Submitted:
05 May 2023
Posted:
05 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Clinical trial
2.2. Photograph assessments
2.3. Calculations
3. Results
3.1. Incidence of new wounds
3.2. Outcomes of the new wounds
3.3. Comparison of new versus baseline wounds
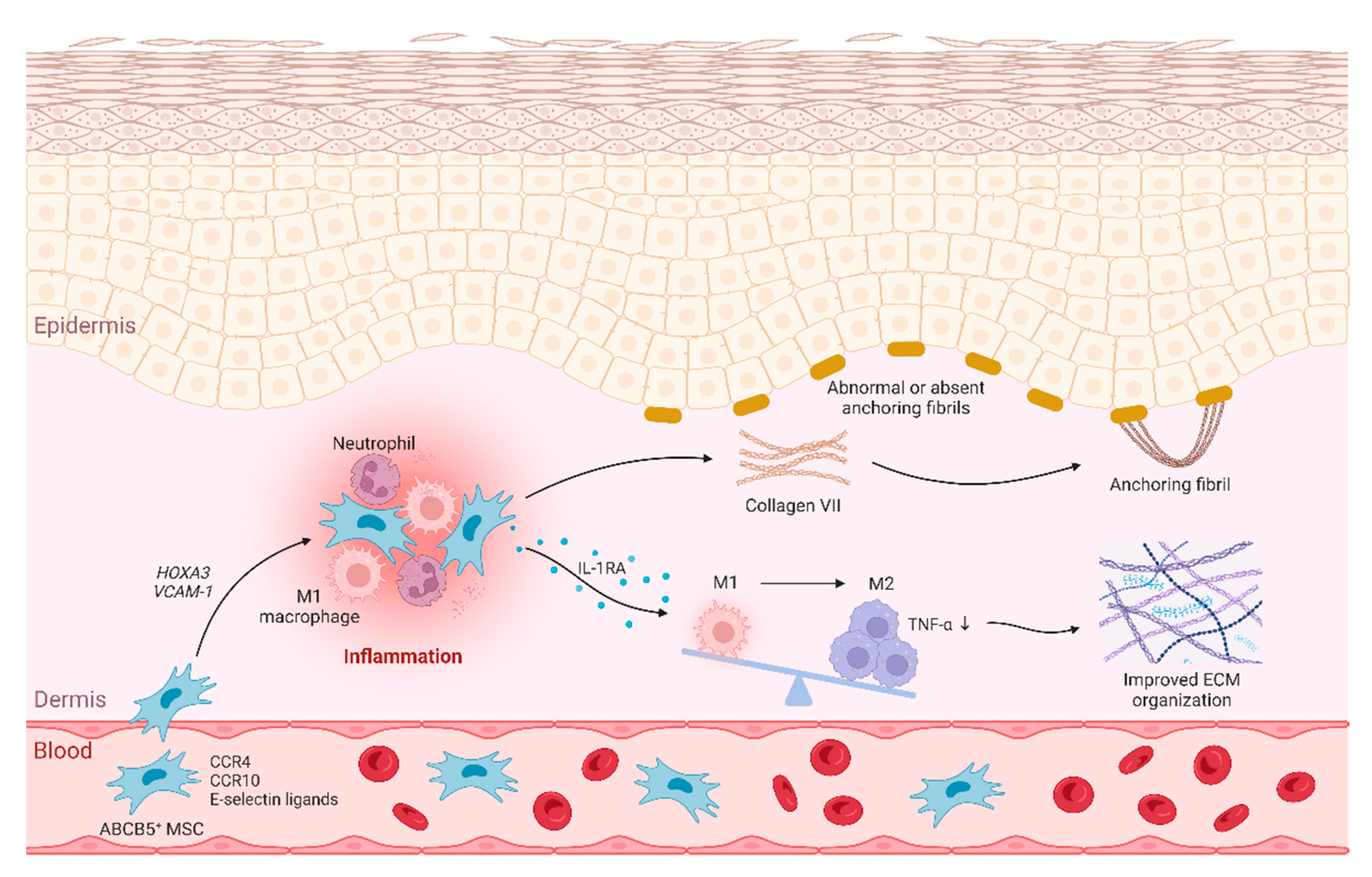
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, J.Y.; Marinkovich, M.P.; Lucas, E.; Gorell, E.; Chiou, A.; Lu, Y.; Gillon, J.; Patel, D.; Rudin, D. A systematic literature review of the disease burden in patients with recessive dystrophic epidermolysis bullosa. Orphanet J Rare Dis 2021, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, G.; Cao, Q.; Padron, Z.M.; South, A.P. Impaired Wound Healing, Fibrosis, and Cancer: The Paradigm of Recessive Dystrophic Epidermolysis Bullosa. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Nyström, A.; Bruckner-Tuderman, L.; Kiritsi, D. Dystrophic Epidermolysis Bullosa: Secondary Disease Mechanisms and Disease Modifiers. Front Genet 2021, 12, 737272. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.S.; Antoniou, M.N.; McGrath, J.A.; Lwin, S.M. The potential of gene therapy for recessive dystrophic epidermolysis bullosa. Br J Dermatol 2022, 186, 609–619. [Google Scholar] [CrossRef]
- Welponer, T.; Prodinger, C.; Pinon-Hofbauer, J.; Hintersteininger, A.; Breitenbach-Koller, H.; Bauer, J.W.; Laimer, M. Clinical Perspectives of Gene-Targeted Therapies for Epidermolysis Bullosa. Dermatol Ther (Heidelb) 2021, 11, 1175–1197. [Google Scholar] [CrossRef]
- Bruckner-Tuderman, L. Skin fragility: perspectives on evidence-based therapies. Acta Derm Venereol 2020, 100, adv00053. [Google Scholar] [CrossRef]
- Wally, V.; Reisenberger, M.; Kitzmüller, S.; Laimer, M. Small molecule drug development for rare genodermatoses - evaluation of the current status in epidermolysis bullosa. Orphanet J Rare Dis 2020, 15, 292. [Google Scholar] [CrossRef]
- Ebens, C.L. Deconstructing progressive inflammatory fibrosis in recessive dystrophic epidermolysis bullosa. EMBO Mol Med 2021, 13, e14864. [Google Scholar] [CrossRef]
