Submitted:
05 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
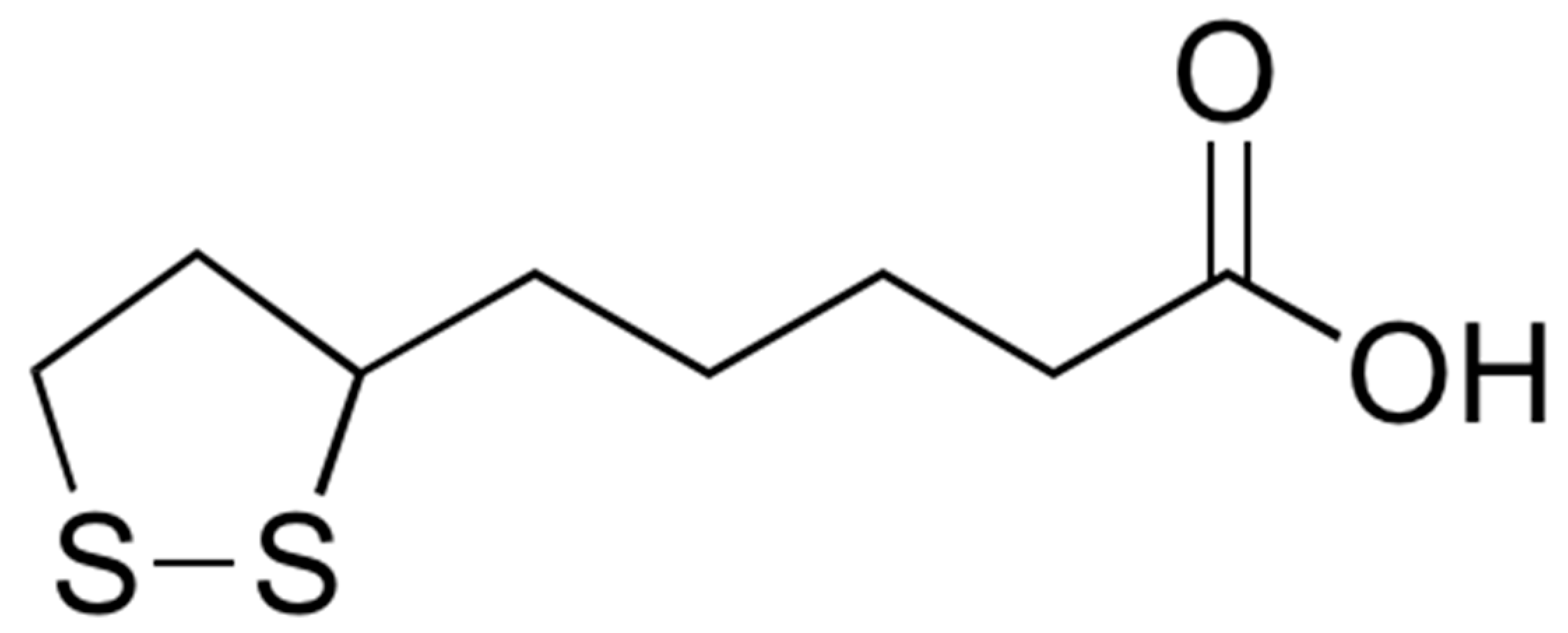
2.1. Synthetic Procedure
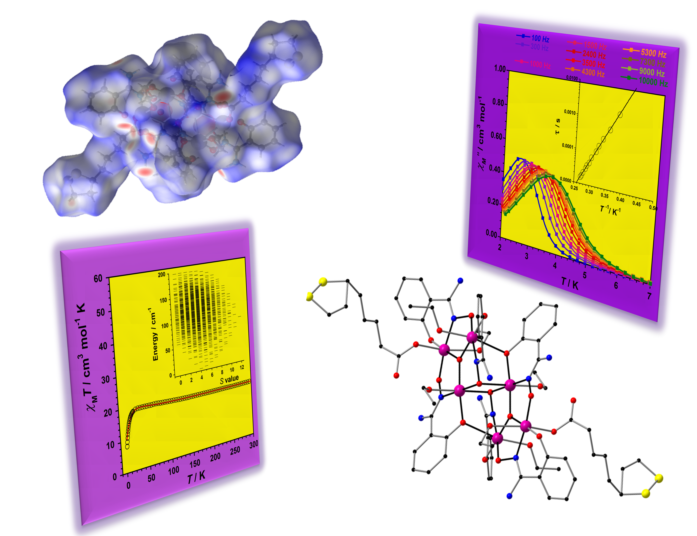
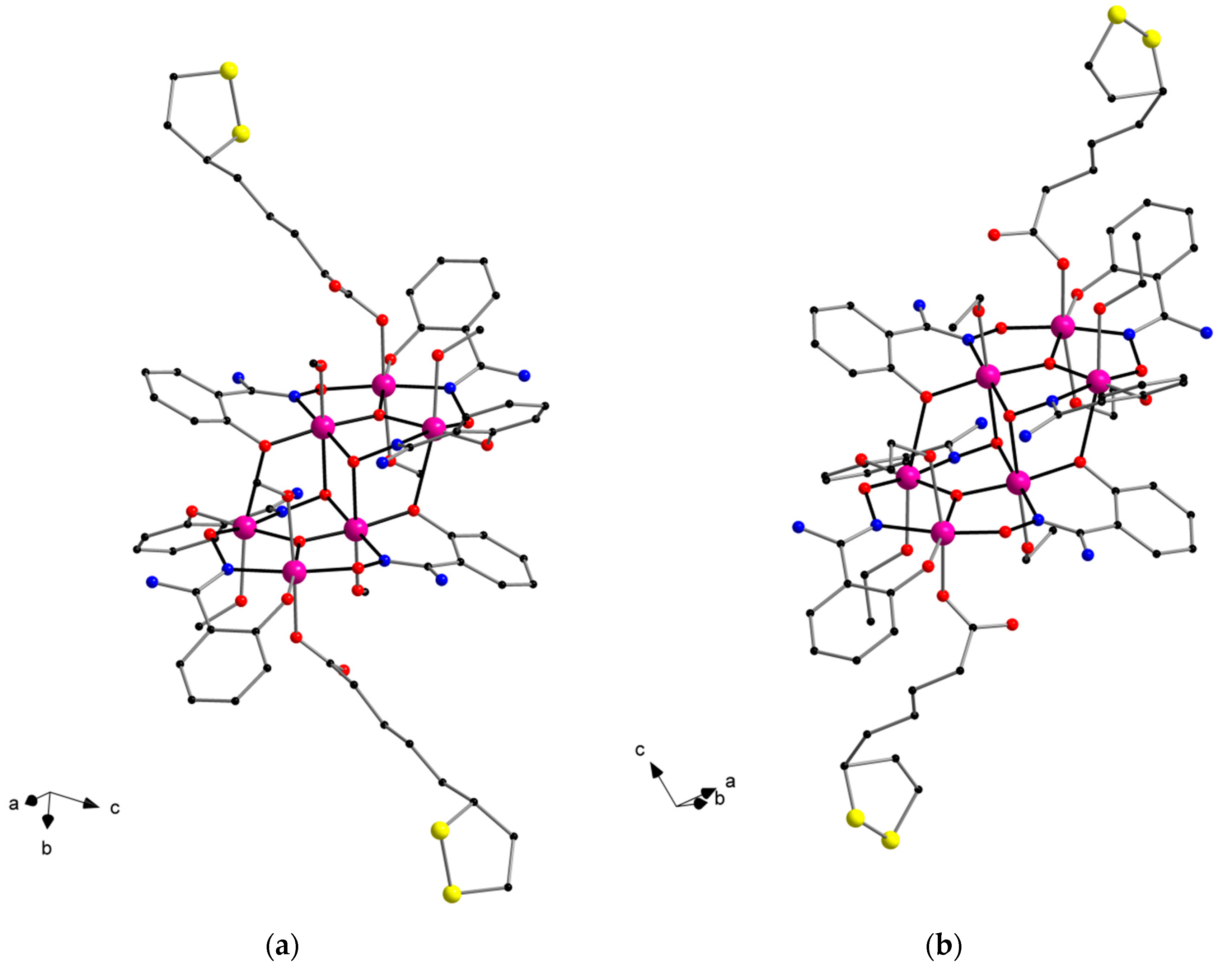
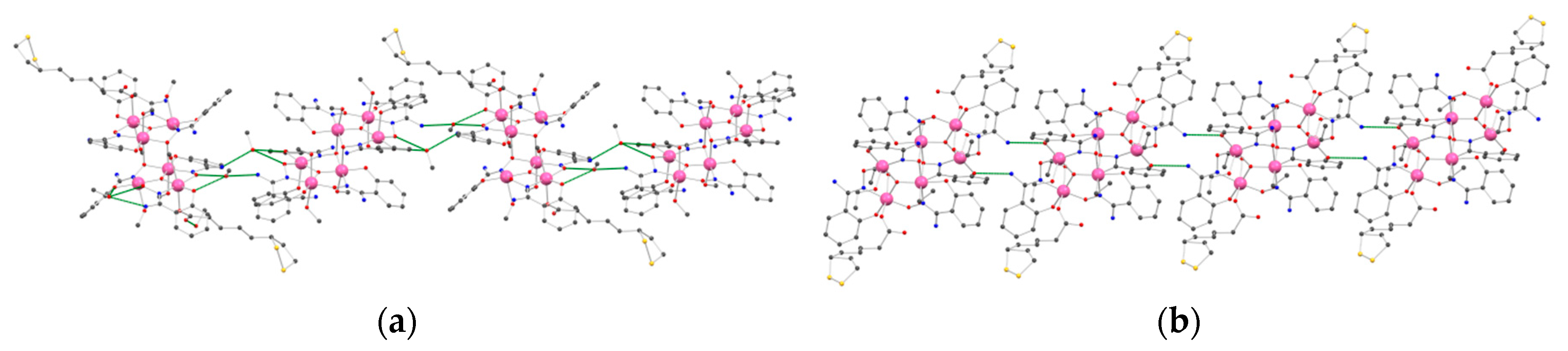
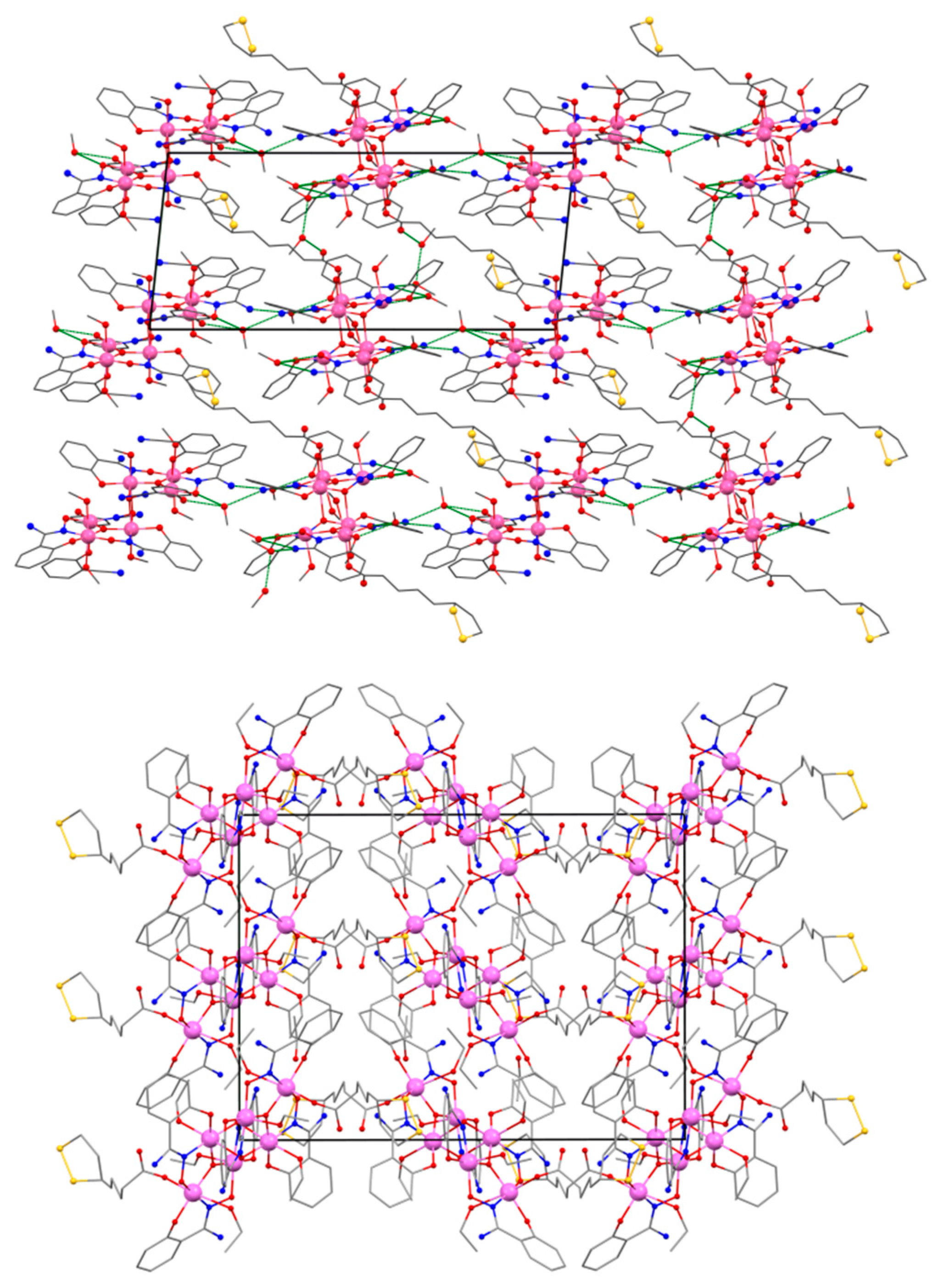
2.2. Description of the Crystal Structures
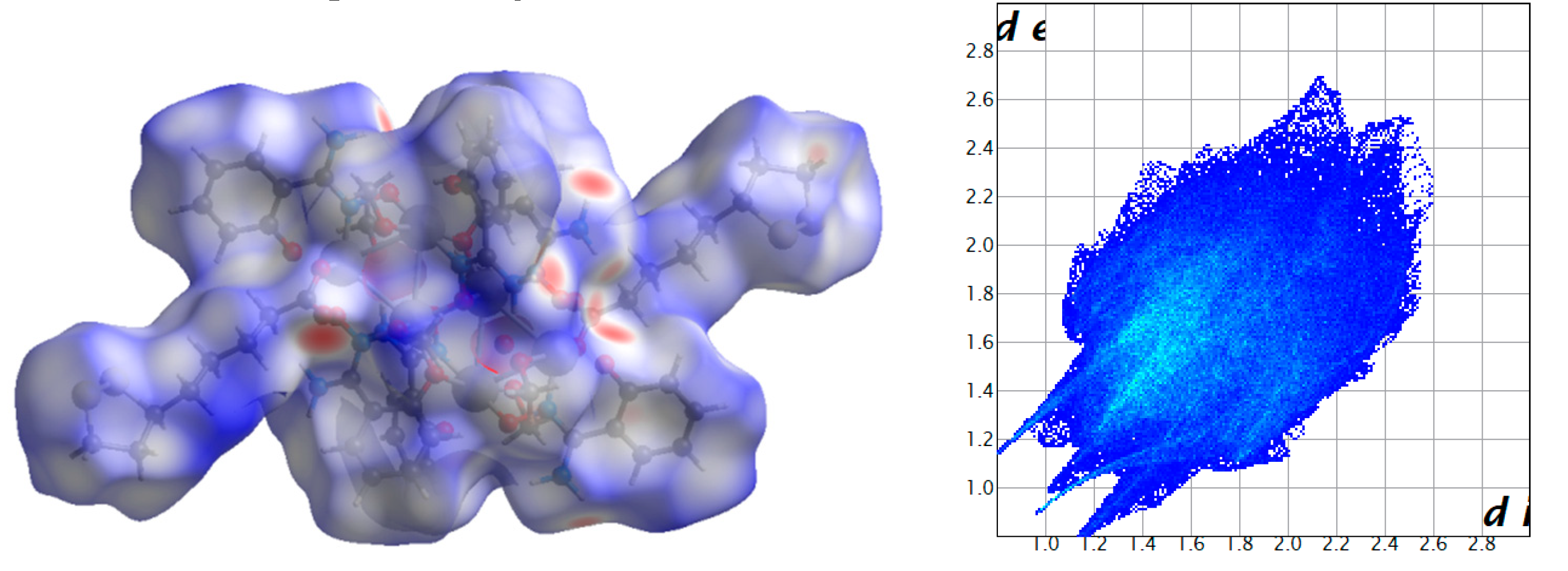
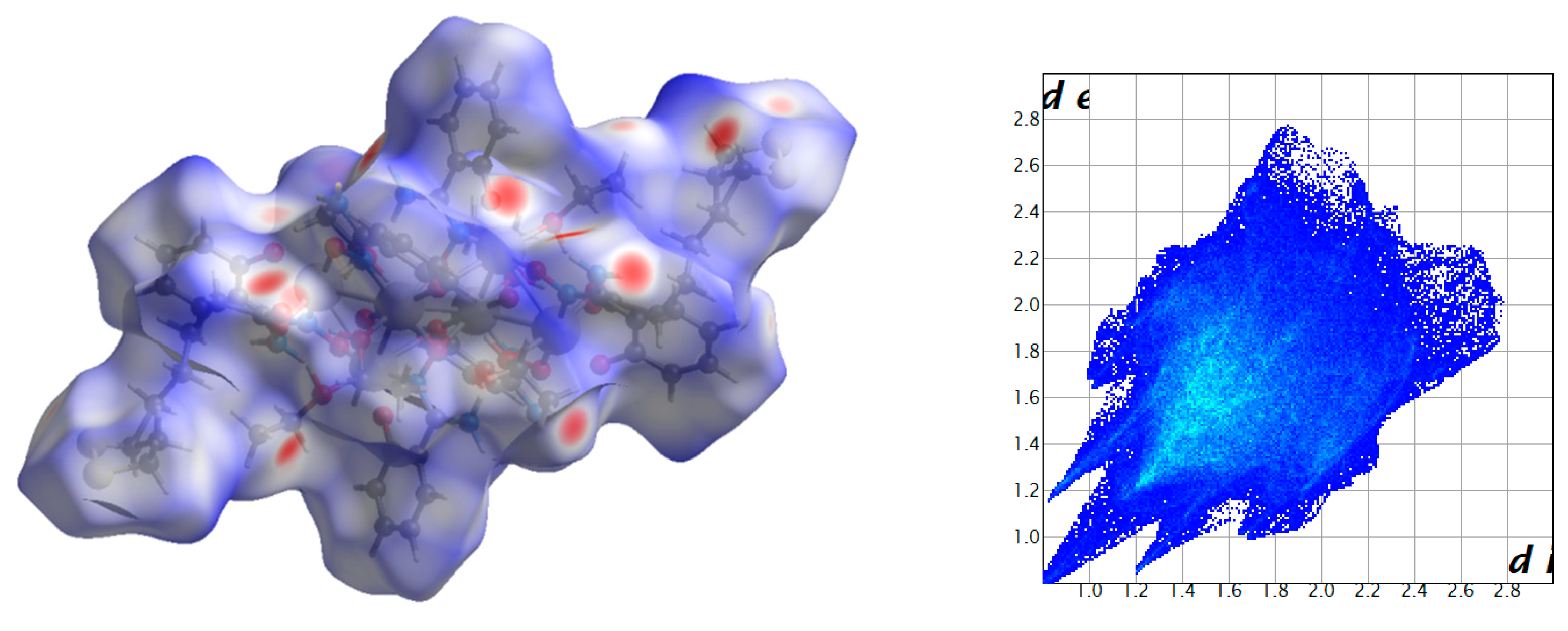
2.3. Analysis of the Hirshfeld Surfaces
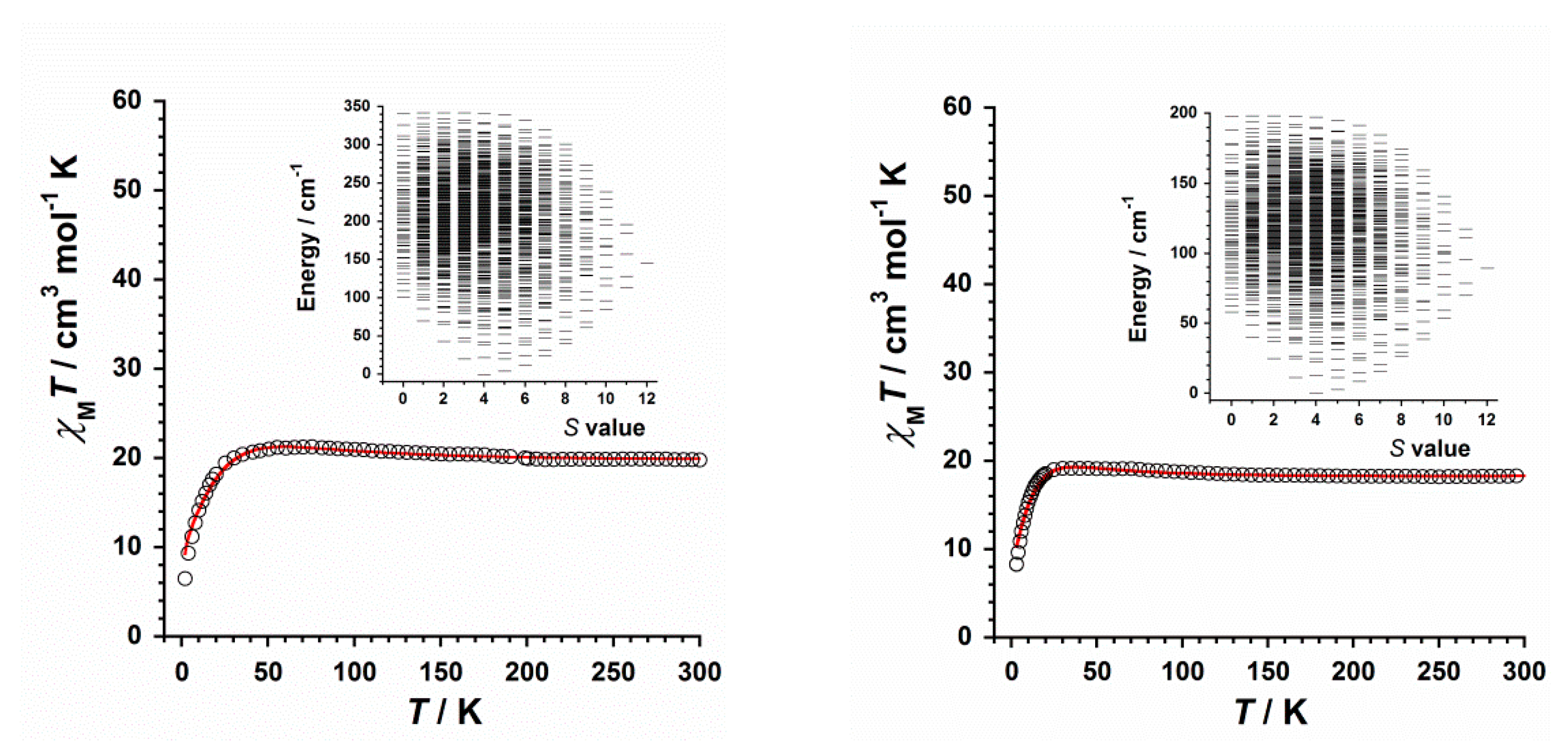
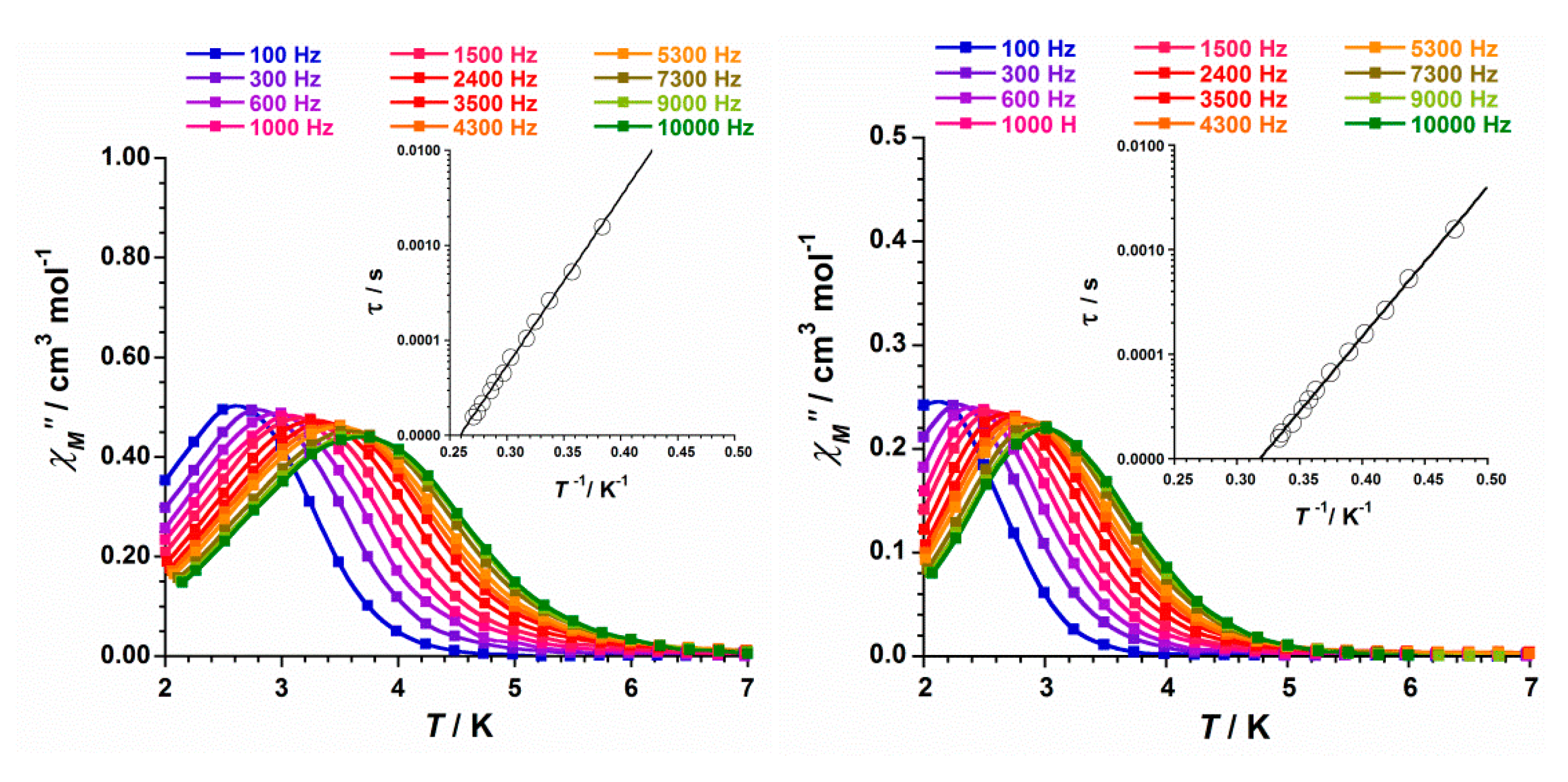
2.4. Study of the Magnetic Properties
3. Experimental Section
3.1. Preparation of the Complexes
3.1.1. Synthesis of 1
3.1.2. Synthesis of 2
3.2. X-Ray Data Collection and Structure Refinement
3.3. Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-ac to dysprosium metallocenes, a travel in time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B. M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Gebrezgiabher, M.; Bayeh, Y.; Gebretsadik, T.; Gebreslassie, G.; Elemo, F.; Thomas, M.; Linert, W. Lanthanide-Based Single-Molecule Magnets Derived from Schiff Base Ligands of Salicylaldehyde Derivatives. Inorganics 2020, 8, 66. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Castro, I.; Lloret, F.; Martínez-Lillo, J. Field-induced slow relaxation of magnetisation in two one-dimensional homometallic dysprosium(III) complexes based on alpha- and beta-amino acids. Dalton Trans. 2020, 49, 9155–9163. [Google Scholar] [CrossRef] [PubMed]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 2011, 40, 3336–3355. [Google Scholar] [CrossRef]
- Cornia, A.; Pierre Seneor, P. The molecular way. Nat. Mater. 2017, 16, 505–506. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic polyoxometalates: from molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef]
- Tyagi, P.; Li, D.; Holmes, S.M.; Hinds, B.J. Molecular Electrodes at the Exposed Edge of Metal/Insulator/Metal Trilayer Structures. J. Am. Chem. Soc. 2007, 129, 4929–4938. [Google Scholar] [CrossRef] [PubMed]
- Komeda, T.; Isshiki, H.; Liu, J.; Zhang, Y.-F.; N. Lorente, N.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Observation and electric current control of a local spin in a single-molecule magnet. Nat. Commun. 2011, 2, 217. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gu, X.; Zhu, X.; Sun, X. Recent Advances in Molecular Spintronics: Multifunctional Spintronic Devices. Adv. Mater. 2019, 31, 1805355. [Google Scholar] [CrossRef] [PubMed]
- Lewkowitz, M.; Adams, J.; Sullivan, N.S.; Wang, P.; Shatruk, M.; Zapf, V.; Arvij, A.S. Direct observation of electric field-induced magnetism in a molecular magnet. Sci Rep. 2023, 13, 2769. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Raptopoulou, C.P.; Terzis, A.; Lloret, F.; Vicente, R.; Perlepes, S.P.; Escuer, A. Hexanuclear manganese(III) single-molecule magnets. Angew. Chem. Int. Ed. 2004, 43, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Vinslava, A.; Wood, P.A.; Parsons, S.; Wernsdorfer, W.; Christou, G.; Perlepes, S.P.; Brechin, E.K. A Single-Molecule Magnet with a “Twist”. J. Am. Chem. Soc. 2007, 129, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Vinslava, A.; Wernsdorfer, W.; Moggach, S.; Parsons, S.; Perlepes, S.P.; Christou, G.; Brechin, E.K. A Record Anisotropy Barrier for a Single-Molecule Magnet. J. Am. Chem. Soc. 2007, 129, 2754–2755. [Google Scholar] [CrossRef]
