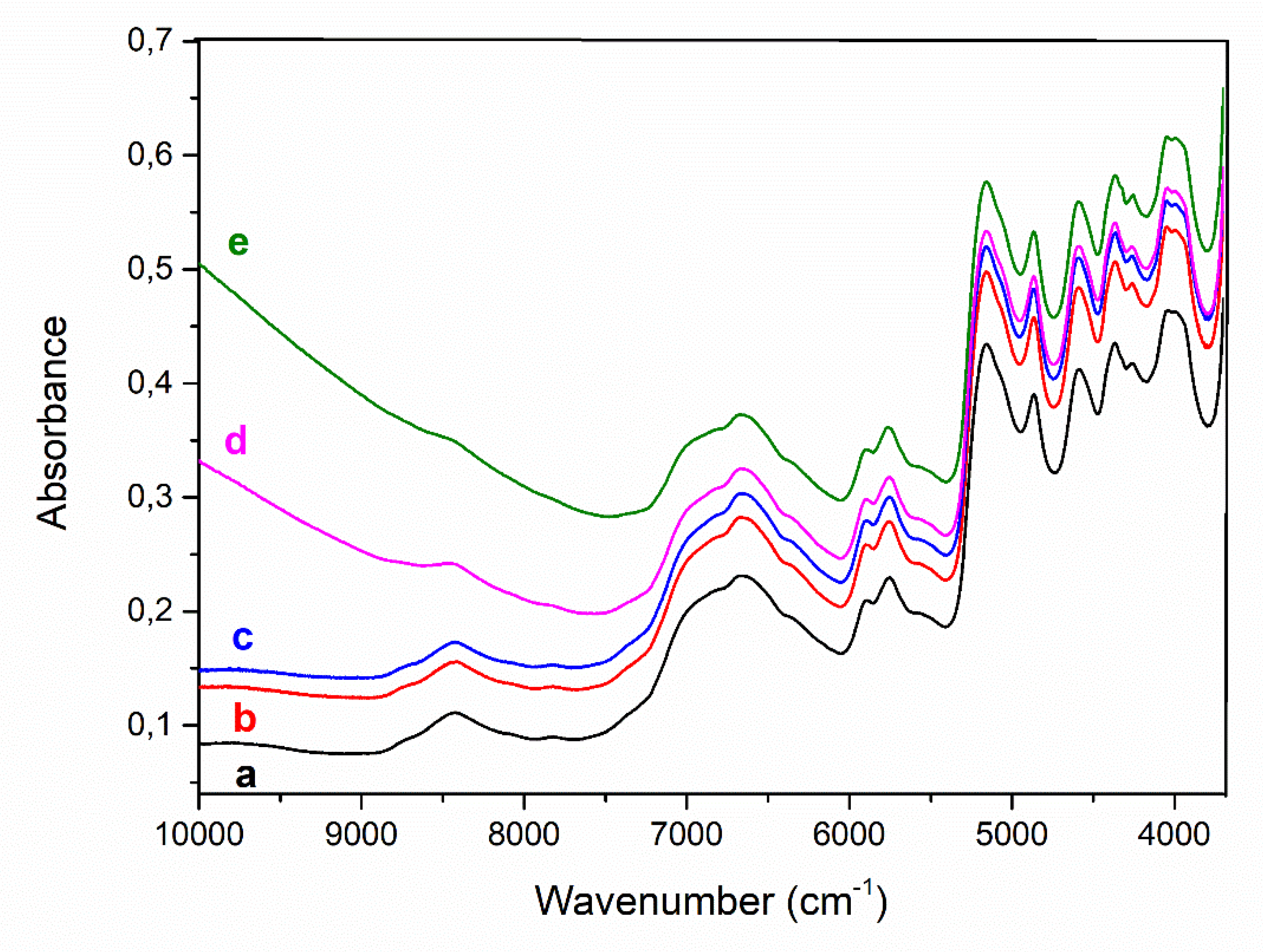
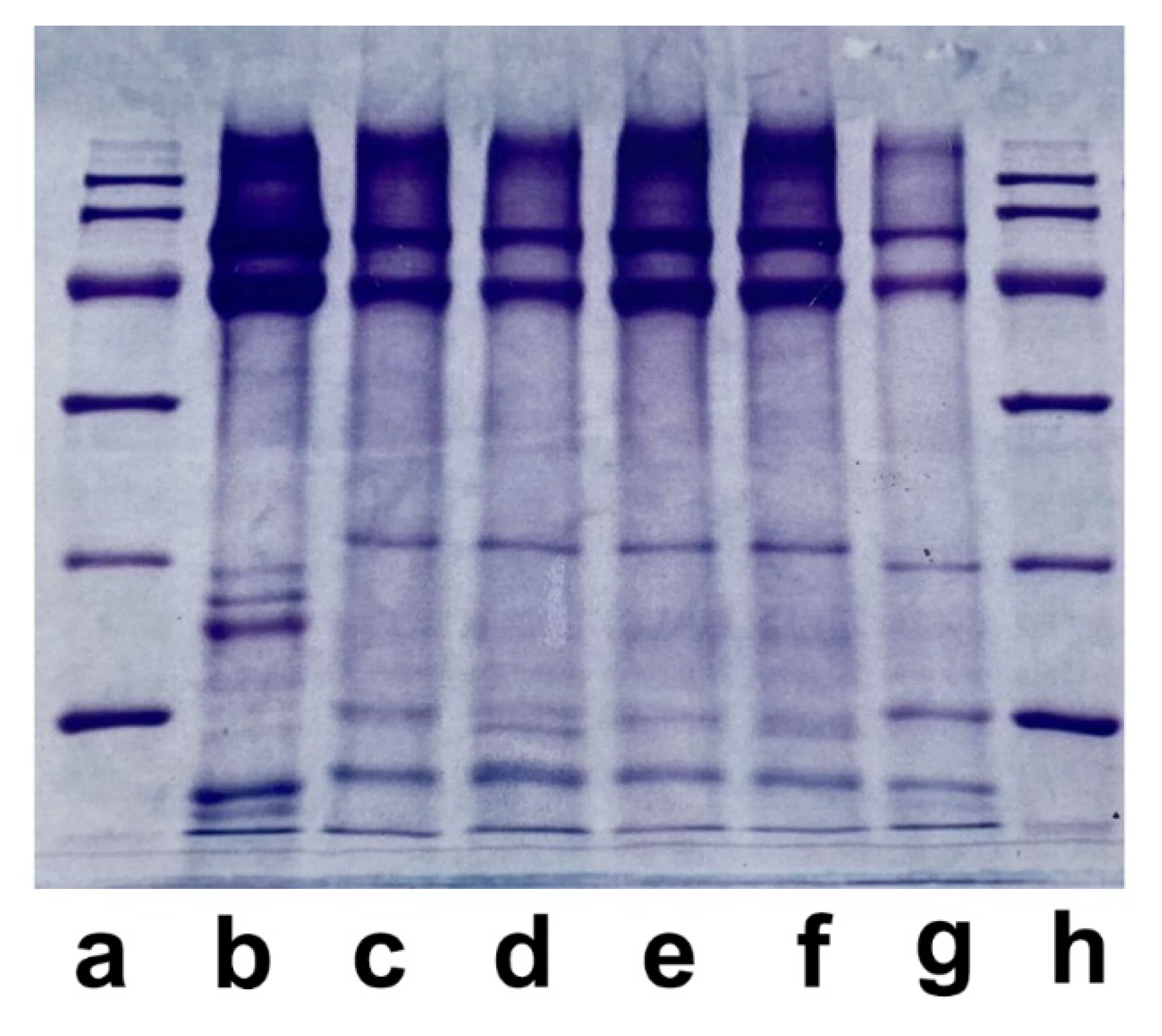
| Fiber | Main breeding countries | Coat or undercoat | Finess | Natural color | Reference |
|---|---|---|---|---|---|
| cashmere | China, Mongolia, Afghanistan and Iran | undercoat | 15–19 μm | white, gray and brown | [11] |
| mohair | South Africa and the U.S.A. | coat | not so fine | white and glossy | [12] |
| cashgora | Australia and New Zealand | coat | 18 to 23 μm | white | [13] |
| camel | China, Mongolia, Iran, Afghanistan, Russia, New Zealand and Australia | undercoat | fine | golden tan | [11] |
| lama | South America | coat | 10-44 μm | various colors, sometimes brown | [14,15,16] |
| alpaca | South America |
coat | 20 -40 μm | Grey, fawn white, black, cafe ́, etc | [14,15,16] |
| vicuña | Perù, Bolivia and Argentina | undercoat | 13-14 μm | from golden to cinnamon | [14,15] |
| guanaco | South America | undercoat | fine | light brown | [14,15] |
| yak | China, Afghanistan, Nepal, and other Asian countries | undercoat | 15-20 μm | dark brown | [17] |
| angora | China | coat | fine | white | [18] |
| Fiber | Cuticular cells thickness | Cuticular cells morphology | Medulla | Pigments | Reference |
| wool | ≥ 0.6 µm | cuticular cells quite close along the fiber axis | absent in fine wool | usually absent | [24,25,26] |
| cashmere | ≤ 0.5 µm | distant and smooth cuticular cells margins | usually absent | sparsely distributed when present | [10,23,27] |
| mohair | ≤ 0.5 µm | distant cuticular cells margins | absent | absent | [26] |
| cashgora | ≤ 0.5 µm | distant cuticular cells margins | absent | absent | [28] |
| camel | ≤ 0.5 µm | high cuticular cell margins slope | usually absent | present | [12] |
| lama | ≤ 0.5 µm | smooth cuticular cells margins | fragmental medulla | present | [12] |
| alpaca | ≤ 0.5 µm | smooth cuticular cells margins | fragmental medulla | present | [12] |
| vicuña | ≤ 0.5 µm | smooth cuticular cells margins | fragmental medulla | present | [12] |
| guanaco | ≤ 0.5 µm | smooth cuticular cells margins | fragmental medulla | present | [12] |
| yak | ≤ 0.5 µm | distant and smooth cuticular cells margins | usually absent | distributed in string | [28] |
