1. Introduction
The presence of heavy metals in wastewater as a result of rapid industrialization is a common phenomenon these days which leads to the pollution of the environment. These heavy metals which are generally categorized as density greater than 5 g/cc are toxic and carcinogen, generated by electroplating, electrolytic deposition, conversion coating, anodizing coating, melting and etching industries, and petroleum refining [
1]. According to Environment Protection Act (EPA), heavy metals such as Pt, Pd, Ag, Cu, Cd, Pb, Ni, Co, Zn, and Cr are emerging contaminants, whose concentration is in the trace level in wastewater and are thus not completely removed by any conventional wastewater treatment processes [
2,
3,
4,
5,
6,
7]. Among the heavy metals, Co (II) is a priority pollutant, due to its high toxicity at elevated concentration [
8], responsible for causing hypertension, nausea, pulmonary disease, reproductory problems, hyper glycaemia, and mutations in living cells.
Low iron laterite clay based geo polymer has been tried for Co
2+ removal [
9]. The adsorption performance of composite Bis-calix towards Co
2+ remediation was benchmarked at 8.43% at 25
0C [
10]. Clay minerals have garnered the attention of researchers as a potential adsorbent for its characteristics to hold cations and heavy metal ions through cation exchange (CEC) mechanism, abundant high surface area, layered structure, and ease of developing tailor made nano composites with significantly less loading [
11,
12]. In one of the studies, modified kaolinite showed an adsorption of 11 mg/g whereas modified bentonite a value of 138.17 mg/g for Co (II) ion uptake [
13]. The adsorption of Ni
2+ on natural clay accounted to be 70-75%, adsorption of 12.89 mg/g, within a range between 30 to 130 minutes [
14]. Among a plethora of adsorbents tried for Co (II) and Ni (II) removal, special emphasis was laid on materials being inexpensive, easy availability and excellent removal performance, the success of which depended heavily on the surface area of the adsorbents and the solution pH.
Adsorption process, though versatile for wastewater remediation, suffers from selectivity, sludge formation, lack of efficiency in removal for very low metal concentration and non-availability of columns and batches on commercial level [
15].
The need of the hour is to develop a hybrid reactor integrating the functions of adsorption and separation with minimal environmental footprint [
16,
17,
18,
19]. The common problem with adsorbents in membranes is fouling, which can be tackled by optimization that can help the utilization on a large scale. The impetus for the present work lies in the fact that to date, there has yet to be a complete review of Co
2+ and Ni
2+ ions removal in literature. The main objective of the work is to prepare an adsorbent membrane with a high surface area and the adsorptive ability for trace metal ions removal. Montmorillonite (Mt) was functionalized with poly dimethyl siloxane (PDMS), and the resulting material was characterized by Fourier Transform Infra-Red Spectroscopy (FTIR), Brunauer Emmett Teller (BET), and X-Ray Diffraction (XRD).
2. Materials and Methods
Materials
The materials used in this study, such as Montmorillonite K10 with CEC of 80-100 meq/g, cetyl trimethyl ammonium bromide (CTAB), polyvinyl alcohol (PVA), and Ni (II) chloride hexahydrate were supplied by Himedia. Cobalt Nitrate and poly dimethyl siloxane (PDMS) were bought from Sigma Aldrich.
Methods
Preparation of Functionalized Mt with PDMS
Mt was dispersed in a CTAB solution to make organophilic clay. After drying, about 5 mL of PDMS was added to get the silylated clay (S2). The resulting material was dried at room temperature.
Preparation of Flat Disk Membrane Support
The Mt was fabricated into flat disks of 25 mm diameter and 2 mm thickness, sintered at 600
0C in a muffle furnace with a heating and cooling rate 0f 20
0C/min and was kept on hold for 30 minutes [
20]
Preparation of coated membrane
About 5 g of PDMS treated clay (S2) was added to the PVA solution (5% w/v). The prepared Mt disk membranes were dip coated in the resulting solution at a dipping and withdrawal rate of 150 mm/sec with a specific hold time for uniform coating. They were then dried in air for one hour for the rejection test to be carried out.