- Milios, C.J.; Piligkos, S.; Brechin, E.K. Ground state spin-switching via targeted structural distortion: Twisted single-molecule magnets from derivatised salicylaldoximes. Dalton Trans. 2008, 14, 1809–1817. [Google Scholar] [CrossRef]
- Inglis, R.; Milios, C.J.; Jones, L.F.; Piligkos, S.; Brechin, E.K. Twisted molecular magnets. Chem. Commun. 2012, 48, 181–190. [Google Scholar] [CrossRef]
- Kalofolias, D.A.; Flamourakis, A.G.; Siczek, M.; Lis, T.; Milios, C.J. A bulky oxime for the synthesis of Mn(III) clusters. J. Coord. Chem. 2015, 68, 1–20. [Google Scholar] [CrossRef]
- Tomsa, A.-R.; Martínez-Lillo, J.; Li, Y.; Chamoreau, L.-M.; Boubekeur, K.; Farias, F.; Novak, M.A.; Cremades, E.; Ruiz, E.; Proust, A.; et al. A new family of oxime-based hexanuclear manganese(III) single-molecule magnets with high anisotropy energy barriers. Chem. Commun. 2010, 46, 5106–5108. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, C.-M.; Kou, H.-Z. Porous Coordination Polymers Based on {Mn6} Single-Molecule Magnets. Inorg. Chem. 2016, 55, 5880–5885. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Chamoreau, L.-M.; Proust, A.; Verdaguer, M.; Gouzerh, P. Hexanuclear manganese(III) single-molecule magnets from derivatized salicylamidoximes. C. R. Chim. 2012, 15, 889–894. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Tomsa, A.-R.; Li, Y.; Chamoreau, L.-M.; Cremades, E.; Ruiz, E.; Barra, A.-L.; Proust, A.; Verdaguer, M.; Gouzerh, P. Synthesis, crystal structure and magnetism of new salicylamidoxime-based hexanuclear manganese(III) single-molecule magnets. Dalton Trans. 2012, 41, 13668–13681. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Dolan, N.; Brechin, E.K. A cationic and ferromagnetic hexametallic Mn(III) single-molecule magnet based on the salicylamidoxime ligand. Dalton Trans. 2013, 42, 12824–12827. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Dolan, N.; Brechin, E.K. A family of cationic oxime-based hexametallic manganese(III) single-molecule magnets. Dalton Trans. 2014, 43, 4408–4414. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Cano, J.; Wernsdorfer, W.; Brechin, E.K. The Effect of Crystal Packing and ReIV Ions on the Magnetisation Relaxation of [Mn6]-Based Molecular Magnets. Chem. Eur. J. 2015, 21, 8790–8798. [Google Scholar] [CrossRef]