| angora | ≤ 0.5 µm | chevron cuticular cells patterns | Ladder type of medulla | absent | [12] |
| Animal fibers | Accuracy (%) | Fiber processsing stage | Imaging type | References | Year |
|---|---|---|---|---|---|
| wool, cashmere | 94.39 | fiber | SEM | [35] | 2023 |
| wool, cashmere | 98.95 | fiber | SEM | [36] | 2022 |
| wool, cashmere | up to 91 | fiber | SEM and LM | [37] | 2022 |
| wool, cashmere | 95.2 | fiber | SEM | [38] | 2022 |
| wool, cashmere | 96.67 | fiber | LM | [39] | 2022 |
| wool, cashmere, yellow wool, goat hair |
99.15 | fiber | LM | [40] | 2022 |
| wool, cashmere | 90 | fiber | SEM | [41] | 2021 |
| wool, cashmere | 98.7 | fiber | LM | [42] | 2021 |
| wool, cashmere | 97.1 | fiber | SEM | [43] | 2021 |
| wool, cashmere | up to 90 | fiber | LM | [44] | 2021 |
| wool, cashmere | 97.1 | fiber | LM | [45] | 2021 |
| wool, cashmere | 93.33 | fiber | SEM | [46] | 2021 |
| wool, cashmere | 94.2 | fiber | LM | [47] | 2020 |
| wool, mohair | 99.8 | fiber | LM | [48] | 2020 |
| wool, cashmere and wool cashmere blends | recognition highter than 93 | fiber | SEM | [49] | 2019 |
| wool, cashmere | 94.29 | fiber | LM | [50] | 2019 |
| wool, cashmere | 90.07 | fiber | LM | [51] | 2019 |
| wool, cashmere | 95.25 | fiber | LM | [52] | 2019 |
| wool, cashmere | 92.5 | fiber | LM | [53] | 2019 |
| wool, cashmere | 96 | fiber | SEM | [54] | 2019 |
| wool, cashmere and wool cashmere blends | around 90 | fiber | LM | [55] | 2019 |
| wool, cashmere and wool cashmere blends | 97.47 | fiber | LM | [56] | 2019 |
| wool, cashmere and wool cashmere blends | more than 90 | fiber from top | LM | [57] | 2018 |
| wool, cashmere and wool cashmere blends |
up to 95.2 | fiber | LM | [58] | 2018 |
| wool, cashmere | 90 | fiber | LM | [59] | 2018 |
| wool cashmere blends | around 90 | fiber from top | LM | [60] | 2017 |
| wool, cashmere | 81.17 | fiber | LM | [61] | 2015 |
| wool, cashmere | 87.35 | fiber | LM | [62] | 2014 |
| wool, cashmere | above 83 | fiber | SEM | [63] | 2012 |
| wool, cashmere | over 92 | fiber | xxxxxxx | [64] | 2011 |
| wool, cashmere | higher than 93 | fiber | LM | [65] | 2011 |
| wool, cashmere and stretch wool, cashmere | 99 and 81.06 | fiber | xxxxxxx | [66] | 2010 |