Membrane Rejection Test
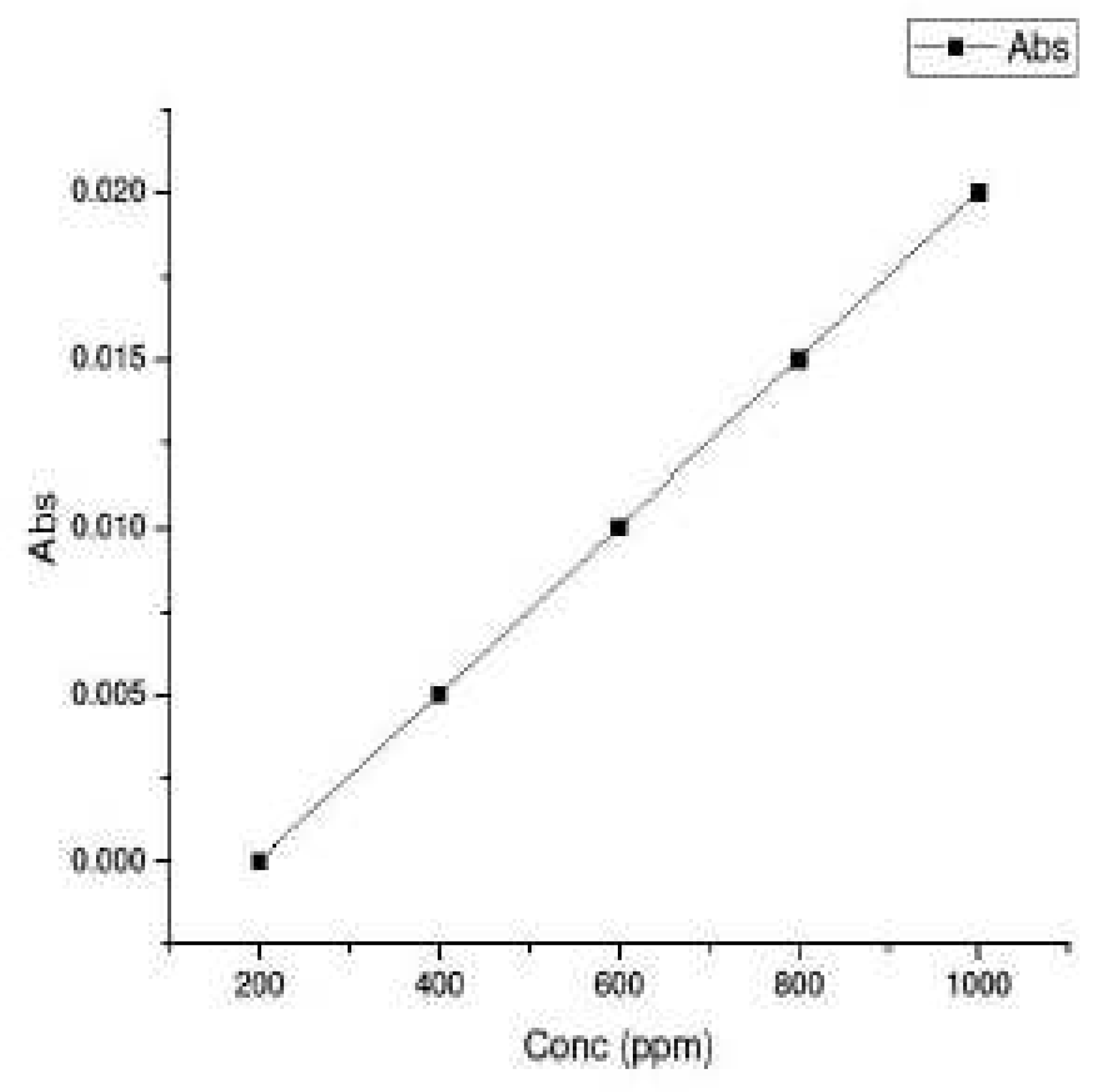
The stock solutions of 2000 ppm each of Co
2+ and Ni
2+ ions were made by dissolving 2 gm of cobalt nitrate and nickel (II) chloride hexahydrate in 1 L of water. Standard solutions of 200ppm, 400 ppm, 600 ppm, and 800 ppm were made upon dilution and were calibrated to get the Lambert-Beer plot as shown in
Figure 5. pH was adjusted to 9 for Co
2+ and 11 for Ni
2+ by adding suitable amount of acid /alkali. It was then subjected to filtration test involving membranes S1 (only Mt) and S2 (treated clay).
