Introduction
KRAS is the most frequently mutated oncogene and in addition mutated in congenital disorders, called RASopathies [
1,
2]. It is not fully understood why
KRAS is more frequently mutated in cancer than the other
RAS genes,
NRAS and
HRAS. Several facets of Ras biology may contribute to the higher exploitation of
KRAS, such as its higher expression level, its specific intracellular trafficking and distribution or its distinct nanoscale organization in the plasma membrane that imposes differential effector usage [
3,
4,
5]. Another less characterized difference is the ability of Ras proteins to impose stemness properties in cells [
6,
7]. Notably, the most common
KRAS splice variant, K-Ras4B (hereafter K-Ras), but not H-Ras, mediates stemness properties via calmodulin (CaM)-dependent non-canonical Wnt-signalling [
6]. In line with this, CaM-inhibitors block stemness properties of
K-RAS-mutant cancer cells [
8,
9]. However, the exact mechanism of how CaM mediates K-Ras-driven stemness is not resolved.
Previous cellular data showed that K-Ras/ CaM complexes are disrupted by phosphomimetic mutations of Ser181 at the C-terminus of K-Ras. Conversely, CaM binding blocked phosphorylation at that site [
10]. Intriguingly, the phosphomimetic mutation of K-RasG12V on Ser181 reduces its ability to drive stemness [
6]. Mutations at this site also modulate the interaction with another trafficking chaperone PDE6D [
11], which traffics several prenylated proteins to stemness mediating organelles [
12]. Hence, CaM may not be alone in mediating the K-Ras-stemness activity.
CaM possesses two Ca
2+-binding lobes, which can each encase 15-20-residue long peptide stretches of classical target proteins in their hydrophobic surfaces [
13]. Classical target peptides are typically helical, positively charged and contain hydrophobic anchor residues. Very similar biochemical characteristics are found in singly lipidated, polybasic termini of prenylated or myristoylated proteins, which have emerged as non-canonical targets of CaM [
14]. CaM facilitates the Ca
2+-dependent cytoplasmic solubilization of K-Ras, by sequestering its farnesyl-tail from the aqueous environment [
15]. This contrasts to the GTP-Arl2/3 triggered release of PDE6D cargo [
16]. PDE6D and CaM share the preference for K-Ras amongst the Ras isoforms, as palmitoylation obstructs access to the hydrophobic pockets, making K-Ras4A, N-Ras and H-Ras clients only in their non-palmitoylated states [
17,
18]. Both trafficking chaperones are found in the cyto- and nucleoplasm, and on centriolar structures, such as the primary cilium and the centrosomes [
16,
19,
20]. Hence, it is plausible to assume that these two chaperones have overlapping, yet distinct roles to coordinate trafficking of prenylated proteins spatio-temporally.
In cell lysates, CaM engages more with GTP-loaded K-Ras than with its inactive counterpart [
17,
21]. Furthermore, complexes between K-Ras, CaM and PI3K p110 subunits have been proposed as being relevant for Akt activation during platelet-derived growth factor receptor (PDGFR)-mediated cell migration [
22,
23]. The fact that the activation state of Ras matters for its interaction with CaM contrasts with in vitro and structural data. Only weak transient contacts of CaM with non-farnesylated K-Ras were observed in NMR-experiments, while the farnesylated poly-lysine stretch of K-Ras comprising residues 180-185 was sufficient for CaM binding [
15,
24]. In vitro data further suggest that the polybasic and farnesylated C-terminus of K-Ras binds to either of the Ca
2+-bound lobes of CaM, but without involvement of the G-domain [
25]. Thus, it appears that the farnesylated C-terminus of K-Ras is sufficient for micromolar binding to CaM. However, in cells there may be CaM/ K-Ras complexes that depend on the activation state of K-Ras.
Inhibitors of CaM alter its conformation, thus preventing binding of canonical target peptides and non-canonical targets [
9,
13,
26,
27]. The covalent CaM inhibitor ophiobolin A disrupts binding of K-Ras to CaM and K-Ras membrane anchorage by irreversibly modifying Lys75, 77 and 148 of CaM [
8,
9,
28]. We recently developed an alternative, less toxic covalent inhibitor of CaM, called Calmirasone1, which is much more suitable for cell biological applications [
9].
Centrin (or caltractin) proteins are highly related to CaM with the same bi-lobal structure, however, only the C-terminal lobe binds and senses Ca
2+ with high affinity [
29]. This leaves the centrin-specific N-terminus free for mediating self-assembled extended structures of centrins, which are Ca
2+ -dependent due to allosteric coupling with the C-terminus [
30]. In humans three centrin paralogs (centrin1-3,
CETN1-3) are known [
31]. While centrin2 and centrin3 are ubiquitously expressed, centrin1 expression is limited to male germ cells, neurons, and ciliated cells [
32]. Centrin2 is probably best known for binding and stabilizing XPC (xeroderma pigmentosum group C), which is involved in DNA repair [
33]. In addition, centrins have been implicated in nuclear pore functions and proteasomal activities [
32]. Like CaM, centrins appear to recognize a hydrophobic motif of 15-20 residues in such classical target proteins [
34].
The activity of centrins can be regulated by several phosphorylation and SUMOylation events [
34]. Nuclear localization of centrin2 is enhanced by its SUMOylation [
35]. Phosphorylation of T118 in the third EF-hand of the centrin2 C-terminal lobe is required for Ca
2+-binding and its centrosomal localization [
32]. Centrins localize to distal and intermediate regions preferentially of the mother centrioles and are part of a set of 14 ancient and highly conserved centriolar proteins [
36,
37]. Hence, loss of centrins broadly affects centriolar functions, including organisation of the microtubule network or overall biogenesis of centrioles [
32]. Based on the essential roles of centrins in uni-cellular organisms that depend on cilia formation, it is plausible to assume that an important role also exists for centrins in vertebrate/ mammalian ciliogenesis [
32]. In line with this, ciliogenesis is reduced upon depletion of centrin2 in hTERT-RPE1 cells [
38].
Given the highly similar bi-lobal structure with hydrophobic binding pockets, we hypothesized that centrins also bind to non-classical targets of CaM, such as K-Ras. Here we show that K-Ras binds to centrin1 in cells in a similar manner to CaM. Our results suggest that binding of K-Ras to these Ca2+-binding proteins in cells is largely independent of the prenylation of K-Ras and involves the G-domain. Given that CaM-inhibitors also affect the K-Ras/ centrin1 interaction and the very similar distribution of centrin1 and CaM throughout the cell cycle, the dependence of K-Ras on either protein may be difficult to determine.
