Submitted:
06 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area and Data
2.2. Method
2.2.1. Climate tendency rate
2.2.2. Climate production potential
2.2.3. Logistic curve
2.3. Data processing
3. Results
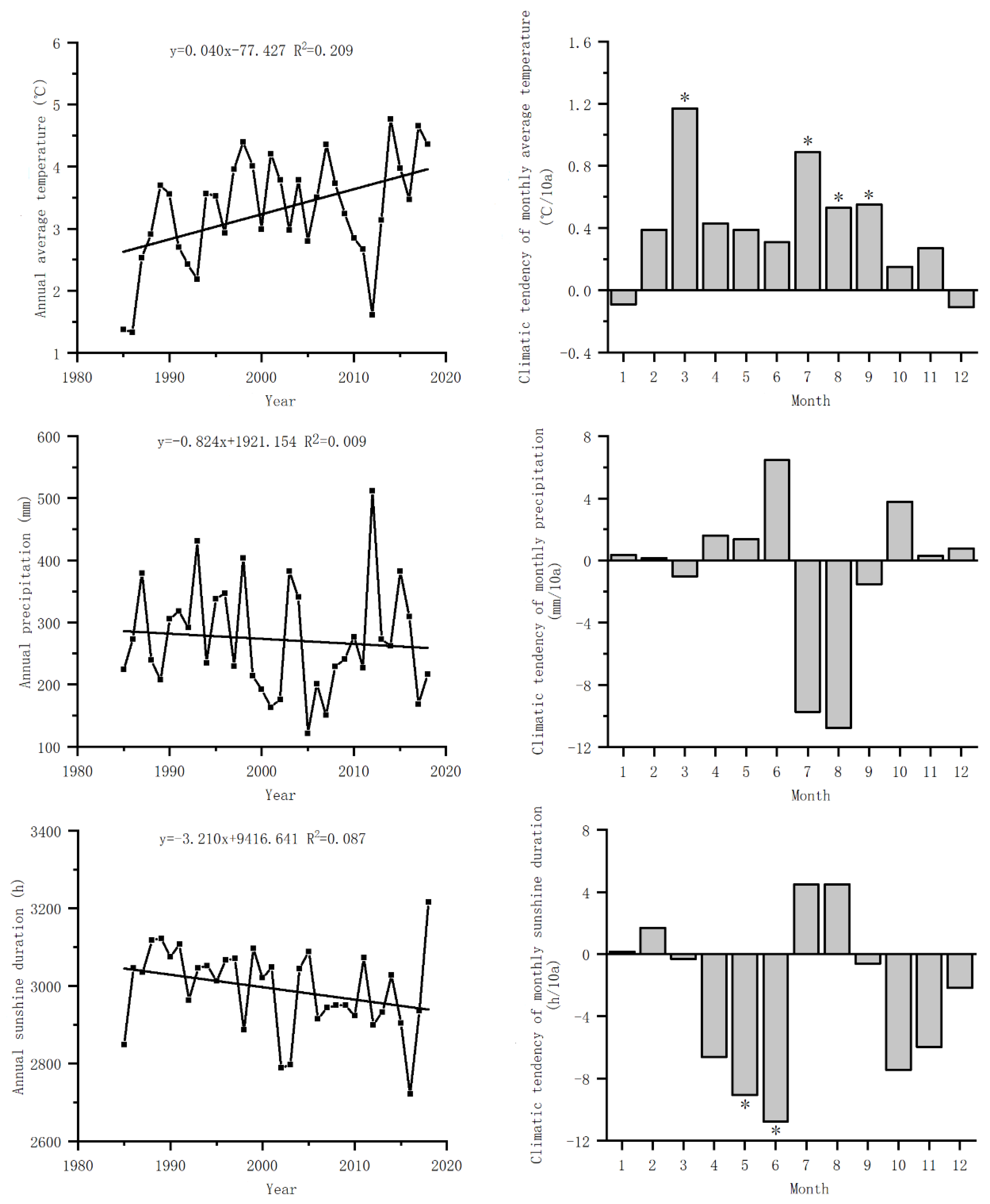
3.1. Climate change trends
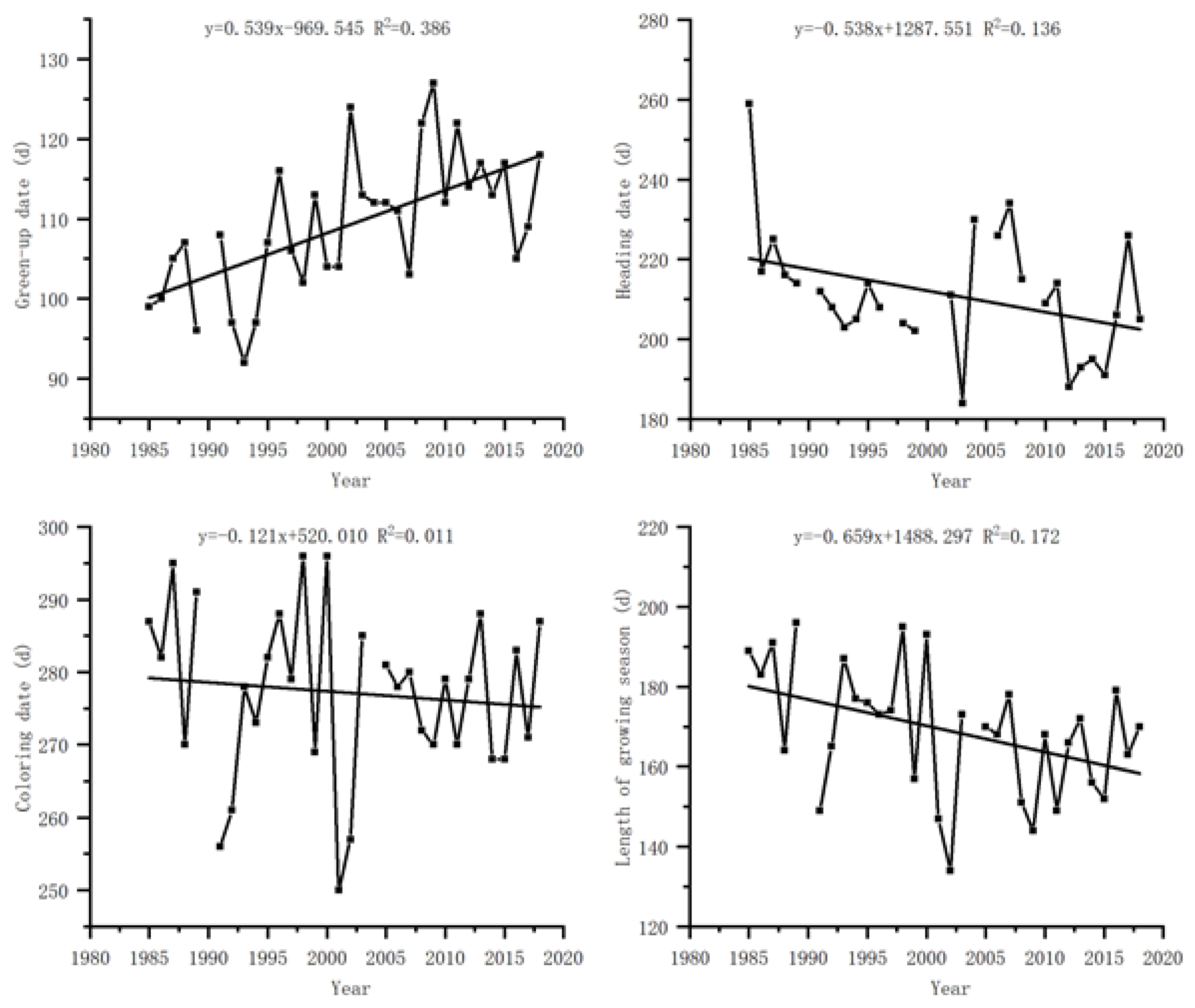
3.2. Phenological change trends of S. krylovii plant
3.3. Relationship between the main phenology and climate production potential
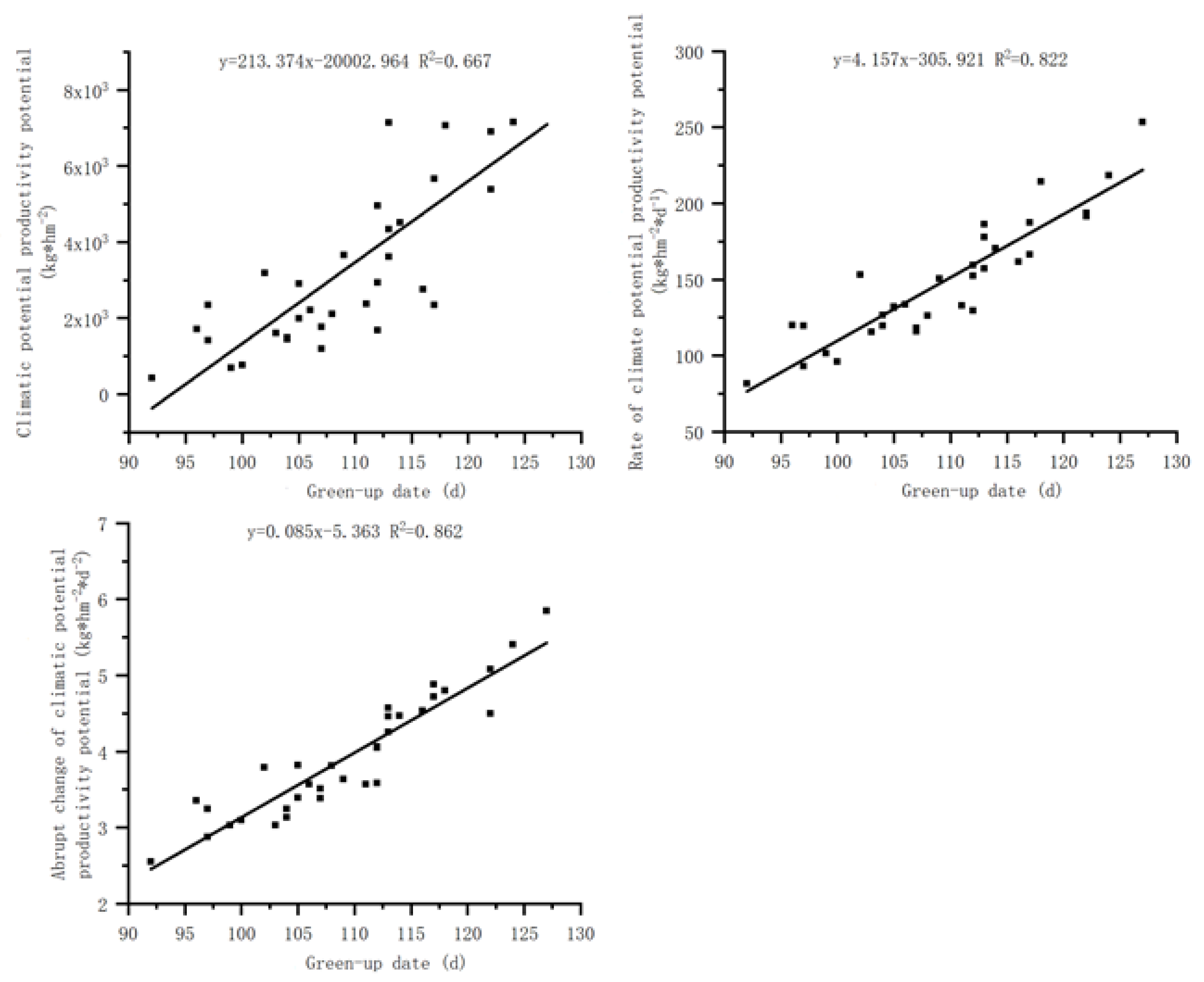
3.3.1. Relationship between the green-up date and climate production potential
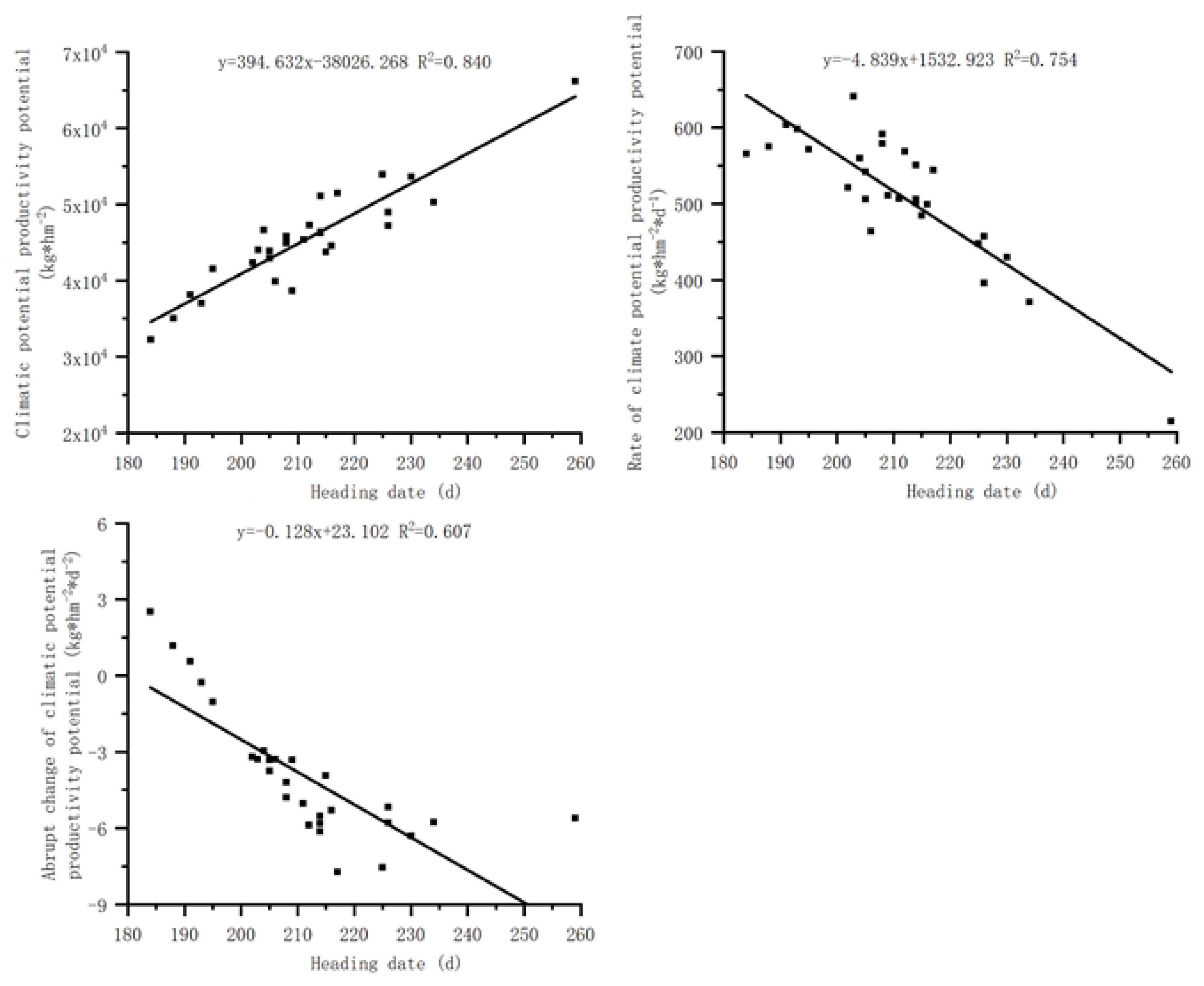
3.3.2. Relationship between the heading date and climate production potential
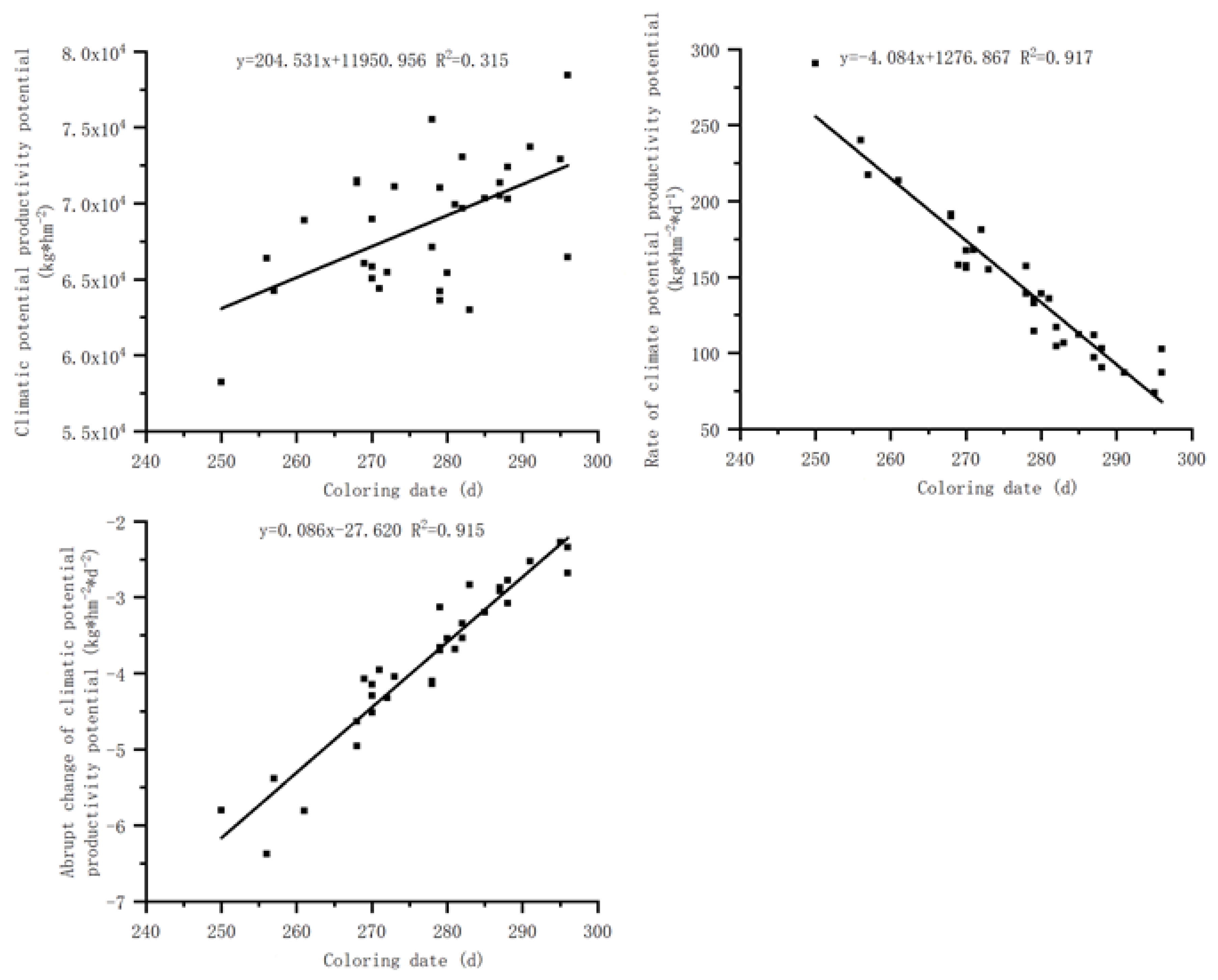
3.3.3. Relationship between the wilting date and climate production potential
4. Discussion and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.X.; Chen, H.L.; Li, Q. Research advances in plant phenology and climate. Acta Eco. Sin. 2010, 30, 0447–0454. (In Chinese) [Google Scholar]