- Rojas-Dotti, C.; Martínez-Lillo, J. Thioester-functionalised and oxime-based hexametallic manganese(III) single-molecule magnets. RSC Adv. 2017, 7, 48841–48847. [Google Scholar] [CrossRef]
- Tyagi, P.; Riso, Ch.; Amir, U. , Rojas-Dotti, C.; Martínez-Lillo, J. Exploring room-temperature transport of single-molecule magnet-based molecular spintronics devices using the magnetic tunnel junction as a device platform. RSC Adv. 2020, 10, 13006–13015. [Google Scholar] [CrossRef]
- Grizzle, A.; D'Angelo, Ch.; Martínez-Lillo, J.; Tyagi, P. Spin state of a single-molecule magnet (SMM) creating long-range ordering on ferromagnetic layers of a magnetic tunnel junction – a Monte Carlo study. RSC Adv. 2021, 11, 32275–32285. [Google Scholar] [CrossRef]
- Mitcov, D.; Pedersen, A.H.; Ceccato, M.; Gelardi, R.M.; Hassenkam, T.; Konstantatos, A.; Reinholdt, A.; Sørensen, M.A.; Thulstrup, P.W.; Vinum, M.G.; Wilhelm, F.; Rogalev, A.; Wernsdorfer, W.; Brechin, E.K.; Piligkos, S. Molecular multifunctionality preservation upon surface