- Rashidghamat, E.; Kadiyirire, T.; Ayis, S.; Petrof, G.; Liu, L.; Pullabhatla, V.; Ainali, C.; Guy, A.; Aristodemou, S.; McMillan, J.R.; et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol 2020, 83, 447–454. [Google Scholar] [CrossRef]
- Petrof, G.; Lwin, S.M.; Martinez-Queipo, M.; Abdul-Wahab, A.; Tso, S.; Mellerio, J.E.; Slaper-Cortenbach, I.; Boelens, J.J.; Tolar, J.; Veys, P.; et al. Potential of systemic allogeneic mesenchymal stromal cell therapy for children with recessive dystrophic epidermolysis bullosa. J Invest Dermatol 2015, 135, 2319–2321. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, S.J.; Kim, S.E.; Kim, K.; Cho, B.; Roh, K.; Kim, S.C. Intravenous allogeneic umbilical cord blood-derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients. JCI Insight 2021, 6, e143606. [Google Scholar] [CrossRef] [PubMed]
- Niebergall-Roth, E.; Frank, N.Y.; Ganss, C.; Frank, M.H.; Kluth, M.A. Skin-Derived ABCB5(+) Mesenchymal Stem Cells for High-Medical-Need Inflammatory Diseases: From Discovery to Entering Clinical Routine. Int J Mol Sci 2022, 24. [Google Scholar] [CrossRef] [PubMed]
- Kiritsi, D.; Dieter, K.; Niebergall-Roth, E.; Fluhr, S.; Daniele, C.; Esterlechner, J.; Sadeghi, S.; Ballikaya, S.; Erdinger, L.; Schauer, F.; et al. Clinical trial of ABCB5+ mesenchymal stem cells for recessive dystrophic epidermolysis bullosa. JCI Insight 2021, 6, e151922. [Google Scholar] [CrossRef] [PubMed]
- Dieter, K.; Niebergall-Roth, E.; Daniele, C.; Fluhr, S.; Frank, N.Y.; Ganss, C.; Kiritsi, D.; McGrath, J.A.; Tolar, J.; Frank, M.H.; et al. ABCB5+ mesenchymal stromal cells facilitate complete and durable wound closure in recessive dystrophic epidermolysis bullosa. Cytotherapy 2023, 25, in, in press. [Google Scholar] [CrossRef] [PubMed]
- Ballikaya, S.; Sadeghi, S.; Niebergall-Roth, E.; Nimtz, L.; Frindert, J.; Norrick, A.; Stemler, N.; Bauer, N.; Rosche, Y.; Kratzenberg, V.; et al. Process data of allogeneic ex vivo-expanded ABCB5+ mesenchymal stromal cells for human use: off-the-shelf GMP-manufactured donor-independent ATMP. Stem Cell Res Ther 2020, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Tappenbeck, N.; Schröder, H.M.; Niebergall-Roth, E.; Hassinger, F.; Dehio, U.; Dieter, K.; Kraft, K.; Kerstan, A.; Esterlechner, J.; Frank, N.Y.; et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy 2019, 21, 546–560. [Google Scholar] [CrossRef]