- Zhu, K.Z.; Wan, M.W. Phenology. Beijing: Science Press, 1973. (In Chinese)
- Zhu, K.Z. A preliminary research of climatic change in China about five thousand years past. Scientia Sin. 1973, 2, 168–189. (In Chinese) [Google Scholar]
- Penuelas, J.; Rutishauser, T.; Filella, I. Ecology. Phenology feedbacks on climate change. Science, 2009, 324, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Gray, J; Friedl, M. A.; Toomey, M; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; Wing, I.S.; Yang, B.; Richardson, A.D. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Li, R.P.; Zhou, G.S.; Zhang, H.L. Research advances in plant phenology. Chin. J. Appl. Ecol. 2006, 17, 541–544. (In Chinese) [Google Scholar]
- Piao, S.L.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Glob. Biogeochem. Cy. 2007, 21, GB3018. [Google Scholar] [CrossRef]
- Jeong, S.J.; Ho, C.H.; Gim, H.J.; Brown, M. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Chang. Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Root, T.; MacMynowski, D.; Mastrandrea, M. Human-modified temperatures induce species changes: Joint attribution. P. Natl. Acad. Sci. USA 2005, 102, 7465–7469. [Google Scholar] [CrossRef]
- Shi, G.H. Phenological variation of main herbages during the last 20 years in the typical steppe of Inner Mongolia Plateau China. Chin. J. Grassl. 2019, 41, 80–88. (In Chinese) [Google Scholar]
- Lesica, P.; Kittelson, P.M. Precipitation and temperature are associated with advanced flowering phenology in a semi-arid grassland. J. Arid Environ. 2020, 74, 1013–1017. [Google Scholar] [CrossRef]
- Zhang, X.B.; Ren, J.R.; Zhang, D.R. Phenological observations onLarix principis-rupprechtii Mayr. in primary seed orchard. J. Forestry Res. 2001, 12, 201–204. [Google Scholar]
- Tao, Z.X.; Wang, H.J.; Liu, Y.C.; Xu, Y.J.; Dai, J.H. Phenological response of different vegetation types to temperature and precipitation variations in northern China during 1982–2012. Int. J. Remote Sens. 2017, 38, 3236–3252. [Google Scholar] [CrossRef]