deposition for a chiral single-molecule magnet. Chem. Sci. 2019, 10, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Dotti, C.; Moliner, N.; Lloret, F.; Martínez-Lillo, J. Ferromagnetic Oxime-Based Manganese(III) Single-Molecule Magnets with Dimethylformamide and Pyridine as Terminal Ligands. Crystals 2019, 9, 23. [Google Scholar] [CrossRef]
- Rojas-Dotti, C.; Moliner, N.; Lloret, F.; Martínez-Lillo, J. Hexanuclear manganese(III) single-molecule magnets based on oxime and azole-type ligands. Polyhedron 2019, 170, 223–231. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 17; University of Western Australia, 2017.
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Ten-Esteve, A.; Ginés-Cárdenas, S.; Castro, I.; Martí-Bonmatí, L.; Martínez-Lillo, J. A Gadolinium(III) Complex Based on the Thymine Nucleobase with Properties Suitable for Magnetic Resonance Imaging. Int. J. Mol. Sci. 2021, 22, 4586. [Google Scholar] [CrossRef]
- Sanchis-Perucho, A.; Orts-Arroyo, M.; Castro, I.; Lloret, F.; Martínez-Lillo, J. Crystal polymorphism in 2,2'-bipyrimidine-based iridium(III) complexes. J. Coord. Chem. 2022, 75, 2495–2507. [Google Scholar] [CrossRef]