Experimental Procedures
Plasmids, siRNAs and Inhibitors
All construct names contain the tag at a position corresponding to its location in the protein sequence, e.g. GFP2-CaM, contains the GFP2-tag at the N-terminus of CaM. All plasmids employed in the study were produced by multi-site gateway cloning [
53]. The human
CALM1 entry clone with L1 – L2 recombination sites was obtained from the NCI RAS Initiative. The K-Ras4b entry clone was procured from Addgene (RAS mutant clone collection, Kit #1000000089). Custom-synthesised entry clones encoding human
CETN1 or the CTK fragment with L1 – L2 recombination sites in pDONR221 vector were commercially obtained from Genecust, France. An LR recombination reaction comprising three entry clones encoding the CMV promoter, a tag (Rluc8 or GFP2) and the protein of interest (CTK, K-Ras wt, CaM and centrin1) and a destination vector, pDest-305 vector was performed to obtain the recombinant plasmids. In a single-site LR recombination reaction, CaM or centrin1 entry clones were combined with the destination vector, pDest-527 to produce bacterial expression plasmids encoding N-terminally His6-tagged CaM and centrin1. The positive clones were selected using ampicillin in
E. coli DH10B. The pmCherry-CaM, pEGFP-centrin1 plasmids and plasmids encoding N-terminal Rluc8 or GFP2-tagged K-RasG12V and H-RasG12V were previously described [
9,
26]. siRNA for
CALM1 (Hs_CALM1_6, SI02224222), and
FNTA (Hs_FNTA_6, SI02661995) were from Qiagen. The siRNA for
CETN1 (ON-TARGETplus SMARTpool siRNA, L-011831-00-0005) and negative control siRNA (ON-TARGETplus Non-targeting pool, D-001810-10-05) were from Dharmacon. Mevastatin (J61357, Alfa Aeser), calmidazolium chloride (sc-201494, Santa Cruz), and ophiobolin A (sc-202266, Santa Cruz) were commercially acquired from the sources given in parenthesis. Calmirasone1 was synthesized as previously described by us [
9].
Protein Sequence Analyses
The protein sequences encoded by
CALM1-3 and
CETN1-3 genes were collected from uniport database (
http://unirprot.org/) and multiple sequence alignment was performed using Clustal Omega (
https://www.ebi.ac.uk/Tools/msa/clustalo/). For paralog number analysis, the protein coding genes of calmodulin and centrin were searched for each species in the NCBI protein database (
https://www.ncbi.nlm.nih.gov/). The
CALM1 or
CETN1 genes were given as search query and orthologs were identified from the annotation pipeline. A process flow was then generated using RefSeq to identify a set of comparable proteins including orthologs and similar proteins. Note that only protein encoding genes were considered and pseudogenes were discarded.
Protein Purification
The His6-tagged human CaM and centrin1 proteins were purified as described previously [
9]. Briefly, the pDest527-His6-CaM or pDest527-His6-centrin1 plasmid transformed
E.coli BL21 (DE3) cells were grown in LB medium supplemented with 100 μg/ml of ampicillin. At 0.4 – 0.6 OD, the culture was induced with 0.5 mM IPTG followed by overnight incubation at 25 °C with shaking. The culture was centrifuged and the obtained cell pellet was suspended in a lysis buffer composed of 20 mM HEPES, pH 7.6, 150 mM NaCl, 5 mM MgCl
2, 0.5 mg/ml lysozyme and 700 units DNase I. 20 ml of lysis buffer was used for cell pellet from 1 l of cell culture. The cells were lysed by sonication and the His-tagged proteins were purified using HisTrapTM HP Prepacked Columns (GE Healthcare) using the chromatography system ÄKTAprime plus (GE Healthcare). The columns were equilibrated with a buffer composed of 50 mM Tris HCl, pH 7.5, 150 mM NaCl, and 35 mM imidazole, and the His-tagged proteins were eluted in an elution buffer containing 250 mM of imidazole. The eluted fractions were dialyzed for 16 h at 4 °C in a buffer composed of 50 mM Tris HCl, pH 7.5, 150 mM NaCl, and 2 mM CaCl
2. Protein concentration was measured by absorbance using a NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific).
Fluorescence Polarisation Binding Assay
Fluorescence polarisation assays were performed as established previously by us [
9,
26]. The fluorescein labelled PMCA- and CaMKII-peptides were custom synthesized by Genscript (USA) and Pepmic (China), respectively. The PMCA peptide was derived from 1086-LRRGQ-ILWFR-GLNRI-QTQIK-1105 of human PMCA and fluorescein was attached to the C-terminal native Lys. The CaMKII peptide sequence was derived from 294-NARRK-LKGAI-LTTML-ATRN-312 of human CaMKII and fluorescein was attached to a non-native cysteine added to the N-terminus. The N-terminal His6-tagged CaM or centrin1 proteins were 2-fold diluted in a buffer composed of 20 mM Tris Cl pH 7.5, 50 mM NaCl, 1 mM CaCl
2 and 0.005% v/v Tween 20 in a black low volume, round bottom 384-well plate (cat. no. 4514, Corning). Then 10 nM of fluorescein-labelled peptide was added to the protein dilution series. The reaction mix was incubated for 20 min at RT before anisotropy measurements.
The Sfi1 peptide was derived from 670-REVAA-RESQH-NRQLL-RGALR-RWK-692 of human Sfi and the fluorescein was attached to the native C-terminal Lys. Sfi1 peptide titration was performed in a buffer composed of 10 mM HEPES pH 7.4, 100 mM CaCl2 and 0.005 % v/v Tween 20. For binding, to a 2-fold dilution series of centrin1, 100 nM of Sfi1 peptide was added and the reaction mix was incubated for 45 min at RT before anisotropy measurements. For measuring the IC50 of inhibitors to centrin1, to the 3-fold dilution series of inhibitors in the assay buffer, a complex of 100 nM fluorescein labelled Sfi1 peptide and 250 nM His-centrin1 was added in 20 µl volume in a 384-well plate. The fluorescence anisotropy was measured after overnight incubation at RT.
The fluorescence anisotropy was measured on a Clariostar (BMG Labtech) plate reader using the fluorescence intensity signal recorded from vertical (I
v)- and horizontal (I
h)- polarized light using a fluorescence polarisation module (λ
excitation 482 ± 8 nm and λ
emission 530 ± 20 nm). Fluorescence anisotropy was calculated from the measured fluorescence intensities, according to,
where r is the fluorescence anisotropy value, I
v and I
h are the fluorescence emission intensities detected with vertical and horizontal polarization respectively. The instrument specific correction factor G(λ) was set to 1, and not determined further. A quadratic equation as described [
54,
55] by others was defined in Prism (GraphPad) and was used to determine the K
D value of the fluorescein tagged peptides to target protein.
