1. Introduction
The uses of the new green inhibitors have recently attracted increasing attention in the technology community. Scientists are tenacious in their pursuit of better and more effective techniques to stop the corrosion of metals in the field of material science and corrosion control [
1,
2,
3].
Since leaves contain corrosion-aggressive substances like tannins, saponins, and flavonoids, among others [
4], the effects of corrosion on building materials and the maintenance or replacement of goods lost or contaminated by corrosion reactions are of remarkable consequence in the food processing industry. More harm than good has been caused by corrosion [
5,
6].
Inhibition is a technique frequently employed to lessen the corrosive attack on metallic materials. Typically, inhibitors are used for this purpose to regulate metal dissolution. Recent investigations on the corrosion of mild steel in acidic solutions have surfaced in the literature. The important materials used in the manufacturing sector are mild steel. Mild Steel is widely used in the construction of machine parts that are employed in manufacturing, processing, and production industries [
7,
8,
9]. The best approach to militate corrosion of these structures is to study the corrosive behavior of this metallic material in the environment concerned to proffer an appropriate method of protection [
10,
11]. Mild steel corrodes when exposed to air and the oxide formed on it is readily broken down, in the presence of moisture, especially if it is not repaired [
12].
Furthermore, because the entire concept of metal protection is based on economic gain and environmental sustainability, the substance used as a metal corrosion inhibitor must be inexpensive, widely available, and environmentally friendly. As a result, research efforts are focused on developing a replacement for inorganic metal corrosion inhibitors. The leaf is a source of inexpensive, readily available, non-toxic green metal corrosion inhibitors. Leaf Products are organic and contain photochemical substances such as tannins, flavonoids, saponins, organic and amino acids, alkaloids, and pigments that can be extracted using simple, low-cost methods. Extracts from various parts of leaves have been widely reported as effective metal corrosion inhibitors in a variety of corrosive media [
13,
14,
15].
Various research works have been carried out in recent times on the use of vegetable extracts as corrosion inhibitors [
16,
17]. The research interest has been necessitated by the fact that the present corrosion inhibitors in the market for the protection of mild steel in the alkaline media are hazardous to the environment and thus compromise safety and sustainability drives [
18]. There is, therefore, the need to develop inhibitors that are eco-friendly and sustainable. It is, however, noteworthy that the results of these studies show that extracts of the leaf are at the top of the list of non-toxic organic that has been used as corrosion inhibitors to replace environmentally hazardous synthetic. They are non-toxic, environmentally friendly, and readily available. Extensive research has been conducted to identify the synergistic effect of other additives on the efficiency of metal corrosion inhibitors. [
19,
20,
21] observed that synergism can improve an inhibitor's inhibitive force, reduce the amount of inhibitor used, and diversify the inhibitor's application in an aggressive environment.
In various years, many researchers, scholars, and organizations globally have carried out a lot of research on the evaluations of plant extracts as corrosion inhibitors, for example, Origanum vulgare [
22], Sapium ellipticum [
23], Persian liquorice [
24], chicory aqueous [
25]. This green approach has received favorable attention owing to its availability, low cost, and to the best of our knowledge little or no negative effect on the environment has been documented. The present study is concerned with an investigation of the inhibiting effect of Dioscorea spp. leafy extracts on the corrosion of mild steel in selected environments by using the weight loss method. It represents a modest effort to contribute to this growing field of research on the inhibitory behavior of leaf extracts on the corrosion of mild steel in selected media.
2. Materials and Methods
The materials that were used for this study include the following: mild steel rods, hacksaw, lathe machine, electronic weighing balance METTLE TOLEDO model ME204E, beakers (100cm3, measuring cylinder (1000cm3), volumetric flask (250cm3), masking tape, sandpaper, nylon thread, hand towel, razor blade, retorts stand, paper sieve, funnel, distilled water, Dioscorea spp. leaf extracts. The chemicals that were used were Acetone, tetraoxosulphate (vi) acid (H2SO4), sodium hydroxide (NaOH), and sodium chloride (NaCl).
Material preparation
This study made use of mild steel rods. The mild steel rods' composition was determined using an Optical Emission Spectrometer, and the mild steel rods were obtained from metal stockists. The chemicals and reagents used in this study were of the highest quality. A lathe machine and a hacksaw were used to machine cylindrical mild steel samples with diameters of 8mm and heights of 16mm. Each coupon was degreased by washing in ethanol, drying in acetone, and storing in a desiccator before being weighed and inserted into the beaker to determine the weight difference.
Preparation of leaf extracts
The leaves were collected in Edda-Echara, Ebonyi State, and identified by a laboratory technologist at Ebonyi State University's Department of Applied Biology in Abakaliki, Nigeria. Using standard laboratory procedures, 20g of Dioscorea spp. leaf extracts were obtained. The leaf extract volumetric concentrations were expressed in milliliters (ml). The concentrations of Dioscorea spp. leaf extracts used in the study were 5 ml, 10 ml, and 15 ml, respectively, while the concentrations of acid, alkali, and salt were 0.5 M and 1.0 M. A total of twenty (20) beakers were rinsed with distilled water and dried in the air before the experimental was set up, to avoid additional water. The coupons were immersed in various media using a nylon thread that was tied to the coupons and hung on a retort stand. Mild steel samples were placed in beakers and left to stand for 28 days (672 hours), with one set withdrawn every 7 days (168 hours). To avoid crevices and galvanic corrosion, none of the coupons were allowed to touch.