- Solis, D.C.; Teng, C.; Gorell, E.S.; Barriga, M.; Nazaroff, J.; Li, S.; Lu, Y.; Bruckner, A.; Marinkovich, M.P.; Tang, J.Y. Classification of 2 distinct wound types in recessive dystrophic epidermolysis bullosa: A retrospective and cohort natural history study. J Am Acad Dermatol 2021, 85, 1296–1298. [Google Scholar] [CrossRef]
- Bruckner, A.L.; Losow, M.; Wisk, J.; Patel, N.; Reha, A.; Lagast, H.; Gault, J.; Gershkowitz, J.; Kopelan, B.; Hund, M.; et al. The challenges of living with and managing epidermolysis bullosa: insights from patients and caregivers. Orphanet J Rare Dis 2020, 15, 1. [Google Scholar] [CrossRef]
- Schräder, N.H.B.; Korte, E.W.H.; Duipmans, J.C.; Stewart, R.E.; Bolling, M.C.; Wolff, A.P. Identifying Epidermolysis Bullosa Patient Needs and Perceived Treatment Benefits: An Explorative Study Using the Patient Benefit Index. J Clin Med 2021, 10, 5836. [Google Scholar] [CrossRef]
- Paller, A.S.; Pope, E.; Rudin, D.; Malyala, A.; Ramsdell, D.; Johnson, R.; Landy, H.; Murrell, D.F. A prospective short-term study to evaluate methodologies for the assessment of disease extent, impact, and wound evolution in patients with dystrophic epidermolysis bullosa. Orphanet J Rare Dis 2022, 17, 314. [Google Scholar] [CrossRef]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Riedl, J.; Pickett-Leonard, M.; Eide, C.; Kluth, M.A.; Ganss, C.; Frank, N.Y.; Frank, M.H.; Ebens, C.L.; Tolar, J. ABCB5+ dermal mesenchymal stromal cells with favorable skin homing and local immunomodulation for recessive dystrophic epidermolysis bullosa treatment. Stem Cells 2021, 39, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Schatton, T.; Yang, J.; Kleffel, S.; Uehara, M.; Barthel, S.R.; Schlapbach, C.; Zhan, Q.; Dudeney, S.; Mueller, H.; Lee, N.; et al. ABCB5 Identifies