Here, Af is the anisotropy value of the free fluorescent probe, Ab is the anisotropy value of the fluorescent probe /protein complex, Lt is the total concentration of the fluorescent probe, KD is the equilibrium dissociation constant, x is total concentration of protein and y is measured anisotropy value. KD is measured in the same unit of x. Note that variations in the active fraction of the home-made proteins and different methods used to determine the protein concentrations the obtained KD values can vary from those reported.
The IC
50 value of inhibitors was determined by plotting the log concentration of inhibitor against fluorescence anisotropy values and fitting the data to log inhibitor
vs. response – variable slope (four parameters) equation in Prism (GraphPad). The IC
50 of the inhibitor was converted into K
d as described earlier using the equation [
56],
where [I]
50 is the concentration of free inhibitor at 50 % displacement, given as
, where [EI]
50 is the concentration of centrin1:inhibitor complex in case of 50 % displacement, [P]
50 is concentration of free probe, F-Sfi1 at 50 % displacement, [E]
0 is concentration of free centrin1 at 0 % displacement, K
D,probe is the dissociation constant of the complex of Sfi1 and centrin1.
Co-Immunoprecipitation Experiments
About 800,000 HEK293-ebna cells were seeded in 60 mm dishes and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % v/v Foetal Bovine Serum (FBS), 2 mM L-glutamine (cat. no. 25030-024, Gibco, Thermo Fisher Scientific) and 1 % v/v penicillin/ streptomycin (cat. no. 15140122, Gibco, Thermo Fisher Scientific) overnight. The next day cells were transiently transfected with 4 µg plasmids encoding the indicated combinations of constructs using jetPRIME (cat. no. 114-75, Polyplus) according to the manufacturer’s instructions. At 48 h post-transfection, the cells were lysed using 200 μl of Lysis buffer (10 mM Tris Cl pH 7.5, 150 mM NaCl, 2 mM CaCl2, 0.2 % v/v NP40) supplemented with protease inhibitor cocktail (cat. no. A32955, Pierce, Thermo Fisher Scientific). After 30 min incubation on ice, the lysate was cleared by centrifugation for 10 min at 4 °C and 17,000 g. The cleared lysate was transferred to a clean tube and 15 µl sample was withdrawn (as “Input” for Western blot analysis). The lysate was diluted with 300 μl of dilution buffer (10 mM Tris Cl pH 7.5, 150 mM NaCl, 2mM CaCl2) supplemented with protease inhibitor cocktail. Then 25 µl of GFP-trap Beads Slurry (ChromoTek GFP-Trap Agarose, cat. no. gta) were added to the diluted lysate and rotated end-over-end for 1 h at 4 ⁰C. Then the beads were washed 3 times with Wash buffer (10 mM Tris Cl pH 7.5, 150 mM NaCl, 2 mM CaCl2, 0.02 % v/v NP40). Bound proteins were eluted by the addition of 2 × Laemlli buffer and boiling for 10 min at 95 ⁰C. The eluted proteins were subsequently analyzed by SDS-PAGE on 10 % acrylamide gels. Proteins were transferred onto a nitrocellulose membrane 0.2 µm (Bio-Rad) using the Trans-Blot Turbo Transfer system (Bio-Rad) and probed with a primary antibody. The primary antibodies employed were anti-GFP (SAB4301138, Sigma-Aldrich, 1:5000), anti-Renilla Luciferase (ab187338, Abcam, 1:3000), anti β-actin (A5441, Sigma- Aldrich, 1:5000). Anti-rabbit IRDye 680RD or anti-mouse IRDye 800CW secondary antibodies (LI-COR) were used to visualize the proteins on an Odyssey CLx system (LI-COR). The relative expression level of proteins was densitometrically quantified from images of membranes analysed using Image Studio software (LI-COR). For the quantitative analysis of the pull-down proteins, the signal of the Rluc8-tagged prey proteins was normalized with the signal from the GFP-tagged bait protein. Next, the signal intensity of the GFP2-K-RasG12V + Rluc8-CaM transfected sample was used to normalize the other samples.
BRET Donor Saturation Titration Assays
The detailed method of our BRET assay can be found in [
9,
57]. Briefly, ~200,000 HEK293-ebna cells were seeded per well of a 12-well plate (cat. No. 665180, Greiner Bio-One) and grown in 1 ml of complete DMEM. The next day ~ 1 µg of BRET sensor plasmids was transfected using 2.5 µl of jetPRIME. For titration curves, the concentration of donor plasmid was kept constant at 25 ng and the concentration of acceptor plasmid was varied from 25 ng to 1000 ng. 24 h after transfection, the cells were treated with inhibitors or vehicle control (DMSO at 0.2 % v/v). The next day, cells were collected in PBS and re-plated in white flat bottom 96-well plates (cat. no. 236108, Nunc, Thermo Fisher Scientific). Then the BRET readings were taken on a Clariostar plate reader (BMG Labtech). First the fluorescence intensity (λ
excitation 405 ± 10 nm and λ
emission 515 ± 10 nm) of GFP2 was measured as it is directly proportional to the acceptor concentration (RFU). Next the BRET readings were taken in well-mode by adding coelenterazine 400a (cat. no. C-320, GoldBio) to a final concentration of 10 μM and the luminescence emission intensities were simultaneously recorded at 410 ± 40 nm and at 515 ± 15 nm corresponding to the signal of donor (RLU) and the BRET signal, respectively. The raw BRET ratio was calculated as the ratio of BRET signal /RLU. The BRET ratio was obtained by subtracting the raw BRET ratio from the background raw BRET ratio measured from cells expressing only the donor. The relative expression is calculated as the ratio of RFU /RLU and denoted as [Acceptor] /[Donor]. The BRET ratio vs [Acceptor] /[Donor] ratio data from typically 3 biological repeats were plotted and the data were fitted by a hyperbolic equation in Prism. The BRETtop value represents the top asymptote of the BRET ratio reached within the defined [Acceptor] /[Donor] ratio. The one phase association equation of Prism 9 (GraphPad) was used to predict the top asymptote Ymax-value, which was taken as the BRETtop. Statistical analysis between the BRETtop values was performed using the Extra sum-of-squares F test.
Dose Response Analysis of Inhibitors and siRNA Knockdown in BRET Assays
For dose response analysis of inhibitors, on day one, ~200,000 HEK293-ebna cells were seeded per well of a 12-well plate (cat. No. 665180, Greiner Bio-One) and grown in complete DMEM. On day two ~ 1 µg of BRET sensor plasmids were transfected at an indicated donor /acceptor plasmid ratio using jetPRIME as mentioned in the corresponding figure legends. On day three, the medium was exchanged with fresh medium containing various doses of inhibitors. After 24 h incubation, on day four, the cells were collected in PBS and the BRET assay was performed. The log inhibitor vs BRET ratio was plotted, and the data were fitted by a log (inhibitor) vs. response variable slope (four parameters) equation of Prism and the IC50 values were calculated.