- Pennington, D.D.; Collins, S.L. Response of an aridland ecosystem to interannual climate variability and prolonged drought. Landscape Ecol. 2007, 22, 897–910. [Google Scholar] [CrossRef]
- Hu, Y.; Li, P.; Yang, J.G. Applied Meteorology (2nd edition). Beijing: China Meteorological Press, 2005.
- Way, D.A.; Montgomery, R.A. Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant Cell Environ. 2015, 38, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Yokozawa, M.; Zhang, Z.; Hayashi, Y.; Ishigooka, Y. Land surface phenology dynamics and climate variations in the North East China Transect (NECT), 1982–2000. Int. J. Remote Sens. 2008, 29, 5461–5478. [Google Scholar] [CrossRef]
- Fu, Y.H.; Zhao, H.F.; Piao, S.L.; Peaucelle, Marc; Peng, S. S.; Zhou, G.Y.; Ciais, P.; Huang, M.T.; Menzel, A.; Peñuelas, J.; Song, Y.; Vitasse, Y.; Zeng, Z.Z.; Janssens, I.A. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Li, X.X.; Zhou, X.C.; Geng, X.J.; Guo, Y.H.; Zhang, Y.R. Progress in plant phenology modeling under global climate change. Sci. Chin. Earth Sci. 2020, 63, 1237–1247. [Google Scholar] [CrossRef]