| wool, cashmere | xxxxxxx | fiber | SEM | [67] | 2010 |
| wool, cashmere blends | xxxxxxx | yarn | LM | [68] | 2010 |
| wool, cashmere | until 98.75 | fiber | LM | [69] | 2008 |
| wool, mohair | xxxxxxx | fiber | LM | [70] | 2002 |
| wool, mohair | 88 | fiber | LM | [71] | 2001 |
| wool, cashmere | until 97.5 | fiber | SEM | [72] | 2000 |
| wool, cashmere | xxxxxxx | fiber | SEM | [73] | 1997 |
| Fibers | Analytical method |
Identification or quantification | Accuracy | Fiber processing stage | References | Year |
|---|---|---|---|---|---|---|
| wool, mohair | raman spectroscopy and ratiometric analysis |
identification | xxxxxxx | fiber | [89] | 2022 |
| shahtoosh, cashmere, angora rabbit | FTIR and chemometry | identification | 100% | xxxxxxx | [90] | 2022 |
| wool, cashmere, wool/cashmere blend | NIR spectroscopy | identification | 93.33% for cashmere and 96.60 for cashmere wool blend | textiles from market | [91] | 2019 |
| cotton, tencel, wool, cashmere, PET, PLA, PP | NIR spectroscopy | identification | 100% identification | fiber sliver by carding | [92] | 2019 |
| wool, cashmere, rabbit, camel | NIR spectroscopy | identification | 100% sensitivity and 100% specificity | fiber | [93] | 2019 |
| wool, cashmere, qiviut, bison, vicuña | FTIR | identification | xxxxxxx | fiber | [94] | 2018 |
| wool cashmere blends | NIR spectroscopy | quantification | SEP of cashmere content 0.5% | fiber | [95] | 2017 |
| wool/cotton, wool/mohair, wool/spandex, wool/silk and wool/cashmere blends |
NIR spectroscopy | blend identification | from 100% to 85% | fabric | [96] | 2016 |
| wool cashmere blend | NIR spectroscopy | quantification | RMSEP: 2.8% | fiber | [97] | 2014 |
| wool, cashmere, yak, angora rabbit and wool cashmere blends | NIR spectroscopy | identification and quantification | percentages of recognition and rejection of 98-100%. SEP: 13.10 for wool/cashmere blend |
combed sliver | [98] | 2013 |
| wool, cashmere,PET, PA, PU, silk, flax, linen, cotton, viscose, cotton-flax blending, PET-cotton blending, and wool-cashmere blending |
NIR spectroscopy | identification | 100% discrimination between wool and cashmere | fiber, yarn, fabric | [99] | 2010 |