For studying the effect of siRNA-mediated knockdown, on day one the HEK293-ebna cells were seeded in 12-well plates in 1 ml of growth medium. On day two, cells were transfected with 100 nM of siRNA per well using 3.5 µl Lipofectamine RNAiMAX (cat. no. 13778, Thermo Fisher Scientific) and Opti-MEM medium (cat. no. 31985062, Gibco, Thermo Fisher Scientific) as vehicle. On day three, the medium was exchanged and the cells were transfected with ~ 1 µg of BRET sensor plasmids using 3 µl jetPRIME reagent and expressed for 48 h. The transfected donor /acceptor plasmid ratio is indicated in corresponding figure legends. On day five, the BRET assay was performed as indicated above.
3D Spheroid Assay
MDA-MB-231 and MCF-7 cells were seeded in 12-well plates (cat. No. 665180, Greiner Bio-One) and transfected with either 100 nM negative control siRNA or siRNA targeting CALM1 or CETN1 using Lipofectamine RNAiMAX. The next day, cells were collected by trypsinization and re-plated into low attachment, suspension cell culture 96-well plates (cat. no. 655185, Cellstar, Greiner Bio-One) for 3D spheroid suspension culture. About 1,000 MDA-MB-231 or 2,500 MCF-7 cell were seeded per well of the 96-well plate in 50 µl of RPMI medium (cat. no. 52400-025, Gibco, Thermo Fisher Scientific) or DMEM, respectively, containing 0.5% v/v MethoCult (cat. no. SFH4636, Stemcell technologies), 1x B27 (cat. no. 17504044, Gibco, Thermo Fisher Scientific), 25 ng/ml EGF (cat. no. E9644, Sigma-Aldrich), and 25 ng/ml FGF (cat. no. RP-8628, Thermo Fisher Scientific). Cells were incubated in a cell culture incubator for 6 days and fresh growth medium was supplemented on the third day. After six days of incubation, a 10 % final volume of the alamarBlue reagent (cat. no. DAL1025, Invitrogen, Thermo Fisher Scientific) was added to each well and incubated for 4 h at 37 °C. Then the fluorescence intensity (λexcitation 560 ± 5 nm and λemission 590 ± 5 nm) was measured using the Clariostar plate reader. The obtained fluorescence intensity data were normalized to negative control siRNA corresponding to 100 % sphere formation.
Confocal Microscopy
HeLa cells were seeded on glass coverslips 1.5H (cat. no. LH22.1, Carl Roth) in 6-well plates (cat. no. 657160, Cellstar, Greiner Bio-One) and grown in complete DMEM for 24 h. The next day the cells were transiently co-transfected with pmCherry-CaM and pmGFP-K-RasG12V using jetPRIME. 48 h after transfection, cells were fixed using 4% v/v formaldehyde (cat. no. 43368, Alfa Aesar) in PBS for 10 min at room temperature. The fixation solution was then replaced with PBS-Tween 0.05% v/v (cat. no. 9127.1, CarlRoth). After permeabilization in PBS-Triton X100 0.5% v/v (cat. no. T8787, Merck) for 10 minutes and blocking for 30 min in 2 % v/v solution of BSA (A6588, Applichem) in PBS, the cells were incubated for 1 h at room temperature with primary antibody against centrin1 (rabbit polyclonal, cat no.12794-1-AP, Proteintech). After washing with PBS-Tween 0.05% v/v, the secondary antibody AlexaFluor 667 goat anti-rabbit (cat no. A21244, Life Technologies) was applied for 1 h at room temperature. DNA was stained with a 1 mg/ml solution of DAPI (cat. no. D1306, Thermo Fisher Scientific) in PBS for 10 min. The coverslips were mounted onto glass slides using Vectashield (cat. no. H-1000, Vector Laboratories). Images were captured on a spinning disk confocal microscope (Andor, Oxford Instruments), fitted with a Zyla 5.5 sCMOS camera (Andor, Oxford Instruments), using a plan APO 60×/ 1.40 Ph3 DM oil immersion objective (Nikon) and NIS-Elements Imaging Software (Nikon).
Data and Statistical analysis :Prism 9 (GraphPad) was used for the preparation of plots, data and statistical analysis. The number of independent biological repeats (n) and type of statistical analysis used are indicated in the corresponding figure legends. A p-value < 0.05 is considered statistically significant, and the statistical significance levels are annotated as follows: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001, or ns = not significant.
Figure 1.
Despite its high similarity to CaM, centrin1 does not recognize CaM-target peptides. (
A) Multiple sequence alignment of human CaM (
CALM1-3) and centrin (
CETN1-3) protein paralogs, designated by the encoding gene names. The Ca
2+-binding residues are highlighted in cyan. Lysines 75, 77 and 148 of CaM that become covalently modified by CaM inhibitor ophiobolin A, are highlighted in yellow. The same highlight was used for lysine residues at similar positions in centrin1 and centrin2, while no such lysine residues could be identified for centrin3. Note that the CaM protein numbering is started from Ala, as the N-terminal, native Met is removed in most organisms [
52]. (
B) Structures of human CaM (PDB ID 1CLL) and human centrin1 (PDB ID 2GGM). Calcium ions are marked as green spheres. (
C) Analysis of the number of paralog coding genes of
CALM1-3 and
CETN1-3 in different species. Data were curated from the NCBI protein database. (
D-F) Binding of 10 nM fluorescein labelled F-CaMKII and F-PMCA (D, E) or 100 nM F-Sfi1 (F) peptides to His-tagged human CaM or centrin1 was detected using fluorescence anisotropy measurements.
Figure 1.
Despite its high similarity to CaM, centrin1 does not recognize CaM-target peptides. (
A) Multiple sequence alignment of human CaM (
CALM1-3) and centrin (
CETN1-3) protein paralogs, designated by the encoding gene names. The Ca
2+-binding residues are highlighted in cyan. Lysines 75, 77 and 148 of CaM that become covalently modified by CaM inhibitor ophiobolin A, are highlighted in yellow. The same highlight was used for lysine residues at similar positions in centrin1 and centrin2, while no such lysine residues could be identified for centrin3. Note that the CaM protein numbering is started from Ala, as the N-terminal, native Met is removed in most organisms [
52]. (
B) Structures of human CaM (PDB ID 1CLL) and human centrin1 (PDB ID 2GGM). Calcium ions are marked as green spheres. (
C) Analysis of the number of paralog coding genes of
CALM1-3 and
CETN1-3 in different species. Data were curated from the NCBI protein database. (
D-F) Binding of 10 nM fluorescein labelled F-CaMKII and F-PMCA (D, E) or 100 nM F-Sfi1 (F) peptides to His-tagged human CaM or centrin1 was detected using fluorescence anisotropy measurements.