- Hänninen, H.; Kramer, K.; Tanino, K.; Zhang, R.; Wu, J.S.; Fu, Y.H. Experiments are necessary in process–based tree phenology modelling. Trends Plant Sci. 2019, 24, 199–209. [Google Scholar] [CrossRef]
- Hu, M.X.; Zhou, G.S. Phenological change and its ecophysiological mechanism of spring maize responding to drought at jointing stage and rewatering. Acta Eco. Sin. 2020, 40, 274–283. (In Chinese) [Google Scholar]
- Dai, W.J.; Jin, H.Y.; Zhang, Y.H. Advances in plant phenology. Acta Ecol. Sin. 2020, 40, 6705–6719. (In Chinese) [Google Scholar]
- Hossain, A.; Teixeira da Silva, J.A.; Lozovskaya, M.V.; Zvolinsky, V.P. High temperature combined with drought affect rainfed spring wheat and barley in South-Eastern Russia: I. Phenology and growth. Saudi. J. Biol. Sci. 2012, 19, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yang, X.G.; Sun, S. Comparison of potential yield and resource utilization efficiency of main food crops in three provinces of Northeast China under climate change. Chin. J. Appl. Ecol. 2015, 26, 3091–3102. (In Chinese) [Google Scholar]
- Zhou, G.S. , Song, X.Y., Zhou, M.Z.; Zhou, L.; Ji, Y.H. Advances in influencing mechanism and model of total climatic production factors of plant phenology change. Sci. Sin. Vitae. 2023, 53, 380–389. (In Chinese) [Google Scholar] [CrossRef]
- Lee, R.; Yu, F.; Price, K.P. Evaluating vegetation phenological patterns in Inner Mongolia using NDVI time-series analysis. Int. J. Remote Sens. 2002, 23, 2505–2512. [Google Scholar] [CrossRef]
- Sui, X.H.; Zhou, G.S.; Zhuang, Q.L. Sensitivity of carbon budget to historical climate variability and atmospheric CO2 concentration in temperate grassland ecosystems in China. Clim. Chang. 2013, 117, 259–272. [Google Scholar] [CrossRef]
- Inner Mongolia Ningxia Comprehensive Expedition, Chinese Academy of Sciences. Vegetation of Inner Mongolia, Beijing: Science Press. 1985.