3. Results
3.1. Instrumentation
XRD was done using Bruker AXS D8 advance powder diffractometer equipped with Cu-Kα generator (λ=1.5405600 A
0). The generator tension was 35KV. IR was done on Thermo Nicolet Avatar 370 in the spectral range of 4000-400 cm
-1. BET surface area of samples was characterized by Nova 1000 Quantachrome Instrument by N
2 sorption at 77.35 K. The concentration of the permeate samples from filtration experiment was measured using Thermoscientific UV-Vis Spectrophotometer in the visible range of 400-800 nm. The micrograph of the cross section of membranes was taken using High Resolution Hitachi S-4800 Scanning Electron Microscope. The membrane filtration unit consisted of a cylindrical chamber with a membrane adapter connected to a pressure gauge of 72 psi with a peristaltic pump Model No RH-P120 VS [
20].
4. Discussion
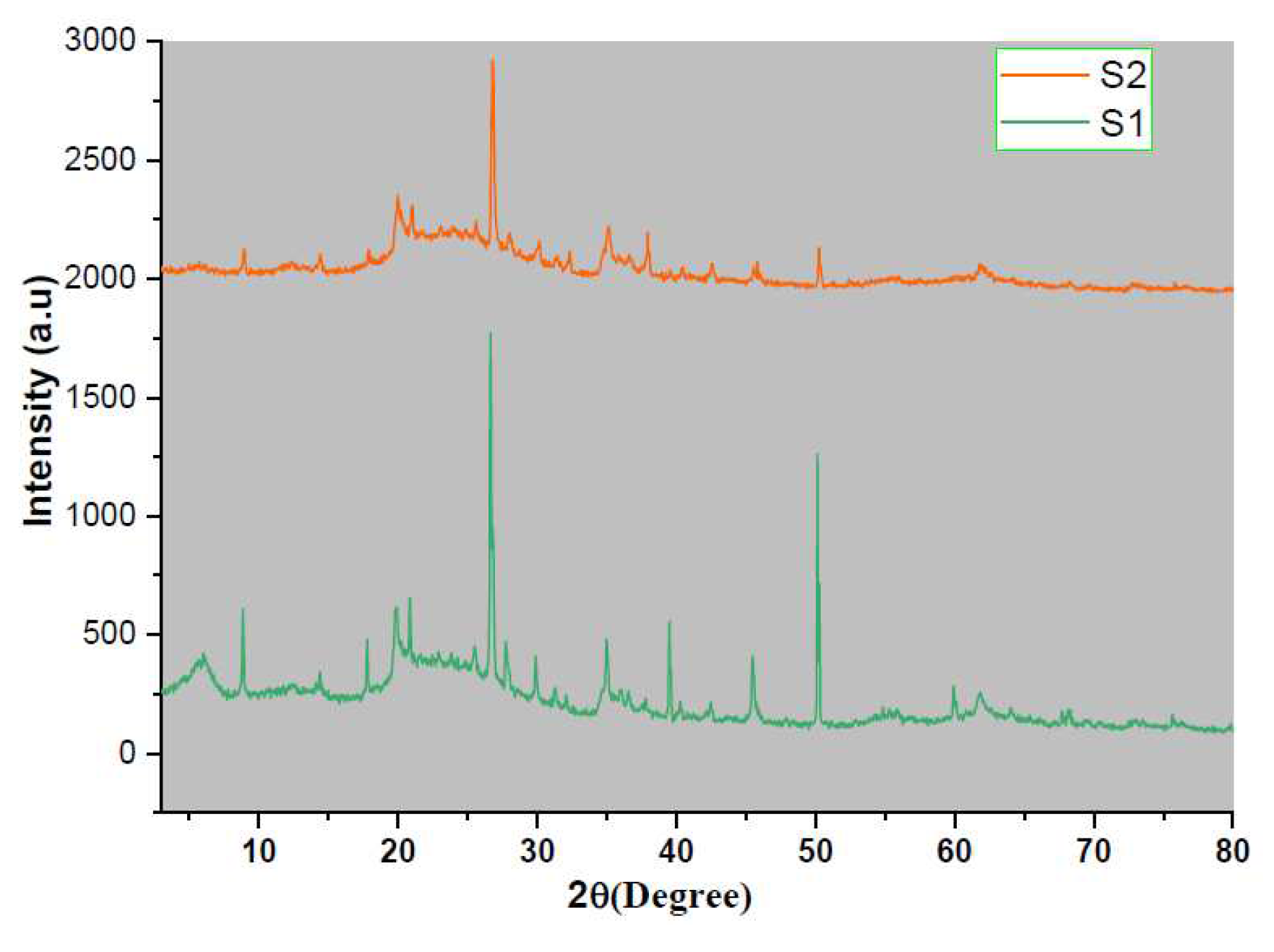