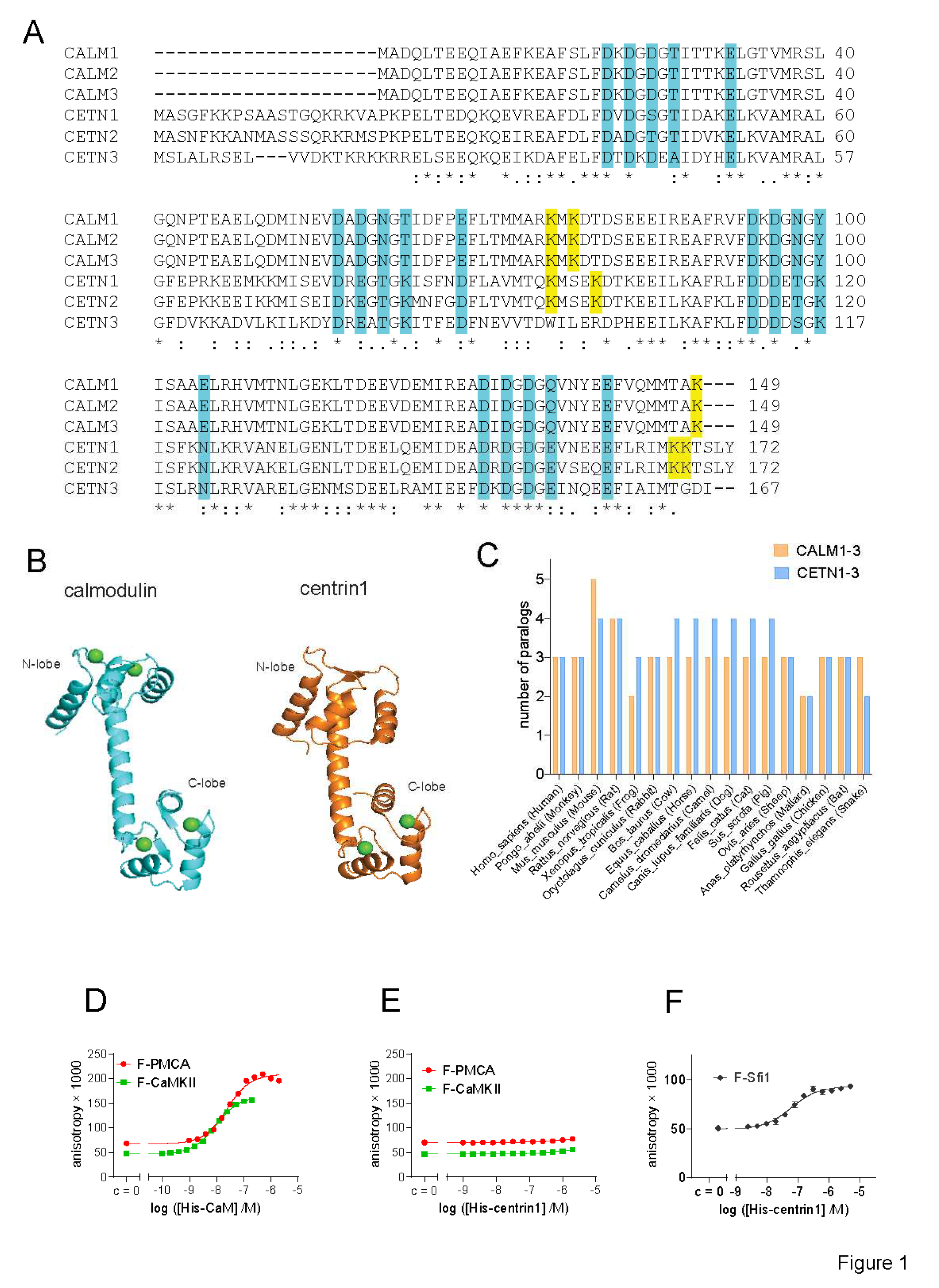
Figure 2.
The interaction of CaM or centrin is increased with active K-Ras. (A) Co-immunoprecipitation of Rluc8-CaM or Rluc8-centrin1 with GFP2-K-RasG12V or GFP2-K-Ras wt. Pull-down was performed using lysates of HEK293-ebna cells transfected with combinations of GFP2-K-RasG12V/ Rluc8-CaM, GFP2-K-RasG12V/ Rluc8-centrin1, GFP2-K-Ras/ Rluc8-CaM, GFP2-K-Ras/ Rluc8-centrin1 and GFP2/ Rluc8 and expressed for 48 h. The GFP2-tagged protein was bound using GFP-beads and the samples were analysed using anti-Rluc8 and anti-GFP antibodies. (B) Immunoprecipitated Rluc8-tagged protein signals were normalized to GFP-tagged protein signals. The signal intensity of the GFP2-K-RasG12V/Rluc8-CaM transfected sample was set to 1 in each experiment and was used to normalize the other samples. The plot shows mean ± SEM and the statistical analysis was performed using one-way ANOVA test. (C, D) Interaction of Rluc8-K-Ras wt, Rluc8-K-RasG12V, and Rluc8-K-RasG12V-D38A with GFP2-CaM (C) or GFP2-centrin1 (D). All samples were treated with 0.2 % v/v DMSO for 24 h, n = 3. Statistics of BRETtop values was analysed using the F-test. BRET donor protein is boxed purple, acceptor protein is boxed green.
Figure 2.
The interaction of CaM or centrin is increased with active K-Ras. (A) Co-immunoprecipitation of Rluc8-CaM or Rluc8-centrin1 with GFP2-K-RasG12V or GFP2-K-Ras wt. Pull-down was performed using lysates of HEK293-ebna cells transfected with combinations of GFP2-K-RasG12V/ Rluc8-CaM, GFP2-K-RasG12V/ Rluc8-centrin1, GFP2-K-Ras/ Rluc8-CaM, GFP2-K-Ras/ Rluc8-centrin1 and GFP2/ Rluc8 and expressed for 48 h. The GFP2-tagged protein was bound using GFP-beads and the samples were analysed using anti-Rluc8 and anti-GFP antibodies. (B) Immunoprecipitated Rluc8-tagged protein signals were normalized to GFP-tagged protein signals. The signal intensity of the GFP2-K-RasG12V/Rluc8-CaM transfected sample was set to 1 in each experiment and was used to normalize the other samples. The plot shows mean ± SEM and the statistical analysis was performed using one-way ANOVA test. (C, D) Interaction of Rluc8-K-Ras wt, Rluc8-K-RasG12V, and Rluc8-K-RasG12V-D38A with GFP2-CaM (C) or GFP2-centrin1 (D). All samples were treated with 0.2 % v/v DMSO for 24 h, n = 3. Statistics of BRETtop values was analysed using the F-test. BRET donor protein is boxed purple, acceptor protein is boxed green.