- Yuan, W.P.; Zhou, G.S.; Wang, Y.H.; Wang, Y.S. Simulating phenological characteristics of two dominant grass species in a semi-arid steppe ecosystem. Ecol. Res. 2007, 22, 784–791. [Google Scholar] [CrossRef]
- Parry, M.; Canziani, O.; Palutikof, J. Intergovernmental Panel on Climate Change Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. UK: Cambridge University Press, 2007.
- China Meteorological Administration. Specification for agrometeorological observation, Beijing: China Meteorological Press. 1993.
- Weng, D. Climatological calculation methods for total radiation. Acta Meteorol. Sin., 1964, 3, 304–315. (In Chinese) [Google Scholar]
- Wang, B.; Zhang, F. Solar energy Resources in China. Acta Energiae Solaris Sin. 1980, 1, 1–9. (In Chinese) [Google Scholar]
- Guo, J.; Gao, S.; Liu, L. Climatic productivity of forage grass and its restricting factors in north region of China. Chin. J. eco-agricul. 2002, 3, 48–50. (In Chinese) [Google Scholar]
- Kanniah, K.D.; Beringer, J.; Hutley, L.B.; Tapper, N.J.; Zhu, X. Evaluation of Collections 4 and 5 of the MODIS Gross Primary Productivity product and algorithm improvement at a tropical savanna site in northern Australia. Remote Sens. Environ. 2009, 113, 1808–1822. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, G.S.; Wang, Y. Phenological calendar of stipa krylovii steppe in Inner Mongolia, China and its correlation with climatic variables. Acta Phytoecol. Sin. 2008, 32, 1312–1322. (In Chinese) [Google Scholar]
- Wang, H.J.; Wu, C.Y.; Ciais, P.; Peñuelas, J.; Dai, J.H.; Fu, Y.H.; Ge, Q.S. Overestimation of the effect of climatic warming on spring phenology due to misrepresentation of chilling. Nat. Commun. 2020, 11, 4945. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L. Non-linear response of dominant plant species regreening to precipitation in mid-west Inner Mongolia in spring. Acta Eco. Sin. 2020, 40, 9120–9128. (In Chinese) [Google Scholar]
- Zhang, X.Y.; Friedl, M.A.; Schaaf, C.B.; Strnhler, A.H. Monitoring the response of vegetation phenology to precipitation in Africa by coupling MODIS and TRMM instruments. J. Geo. Res. Atmos. 2005, 110, D12103. [Google Scholar] [CrossRef]
- Zhang, F. Effects of global warming on plant phenological events in Chana. Acta Geo. Sin. 1995, 50, 402–410. (In Chinese) [Google Scholar]
- Jeong, S.J.; Medvigy, D. Macroscale prediction of autumn leaf coloration throughout the continental United States. Glob. Ecol. Biogeogr. 2014, 23, 1245–1254. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zhou, G.S.; Lv, X.M.; He, Q.J.; Zhou, M.Z. Environmental factors rather than productivity drive autumn leaf senescence: evidence from a grassland in situ simulation experiment. Agr. For. Meteorol. 2022, 37, 109221. [Google Scholar] [CrossRef]