| wool, cashmere and wool/ cashmere blend | NIR spectroscopy | identification and quantification | SEP: 1.2061 | fiber | [100] | 2010 |
| Animal fiber | Identification or quantification | Accuracy | Fiber processing stage | References | Year |
|---|---|---|---|---|---|
| wool/cashmere blend | quantification | results of DNA analysis and LM in fabrics were quite close | fiber, yarn, dyed and finished fabrics | [31] | 2015 |
| rabbit, wool, cashmere, yak, alpaca, duck down | identification of rabbit | good accuracy | fiber | [112] | 2015 |
| wool/cashmere blend | identification | minimum amount of wool detectable in cashmere 9.09% | fiber | [113] | 2015 |
| wool, cashmere | identification | minimum amount of wool detectable in cashmere 11.1% | fiber | [114] | 2015 |
| wool, cashmere | quantification in blend | xxxxxxx | fiber and fabric | [115] | 2014 |
| shahtoosh, cashmere | identification | minimum amount of shahtooosh detectable in cashmere:1% | fiber and processed product | [116] | 2014 |
| wool, cashmere and wool/cashmere blend | identification and quantification in blend | more precise and accurate than traditional microscopic examination | fabric | [117] | 2013 |
| wool, cashmere | identification and quantification in blend | minimum amount of wool detectable in cashmere and viceversa: 11.1% | fiber | [118] | 2012 |
| wool, cashmere and wool/cashmere blend | identification and quantification in blend | minimum amount of wool detectable in cashmere: 1% | fiber | [119] | 2011 |
| cashmere/cashgora,fine wool, yak and camel | identification and quantification in blend | detection limit about 3% for fine wool/cashmere and yak/cashmere blend | untreated and treated (dyed, bleached) samples | [111] | 2009 |
| wool and goat ( cashmere, cashgora, mohair) | distinguishing between sheep and goat fiber | xxxxxxxx | fiber | [120] | 1992 |
| Animal fibers | Protein extraction | Peptide production | Analytical method | Identification or quantification | Accuracy | Fiber processing stage | References | Year |
|---|---|---|---|---|---|---|---|---|
| cashmere, shahtoosh | DTT | sds page and trypsin | Maldi TOF-MS | quantification | minimum amount of shahtoosh detectable in cashmere:5% | raw fiber and fabric | [7] | 2022 |