Immunoregulatory Dermal Cells. Cell Rep 2015, 12, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, J.S.; Rinnerthaler, M.; Trost, A.; Weber, M.; Klausegger, A.; Gruber, C.; Bruckner, D.; Reitsamer, H.A.; Bauer, J.W.; Breitenbach, M. Transcriptome and ultrastructural changes in dystrophic epidermolysis bullosa resemble skin aging. Aging 2015, 7, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, R.; Thriene, K.; Romero-Fernández, E.; Gretzmeier, C.; Kühl, T.; Maler, M.; Nauroy, P.; Kleiser, S.; Rühl-Muth, A.C.; Stumpe, M.; et al. Pro-inflammatory immunity supports fibrosis advancement in epidermolysis bullosa: intervention with Ang-(1-7). EMBO Mol Med 2021, 13, e14392. [Google Scholar] [CrossRef]
- Jiang, D.; Muschhammer, J.; Qi, Y.; Kugler, A.; de Vries, J.C.; Saffarzadeh, M.; Sindrilaru, A.; Vander Beken, S.; Wlaschek, M.; Kluth, M.A.; et al. Suppression of neutrophil-mediated tissue damage - a novel skill of mesenchymal stem cells. Stem Cells 2016, 34, 2393–2406. [Google Scholar] [CrossRef]
- Vander Beken, S.; de Vries, J.C.; Meier-Schiesser, B.; Meyer, P.; Jiang, D.; Sindrilaru, A.; Ferreira, F.F.; Hainzl, A.; Schatz, S.; Muschhammer, J.; et al. Newly defined ATP-binding cassette subfamily B member 5 positive dermal mesenchymal stem cells promote healing of chronic iron-overload wounds via secretion of interleukin-1 receptor antagonist. Stem Cells 2019, 37, 1057–1074. [Google Scholar] [CrossRef]
- Gubinelli, E.; Angelo, C.; Pacifico, V. A case of dystrophic epidermolysis bullosa improved with etanercept for concomitant psoriatic arthritis. Am J Clin Dermatol 2010, 11 Suppl 1, 53–54. [Google Scholar] [CrossRef]
- Maritsi, D.; Martinez, A.E.; Mellerio, J.E.; Eleftheriou, D.; Pilkington, C.A. An unusual case of epidermolysis bullosa complicated by persistent oligoarticular juvenile idiopathic arthritis; lessons to be learned. Pediatr Rheumatol Online J 2011, 9, 13. [Google Scholar] [CrossRef]