- Chen, X.Q.; Li, J. Relationships between Leymus chinensis phenology and meteorological factors in Inner Mongolia grasslands. Acta Ecol. Sin. 2009, 29, 5280–5290. (In Chinese) [Google Scholar]
| Parameters | Definition | Values |
|---|---|---|
| C | Unit conversion factor(MJ·m-2/KJ·g-1→kg·hm-2) | 10000 |
| Ability of photosynthetic fixation of CO2 | 0.95 | |
| Ratio of photosynthetic radiation to total radiation | 0.5 | |
| Quantum efficiency of photosynthesis | 0.224 | |
| Community reflectance | 0.1 | |
| Community leakage rate | 0.04 | |
| Ratio of radiation interception by non-photosynthetic organs | 0.1 | |
| Light saturation limitation | 0.03 | |
| Ratio of respiratory expenditure to photosynthetic products | 0.3 | |
| Plant water content (hay) | 0.1 | |
| Inorganic ash ratio | 0.08 | |
| Economic coefficient | 0.65 | |
| Unit dry matter heat content (KJ·g-1) | 17.77 |
| Year | Logistic curve | Determination coefficients(R2) |
|---|---|---|
| 1985 | 72262.866/(1+548.623*e-0.033t) | 0.998 |
| 1986 | 74654.37/(1+824.629*e-0.035t) | 0.999 |
| 1987 | 74151.926/(1+514.25*e-0.033t) | 0.998 |
| 1988 | 69566.324/(1+531.187*e-0.032t) | 0.998 |
| 1989 | 75977.088/(1+359.302*e-0.031t) | 0.998 |
| 1990 | 77925.061/(1+433.181*e-0.031t) | 0.999 |
| 1991 | 74321.319/(1+684.216*e-0.034t) | 0.999 |
| 1992 | 75164.166/(1+639.736*e-0.033t) | 0.999 |
| 1993 | 78389.704/(1+657.65*e-0.033t) | 0.998 |
| 1994 | 74826.421/(1+330.43*e-0.031t) | 0.998 |
| 1995 | 72703.58/(1+693.954*e-0.034t) | 0.998 |
| 1996 | 74278.724/(1+581.662*e-0.033t) | 0.998 |
| 1997 | 66595.984/(1+357.171*e-0.031t) | 0.998 |
| 1998 | 80381.273/(1+247.249*e-0.029t) | 0.998 |
| 1999 | 71049.169/(1+321.777*e-0.031t) | 0.997 |
| 2000 | 68208.123/(1+300.816*e-0.03t) | 0.996 |
| 2001 | 70380.664/(1+346.159*e-0.03t) | 0.998 |
| 2002 | 71069.31/(1+413.602*e-0.032t) | 0.998 |
| 2003 | 72848.292/(1+451.606*e-0.032t) | 0.998 |
| 2004 | 74846.167/(1+351.334*e-0.03t) | 0.998 |
| 2005 | 73515.12/(1+417.551*e-0.031t) | 0.998 |
| 2006 | 71360.61/(1+445.391*e-0.031t) | 0.998 |
| 2007 | 68455.821/(1+335.983*e-0.03t) | 0.997 |
| 2008 | 71035.352/(1+323.441*e-0.03t) | 0.998 |
| 2009 | 73293.543/(1+348.71*e-0.031t) | 0.998 |
| 2010 | 67186.357/(1+505.09*e-0.032t) | 0.996 |
| 2011 | 70177.565/(1+495.677*e-0.032t) | 0.999 |
| 2012 | 74063.771/(1+401.423*e-0.031t) | 0.998 |
| 2013 | 71732.187/(1+610.204*e-0.033t) | 0.998 |
| 2014 | 76911.24/(1+297.44*e-0.03t) | 0.999 |
| 2015 | 77103.121/(1+423.355*e-0.031t) | 0.999 |
| 2016 | 65493.882/(1+299.812*e-0.03t) | 0.997 |
| 2017 | 68765.746/(1+260.438*e-0.029t) | 0.996 |
| 2018 | 73684.145/(1+237.541*e-0.029t) | 0.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