Figure 1 shows the X-Ray diffraction patterns of PDMS treated Mt (S2) and Mt (S1). S1 shows characteristic Mt reflection peak at 2θ=5.21 A
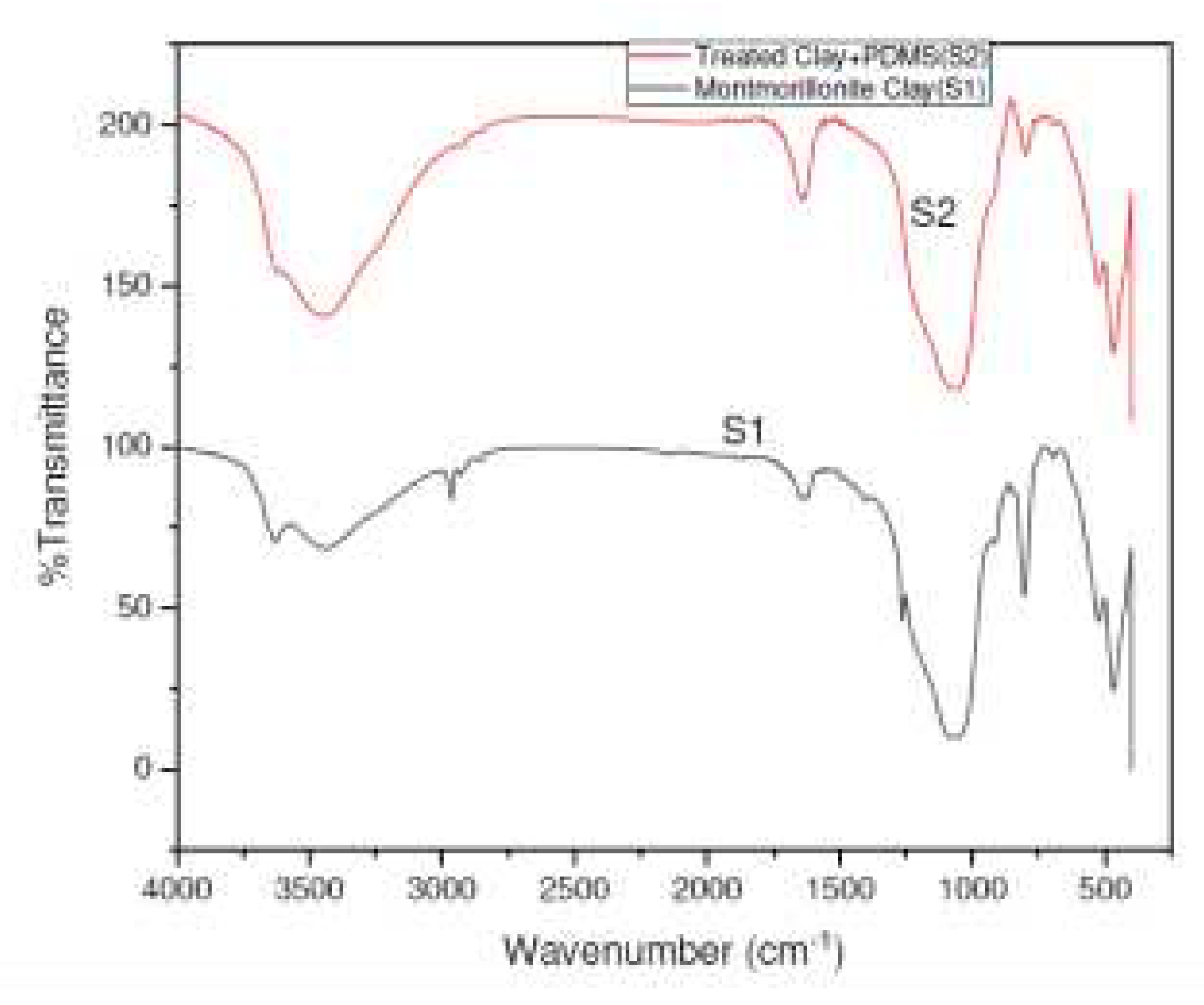
0, which becomes broad and flattened one in S2, indicating complete intercalation and grafting of silanol groups into the interlayer galleries of Mt leading to a delaminated structure and homogeneous structure. FTIR spectra of S1 and S2 were obtained in
Figure 2 to ascertain the formation of silylated clay. The strong band at 3435 cm
-1, corresponding to the stretching vibration of the OH group and the interlayer molecules is completely missing in S2, indicating that surface OH groups of clay were successfully consumed through bonding with Si atom and siloxane moiety of PDMS during surface treatment. Also, the intensity of bonded –OH band at 1628.81 cm
-1 is higher in S2, confirming the above fact. Intense peaks of symmetric Si-O-Si vibrations can be observed at 788 cm
-1 and 790 cm
-1.