| vicuña, alpaca, guanaco, lama | DTT | trypsin | UHPLC MS/MS and chemometry | Identification of guanaco, vicuña, alpaca | 100% discrimination guanaco, vicuña, alpaca | fiber and ancient textiles | [122] | 2021 |
| wool, goat, cattle, camel, human hair | DTT | trypsin | UHPLC-MS ESI-Q-TOF | species-specific marker list improvement | xxxxxxx | ancient raw fibers and ancient textiles | [5] | 2019 |
| wool, cashmere | DTT | trypsin, trypsin-chymotrypsin, trypsin- GLU-C |
NanoLC MS/MS | selection of species unique peptides | xxxxxxx | raw fibers and commercial textiles (for verification) | [127] | 2018 |
| wool, cashmere, yak | DTT | trypsin | UPLC/ESI-MS | quantification | average errors from -3%/-6% to 3%/7% depending on the fiber | fiber, sliver, yarn, fabric | [123] | 2017 |
| wool, cashmere | DTT | trypsin | MALDI-TOF MS | marker identification | xxxxxxx | fiber | [130] | 2016 |
| wool, cashmere, yak | DTT | trypsin | nanoLC MS/MS triple TOF | marker identification, fiber identification and quantification | cashmere percentages are in good agreement with LM results | fiber and fabric | [126] | 2016 |
| wool, cashmere, yak | DTT | sds page and trypsin | MALDI TOF/MS MS | quantification in blend | very good linearity between the composition and the peak area ratio |
fiber and textile | [129] | 2014 |
| cashmere, wool, mohair, yak, camel, angora, alpaca | DTT | trypsin | MALDI-TOF MS and chemometric | identification | RMSE 0.365 for pure fiber RMSE 0.471 for blend |
untreated and treated fibers and 50/50 blend | [131] | 2013 |
| cashmere, yak | mercaptoethanol | trypsin | MALDI TOF MS | identification | xxxxxxx | fiber and fabric | [124] | 2013 |
| wool, cashmere, yak | DTT | trypsin | UPLC/ESI MS UPLC/ESI MS MS |
identification and quantification in blend | limit of detection: 5% | raw, bleached, depigmented, dyed fiber | [32] | 2013 |
| wool, cashmere, yak | DTT | sds page and trypsin | MALDI-TOF MS | specific marker identification for keratin I | xxxxxxx | fiber | [128] | 2012 |
| wool, yak, human, rabbit, dog, mohair, mink, fox | mercaptoethanol | trypsin | MALDI-TOF MS | Identification and quantification | xxxxxxx | raw, dyed, bleached fibers | [125] | 2002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