- SHELXTL-2013/4, Bruker Analytical X-ray Instruments, Madison, WI, 2013.
- Diamond 4.5.0, Crystal Impact GbR, CRYSTAL IMPACT, 2018.
- CrystalMaker 8.5.1, CrystalMaker Software Ltd, Oxford, England.
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal's Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]

| Compound | 1 | 2 |
|---|---|---|
| CCDC | 2253180 | 2253181 |
| Formula | C136H182N26O56S4Mn12 | C72H104N12O27S4Mn6 |
| Fw/g mol−1 | 3864.58 | 2027.55 |
| Crystal system | triclinic | Monoclinic |
| Space group | Pī | C2/c |
| a/Å | 13.048(1) | 20.482(1) |
| b/Å | 13.185(1) | 17.701(1) |
| c/Å | 27.654(2) | 24.934(1) |
| α/° | 91.06(1) | 90 |
| β/° | 99.89(1) | 103.91(1) |
| γ/° | 115.57(1) | 90 |
| V/Å3 | 4204.6(5) | 8774.5(4) |
| Z | 1 | 4 |
| Dc/g cm−3 | 1.526 | 1.535 |
| μ(Mo-Kα)/mm−1 | 1.008 | 1.014 |
| F(000) | 1992 | 4200 |
| Goodness-of-fit on F2 | 1.037 | 1.126 |
| R1 [I > 2σ(I)]/all data | 0.0900/0.1569 | 0.0938/0.1145 |
| wR2 [I > 2σ(I)] /all data | 0.2042/0.2443 | 0.2328/0.2447 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