- Nyström, A.; Thriene, K.; Mittapalli, V.; Kern, J.S.; Kiritsi, D.; Dengjel, J.; Bruckner-Tuderman, L. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol Med 2015, 7, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Kerstan, A.; Niebergall-Roth, E.; Esterlechner, J.; Schröder, H.M.; Gasser, M.; Waaga-Gasser, A.M.; Goebeler, M.; Rak, K.; Schrüfer, P.; Endres, S.; et al. Ex vivo-expanded highly pure ABCB5(+) mesenchymal stromal cells as Good Manufacturing Practice-compliant autologous advanced therapy medicinal product for clinical use: process validation and first in-human data. Cytotherapy 2021, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kerstan, A.; Dieter, K.; Niebergall-Roth, E.; Klingele, S.; Jünger, M.; Hasslacher, C.; Daeschlein, G.; Stemler, L.; Meyer-Pannwitt, U.; Schubert, K.; et al. Translational development of ABCB5+ dermal mesenchymal stem cells for therapeutic induction of angiogenesis in non-healing diabetic foot ulcers. Stem Cell Res Ther 2022, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Uitto, J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin 2010, 28, 93–105. [Google Scholar] [CrossRef]
- Fritsch, A.; Loeckermann, S.; Kern, J.S.; Braun, A.; Bösl, M.R.; Bley, T.A.; Schumann, H.; von Elverfeldt, D.; Paul, D.; Erlacher, M.; et al. A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J Clin Invest 2008, 118, 1669–1679. [Google Scholar] [CrossRef]
- Kühl, T.; Mezger, M.; Hausser, I.; Handgretinger, R.; Bruckner-Tuderman, L.; Nyström, A. High Local Concentrations of Intradermal MSCs Restore Skin Integrity and Facilitate Wound Healing in Dystrophic Epidermolysis Bullosa. Mol Ther 2015, 23, 1368–1379. [Google Scholar] [CrossRef]
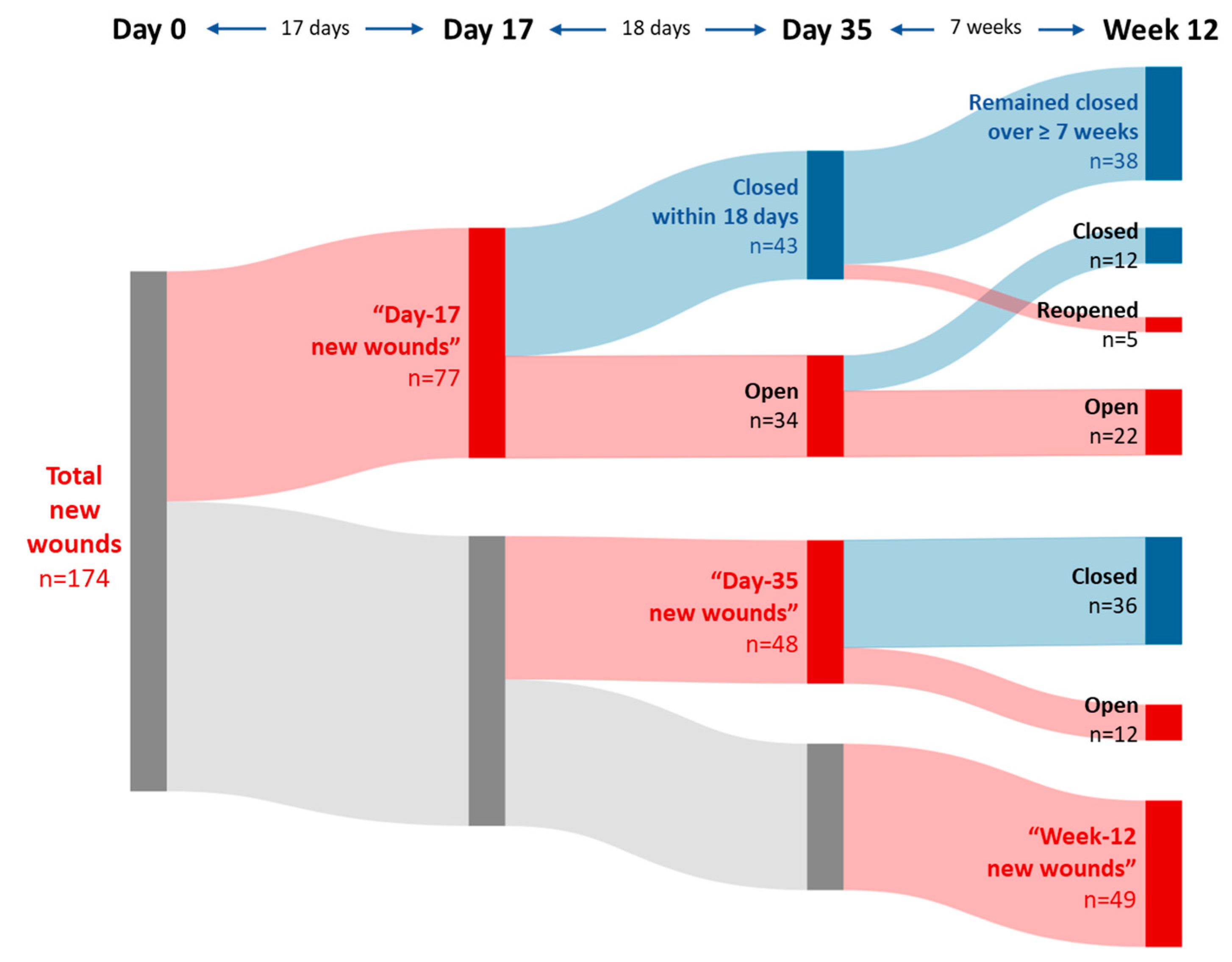
| Parameter | Day-17 new woundsN = 77 | Day-35 new woundsN = 48 | Baseline wounds 1N = 168 | |
|---|---|---|---|---|
| Healing ratio within: | 17 days | 45/168 (27 %) | ||
| 18 days | 43/77 (56 %) | |||
| 35 days | ||||
| 7 weeks | 36/48 (75 %) | |||
| 9.5 weeks | ||||
| 12 weeks | 109/168 (65 %) | |||
| Median time to first wound closure | 18 days 2 | 35 days | ||
| Proportion of durably (≥ 7 weeks) healed wounds 3 | 38/43 4 (88 %) | 69/93 5 (74 %) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