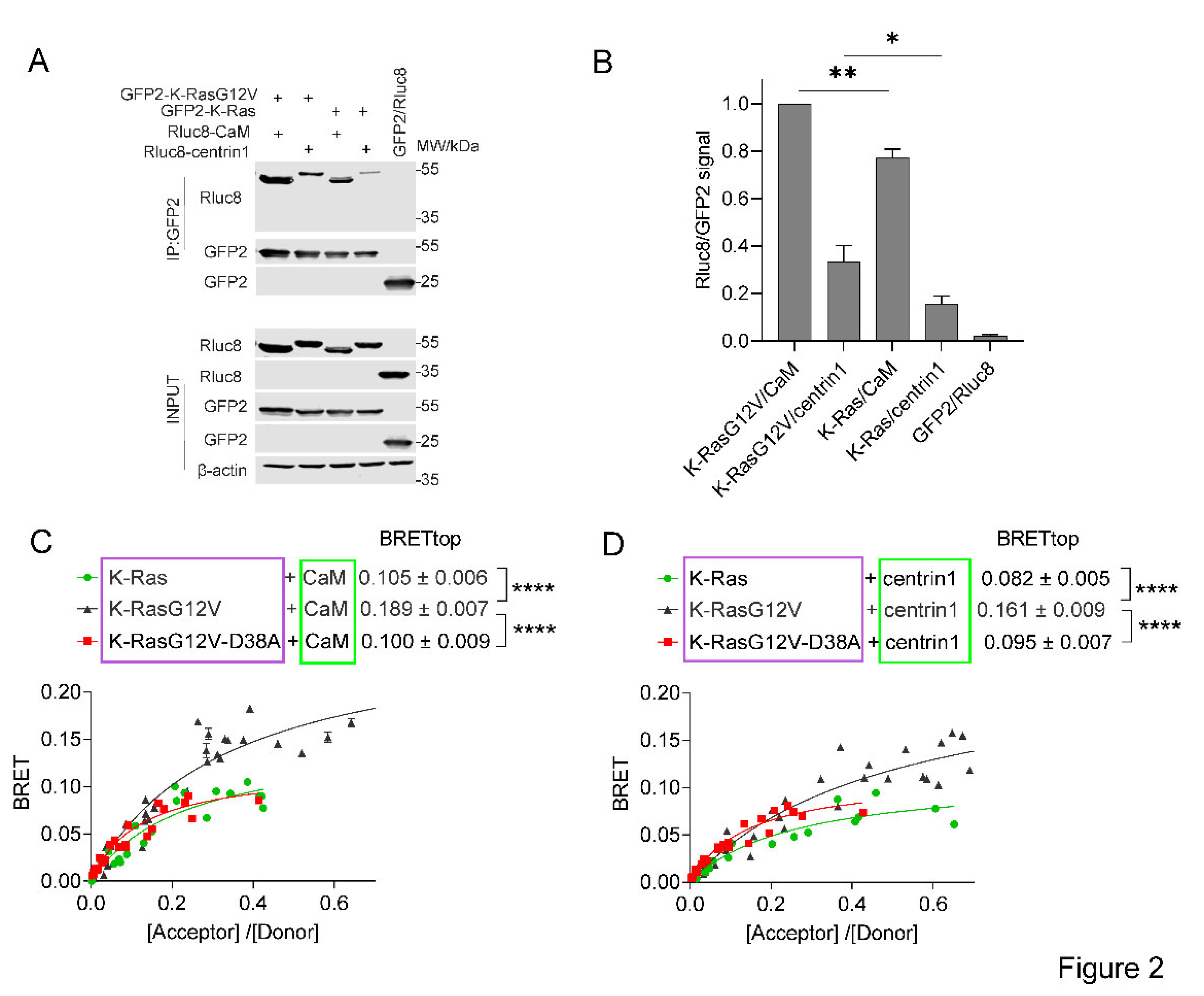
Figure 3.
The interaction of K-Ras with centrin1 is modulated by direct binding of CaM-inhibitors to centrin1. (A) HEK293-ebna cells were transfected with Rluc8-K-RasG12V/ GFP2-centrin1 BRET sensor plasmids for 24 h followed by treatment with ophiobolin A (2.5 µM), calmidazolium (10 µM), calmirsone1 (20 µM) or equal volume of DMSO (0.2% v/v) for another 24 h, n = 3. (B) HEK293-ebna cells were transfected with BRET sensor plasmids Rluc8-K-RasG12V/ GFP2-centrin1 at a ratio of 1/19, respectively, for 24 h followed by a 24 h treatment with 2-fold dilution series of calmidazolium or calmirasone1 ranging from 80 µM to 0.1 µM. Data represent mean ± SEM, n = 2. BRET donor protein is boxed purple, acceptor protein is boxed green. (C) Displacement of fluorescent F-Sfi1 from centrin1 by CaM-inhibitors. The inhibitors were 3-fold diluted in assay buffer, followed by addition of the complex of 100 nM F-Sfi1 and 250 nM His-centrin1. The fluorescence anisotropy was measured after overnight incubation at RT.
Figure 3.
The interaction of K-Ras with centrin1 is modulated by direct binding of CaM-inhibitors to centrin1. (A) HEK293-ebna cells were transfected with Rluc8-K-RasG12V/ GFP2-centrin1 BRET sensor plasmids for 24 h followed by treatment with ophiobolin A (2.5 µM), calmidazolium (10 µM), calmirsone1 (20 µM) or equal volume of DMSO (0.2% v/v) for another 24 h, n = 3. (B) HEK293-ebna cells were transfected with BRET sensor plasmids Rluc8-K-RasG12V/ GFP2-centrin1 at a ratio of 1/19, respectively, for 24 h followed by a 24 h treatment with 2-fold dilution series of calmidazolium or calmirasone1 ranging from 80 µM to 0.1 µM. Data represent mean ± SEM, n = 2. BRET donor protein is boxed purple, acceptor protein is boxed green. (C) Displacement of fluorescent F-Sfi1 from centrin1 by CaM-inhibitors. The inhibitors were 3-fold diluted in assay buffer, followed by addition of the complex of 100 nM F-Sfi1 and 250 nM His-centrin1. The fluorescence anisotropy was measured after overnight incubation at RT.

Figure 4.