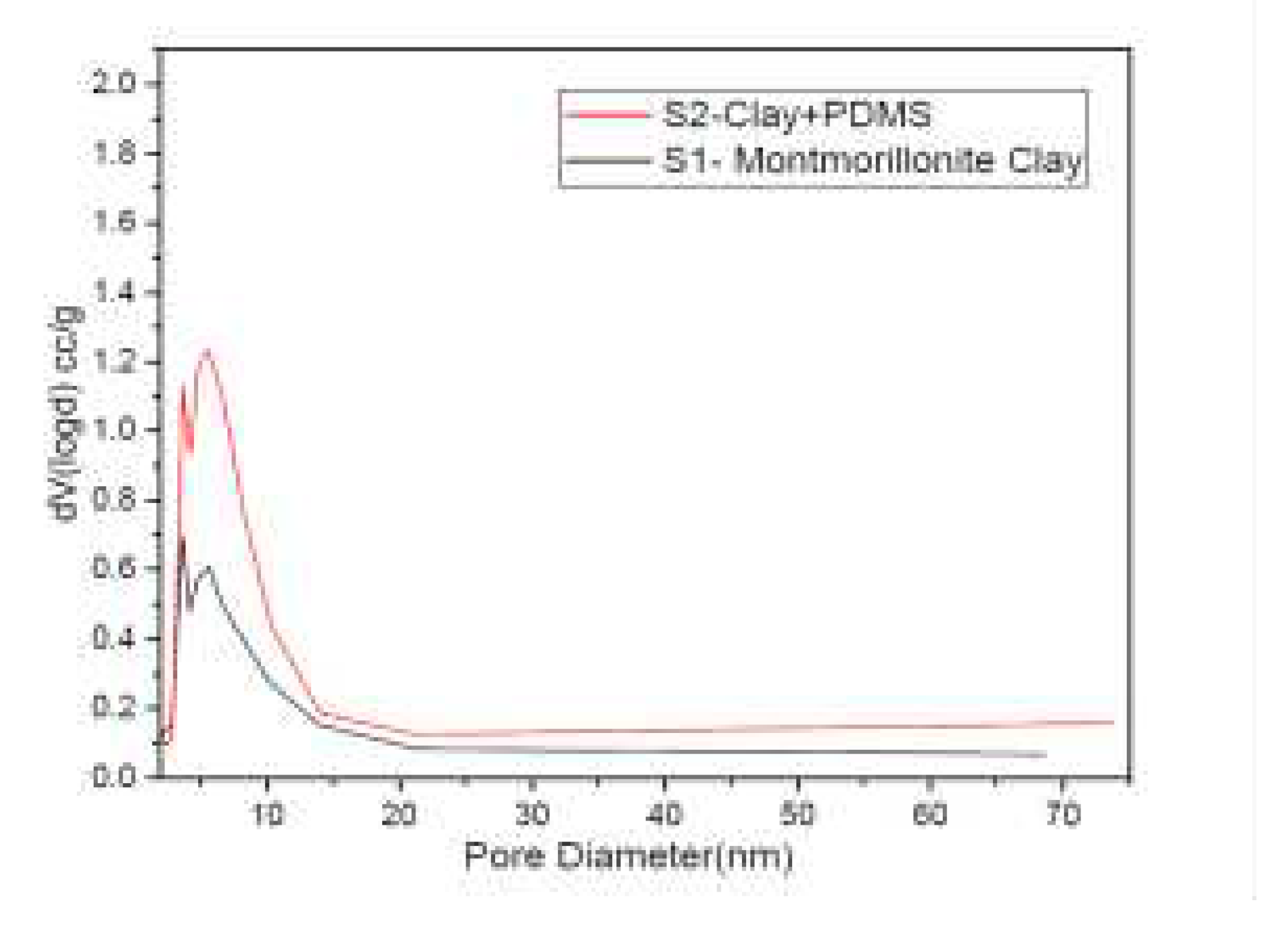
The specific surface areas of S1 and S2 from BET adsorption isotherm were 273 m
2 /g and 443 m
2 /g respectively. S2 also showed a broader pore size distribution than S1, as in
Figure 3, necessary for better flux rate and decreased pressure during the filtration test. This result is congruent with the micrographs obtained in
Figure 4. The porous structure of S2 was clearly indicated with pore size diameter being in the range of 116-163 nm. S2 is thus a macroporous material. Permeates from the membrane filtration test were collected and were calibrated against standard solutions fixing λ
max for Ni
2+ at 725 nm and for Co
2+ at 514 nm. The calibrated Lambert Beer plot is shown in
Figure 5 from which the concentration of the heavy metal ions can be calculated. As can be seen from
Table 1, a higher pH facilitates the ease of removal of the heavy metal ion mainly through complexation. The formation of the green complex on the surface of the membrane is shown in
Figure 6. The best result is shown for Ni
2+ which shows a removal of 75% at pH11 for 800 ppm of the solution.
Figure 3.
Pore Size distribution of Mt (S1) and Treated Clay (S2).
Figure 3.
Pore Size distribution of Mt (S1) and Treated Clay (S2).
Figure 4.
SEM micrograph of Treated Clay (S2) showing macroporosity of the material (163 nm).
Figure 4.
SEM micrograph of Treated Clay (S2) showing macroporosity of the material (163 nm).
Figure 5.
Calibration graph of Aborbance vs concentration for the determination of concentration of Co2+ and Ni2+ ions.
Figure 5.
Calibration graph of Aborbance vs concentration for the determination of concentration of Co2+ and Ni2+ ions.
Figure 6.
Showing Complexation of Ni2+ on the surface of the S2 membrane.
Figure 6.
Showing Complexation of Ni2+ on the surface of the S2 membrane.
When two ions Co
2+ and Ni
2+ ions are there in solution, it is seen from
Table 2 that at higher pH it leads to decrease in the removal percentage. This may be due to the fact that both Co
2+ and Ni
2+ compete for complexation with –OH groups present on the surface of the treated clay, thus resulting in decrease in the removal of the ions [
21].
The introduction of silanol groups acts as a key factor to promote specific interaction with the adsorbate molecules resulting in better permeate flux rates and important industrial applications.
Much research is augured in this area of developing an ideal membrane material with good adsorption and desorption behavior, hydrophilicity, least fouling and better methods of regeneration of the heavy metals from the membranes.
Acknowledgments
The authors are thankful to Chemical Engineering Division, IIT madras for analysis of samples by SEM. Authors are grateful to STIC, Cochin University of Science and Technology, CUSAT, Kochi for analysis of samples by XRD and FTIR, and BIT, Bangalore for providing BET data of samples.