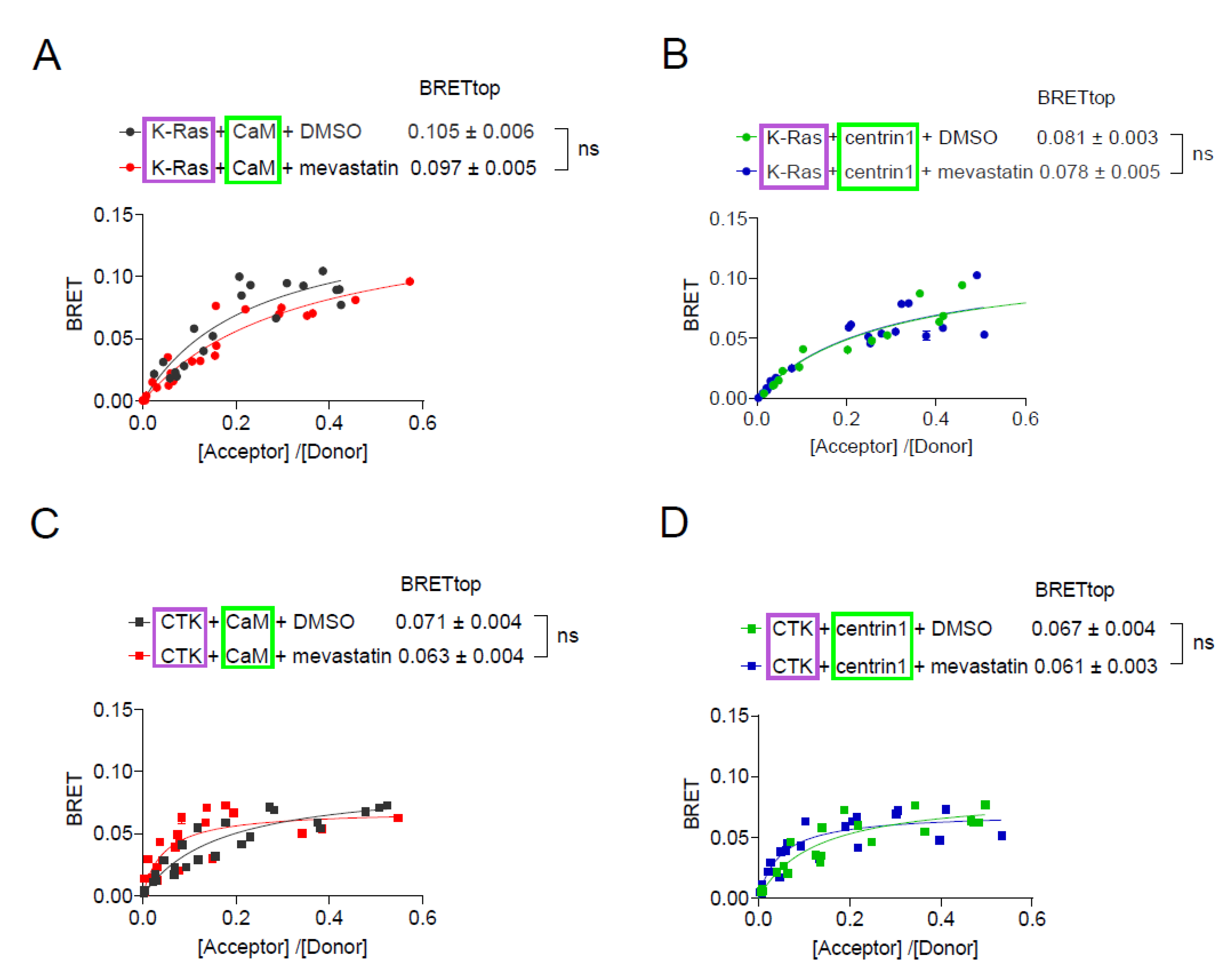
Inhibition of prenylation by mevastatin does not disrupt BRET between K-Ras and CaM or centrin1. (A-D) BRET-sensors Rluc8-K-Ras/ GFP2-CaM (A), Rluc8-K-Ras/ GFP2-centrin1 (B), Rluc8-CTK/ GFP2-CaM (C) and Rluc8-CTK/ GFP2-centrin1 (D) were transfected into HEK293-ebna cells, and cells were treated with 10 µM mevastatin or the vehicle control, DMSO 0.2% v/v for 24 h, n = 3. Statistics of BRETtop values was analysed using the F-test. BRET donor protein is boxed purple, acceptor protein is boxed green.
Figure 4.
Inhibition of prenylation by mevastatin does not disrupt BRET between K-Ras and CaM or centrin1. (A-D) BRET-sensors Rluc8-K-Ras/ GFP2-CaM (A), Rluc8-K-Ras/ GFP2-centrin1 (B), Rluc8-CTK/ GFP2-CaM (C) and Rluc8-CTK/ GFP2-centrin1 (D) were transfected into HEK293-ebna cells, and cells were treated with 10 µM mevastatin or the vehicle control, DMSO 0.2% v/v for 24 h, n = 3. Statistics of BRETtop values was analysed using the F-test. BRET donor protein is boxed purple, acceptor protein is boxed green.
Figure 5.
K-Ras membrane anchorage is selectively affected by CaM-, but less by centrin1-depletion. (A, B) Rluc8-K-Ras was transfected with GFP2-CaM (A) or GFP2-centrin1 (B) plasmids at a donor/acceptor plasmid ratio of 1/5 into HEK293-ebna cells. BRET donor protein is boxed purple, acceptor protein is boxed green. Data represent mean ± SEM, n = 2 to 4. Statistical significance between negative control siRNA and sample siRNA was analysed using Mann-Whitney test. (C, D) HEK293-ebna cells were transfected with 100 nM of negative control siRNA or siFNTA or siCETN1 for 48 h and cell lysates were immunoblotted as indicated. (E, F) HEK293-ebna cells were transfected with 100 nM siRNA for 24 h, followed by BRET sensor transfection. Rluc8-/ GFP2-tagged K-RasG12V (E) or H-RasG12V (F) nanoclustering-BRET sensor plasmids were transfected at a donor/acceptor plasmid ratio of 1/15. BRET donor protein is boxed purple, acceptor protein is boxed green. Data represent mean ± SEM, n = 4. Statistical significance between negative control siRNA and sample siRNA was analysed using Mann-Whitney test.
Figure 5.
K-Ras membrane anchorage is selectively affected by CaM-, but less by centrin1-depletion. (A, B) Rluc8-K-Ras was transfected with GFP2-CaM (A) or GFP2-centrin1 (B) plasmids at a donor/acceptor plasmid ratio of 1/5 into HEK293-ebna cells. BRET donor protein is boxed purple, acceptor protein is boxed green. Data represent mean ± SEM, n = 2 to 4. Statistical significance between negative control siRNA and sample siRNA was analysed using Mann-Whitney test. (C, D) HEK293-ebna cells were transfected with 100 nM of negative control siRNA or siFNTA or siCETN1 for 48 h and cell lysates were immunoblotted as indicated. (E, F) HEK293-ebna cells were transfected with 100 nM siRNA for 24 h, followed by BRET sensor transfection. Rluc8-/ GFP2-tagged K-RasG12V (E) or H-RasG12V (F) nanoclustering-BRET sensor plasmids were transfected at a donor/acceptor plasmid ratio of 1/15. BRET donor protein is boxed purple, acceptor protein is boxed green. Data represent mean ± SEM, n = 4. Statistical significance between negative control siRNA and sample siRNA was analysed using Mann-Whitney test.
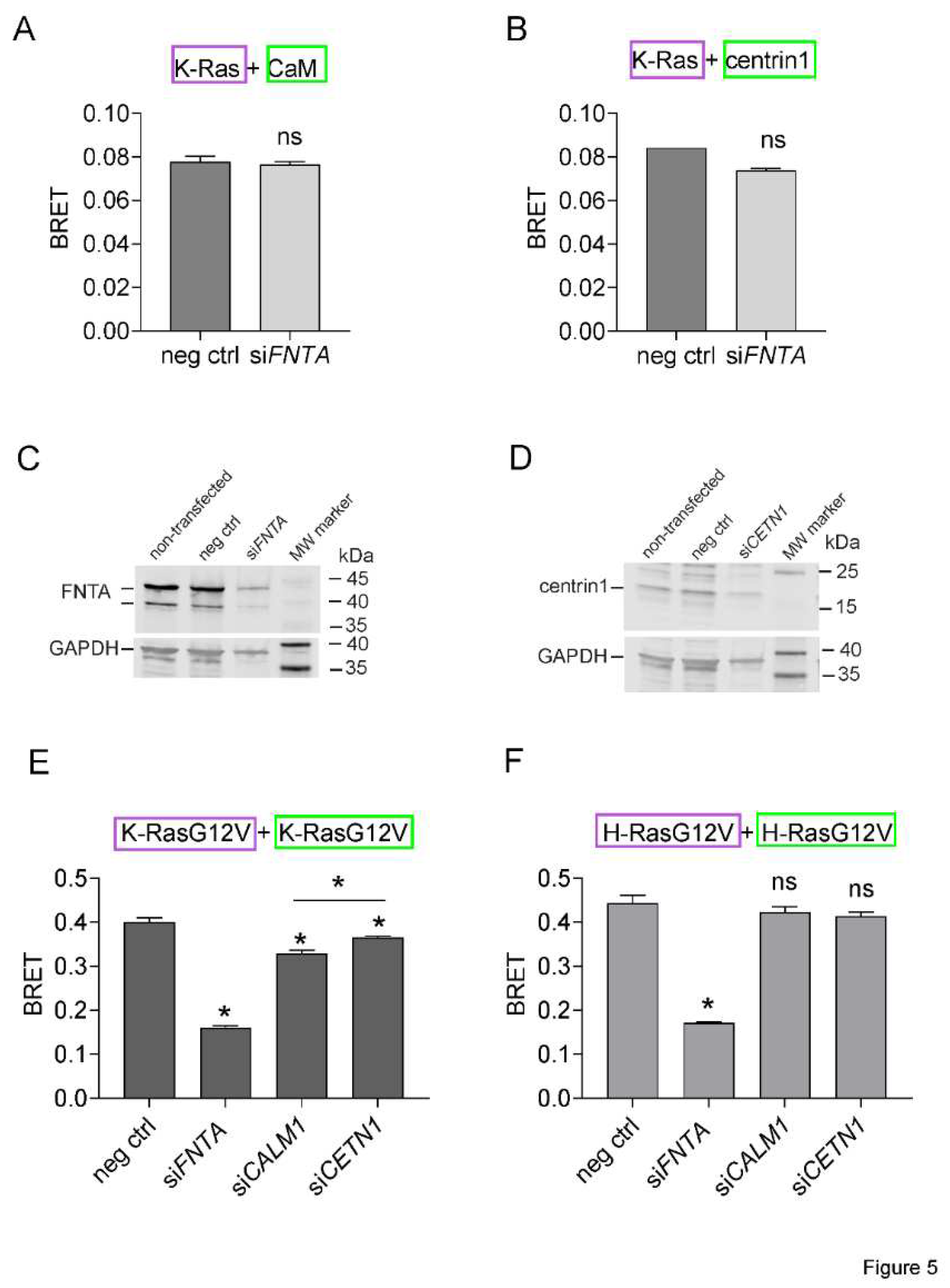
Figure 6.
CaM and centrin1 distribute to the centrosomes and their loss more potently affects spheroid formation of KRAS mutant MDA-MB-231 cells. (A, B) Effect of knockdown of CALM1 and CETN1 on spheroids derived from MDA-MB-231 (A) and MCF-7 (B) cells. The knockdown efficiency was compared to spheroids grown from negative control siRNA transfected cells. Data represent mean ± SEM of 4 biological repeats. Statistical analysis was performed using Mann-Whitney test. (C) Representative images of HeLa cells that were co-transfected with mGFP-K-RasG12V (green) and mCherry-CaM (red). Endogenous centrin1 was immunostained (purple) and DNA was stained using DAPI (blue). Cell-cycle stages are indicated on the left. Scale bar is 5 µm.
Figure 6.
CaM and centrin1 distribute to the centrosomes and their loss more potently affects spheroid formation of KRAS mutant MDA-MB-231 cells. (A, B) Effect of knockdown of CALM1 and CETN1 on spheroids derived from MDA-MB-231 (A) and MCF-7 (B) cells. The knockdown efficiency was compared to spheroids grown from negative control siRNA transfected cells. Data represent mean ± SEM of 4 biological repeats. Statistical analysis was performed using Mann-Whitney test. (C) Representative images of HeLa cells that were co-transfected with mGFP-K-RasG12V (green) and mCherry-CaM (red). Endogenous centrin1 was immunostained (purple) and DNA was stained using DAPI (blue). Cell-cycle stages are indicated on the left. Scale bar is 5 µm.
Table 1.
Comparison of Kd values of CaM-inhibitors with centrin1 and CaM determined by fluorescence anisotropy measurements. The competition assay derived Kd values of inhibitors to CaM were previously reported by us using F-PMCA peptide as probe.
Table 1.
Comparison of Kd values of CaM-inhibitors with centrin1 and CaM determined by fluorescence anisotropy measurements. The competition assay derived Kd values of inhibitors to CaM were previously reported by us using F-PMCA peptide as probe.
| inhibitor |
centrin1 |
CaM |
| Kd (mean ± SEM, n = 2) |
Kd (references) |
| calmidazolium |
1.6 ± 0.3 µM |
13.5 nM [26] |
| W-7 |
18.2 ± 0.3 µM |
1.47 µM[26] |
| ophiobolin A |
49 ± 9 µM |
3.5 µM [9] |
| calmirasone1 |
0.9 ± 0.2 µM |
0.87 µM [9] |