References
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Gil. A et al. A review of organic-inorganic hybrid clay based adsorbents for contaminants removal: Synthesis, perspectives and applications. J. Environ. Chem. Eng 2021, 9, 105808. [CrossRef]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites. Chem. Eng. J. 2020, 420, 127574. [Google Scholar] [CrossRef]
- Es-sahbany. H et al. Investigation of the adsorption of heavy metals (Cu, Co, Ni and Pb) in treatment synthetic wastewater using natural clay as a potential adsorbent (Sale- Morocco). Mater Today: Proceedings 2021, 45, 7290-7298. [CrossRef]
- Thankur. A. K et al. Green adsorbents for the removal of heavy metals from wastewater: A review. Mater Today: Proceedings 2022, 57, 1468-1472. [CrossRef]
- Gafoor, A.; Dhanasekar; Kumar, S.; Sankaran; Sivaranjani; Begum, S.; Rahman, Z. Elimination of nickel (II) ions using various natural/modified clay minerals: A review. Mater. Today: Proc. 2020, 37, 2033–2040. [CrossRef]
- S. S. Dawn; Vishwakarma. V. Recovery and recycle of wastewater contaminated with heavy metals using adsorbents incorporated from waste resources and nanomaterials- A review. Chemosphere 2021, 273, 129677. [CrossRef]
- Islam, A.; Morton, D.W.; Johnson, B.B.; Pramanik, B.K.; Mainali, B.; Angove, M.J. Opportunities and constraints of using the innovative adsorbents for the removal of cobalt(II) from wastewater: A review. Environ. Nanotechnology, Monit. Manag. 2018, 10, 435–456. [Google Scholar] [CrossRef]
- Ghani, U.; Hussain, S.; Amin, N.U.; Imtiaz, M.; Khan, S.A. Laterite clay-based geopolymer as a potential adsorbent for the heavy metals removal from aqueous solutions. J. Saudi Chem. Soc. 2020, 24, 874–884. [Google Scholar] [CrossRef]
- Jlassi, K.; Eid, K.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.M. Data on the fabrication of hybrid calix [4]arene-modified natural bentonite clay for efficient selective removal of toxic metals from wastewater at room temperature. Data Brief 2021, 35, 106799. [Google Scholar] [CrossRef]
- Jlassi. K et al. Calix[4] arene clichéd clay through thiol-yne addition for the molecular recognition and removal of Cd (II) from wastewater. Sep. Purif. Technol 2020, 251, 117383. [CrossRef]
- Hnamte. M; Pulikkal. A. A. Clay Polymer nano composites for water and wastewater treatment: A comprehensive review. Chemosphere 2022, 307, 135869. [CrossRef]
- Bahadur Yadav. V et al. Clay based nanocomposites for removal of heavy metals from water: A review. J. Environ. Manage 2019, 232, 803-817. [CrossRef]
- Es-Sahbany, H.; Berradi, M.; Nkhili, S.; Hsissou, R.; Allaoui, M.; Loutfi, M.; Bassir, D.; Belfaquir, M.; El Youbi, M. Removal of heavy metals (nickel) contained in wastewater-models by the adsorption technique on natural clay. Mater. Today: Proc. 2019, 13, 866–875. [Google Scholar] [CrossRef]
- Gupta, G.; Khan, J.; Singh, N. Application and efficacy of low-cost adsorbents for metal removal from contaminated water: A review. Mater. Today: Proc. 2021, 43, 2958–2964. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Zerafat, M.M.; Mohammadi, Y.; Asadnia, M. Fabrication of tubular ceramic membranes as low-cost adsorbent using natural clay for heavy metals removal. Clean. Eng. Technol. 2022, 10. [Google Scholar] [CrossRef]
- Pagana, A.E.; Sklari, S.D.; Kikkinides, E.S.; Zaspalis, V.T. Microporous ceramic membrane technology for the removal of arsenic and chromium ions from contaminated water. Microporous Mesoporous Mater. 2008, 110, 150–156. [Google Scholar] [CrossRef]
- Katsou. E et al. Use of ultrafiltration membranes and aluminosilicate minerals for Ni removal from industrial wastewater. J. Membr. Sci 2010, 360, 234-249. [CrossRef]
- Shukla, A.; Kumar, A. Separation of Cr(VI) by zeolite–clay composite membranes modified by reaction with NOx. Sep. Purif. Technol. 2007, 52, 423–429. [Google Scholar] [CrossRef]
- Neethu, N.; Choudhury, T. Treatment of Methylene Blue and Methyl Orange Dyes in Wastewater by Grafted Titania Pillared Clay Membranes. Recent Patents Nanotechnol. 2018, 12, 200–207. [Google Scholar] [CrossRef]
- Duan. C et al. Removal of heavy metals from aqueous solution using C- based adsorbents: A Review. J. Water Process. Eng 2020, 37, 101339. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).