Weight loss method
The mild steel sample coupons were weighed using a digital weighing balance, METTLER TOLEDO model ME204E with a least count of 0.0001g, labeled, and immersed in the acid, alkali, and salt with inhibitor test solutions. The weight loss of each sample coupon was calculated and recorded. The weight loss determination and recording were repeated every 68 hours (7 days) for a total of 672 hours (28 days). Each coupon was washed in absolute ethanol, rinsed in distilled water, dried in acetone, and weighed before being measured [
22]. The same experiment was carried out in both the absence and presence of the inhibitor. The corrosion rate of leaf extracts was calculated using Equation 1
where CR is corrosion rate, millimeter per year (mm/yr), K is rate constant equal to 87.6 × 10
4,
is weight loss in mg,
ρ is density of material in gcm
-3 =7.86gcm
-3, T is exposure time in hours, A is exposed area of coupon in cm
2.
3. Results
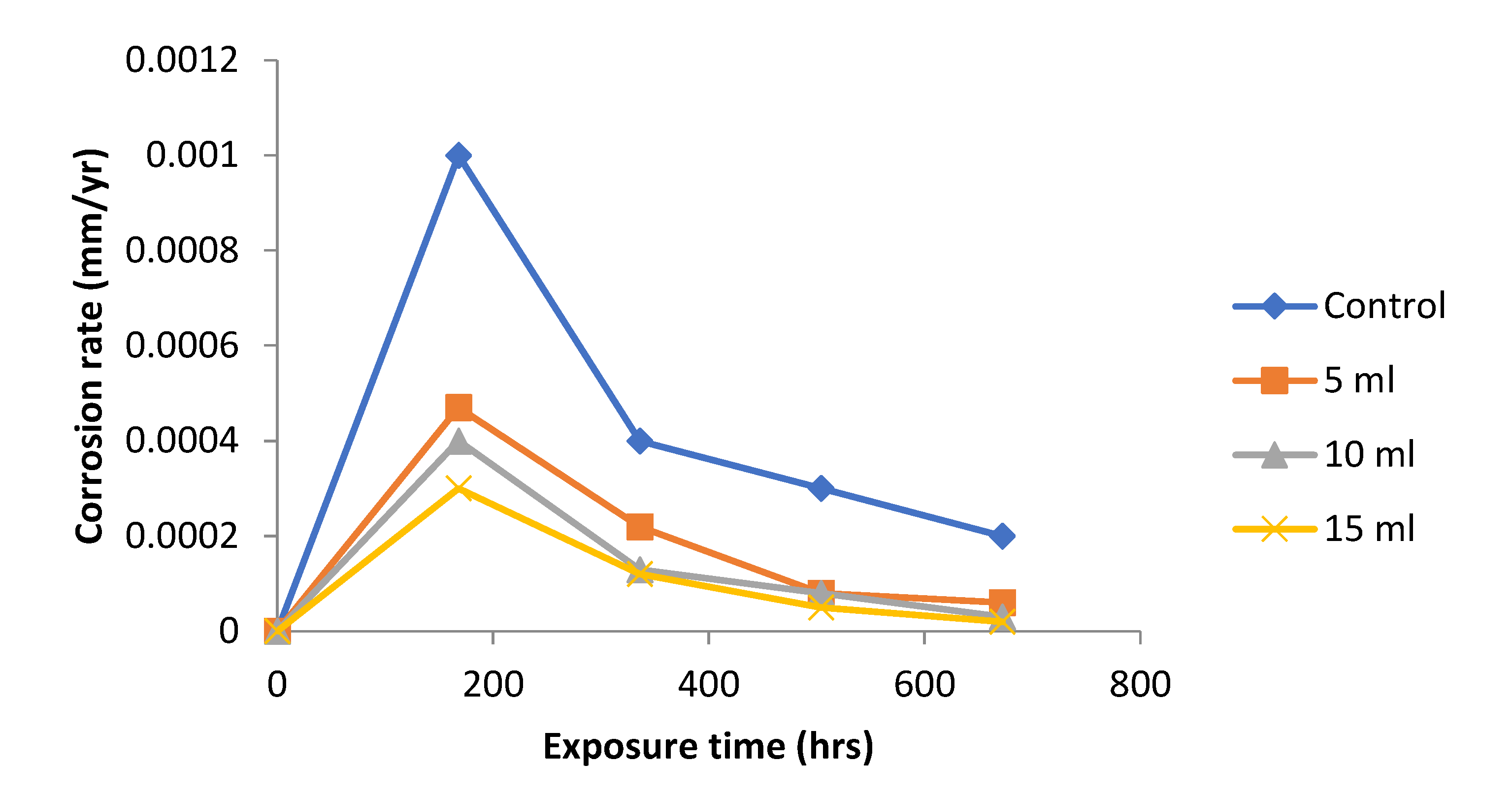
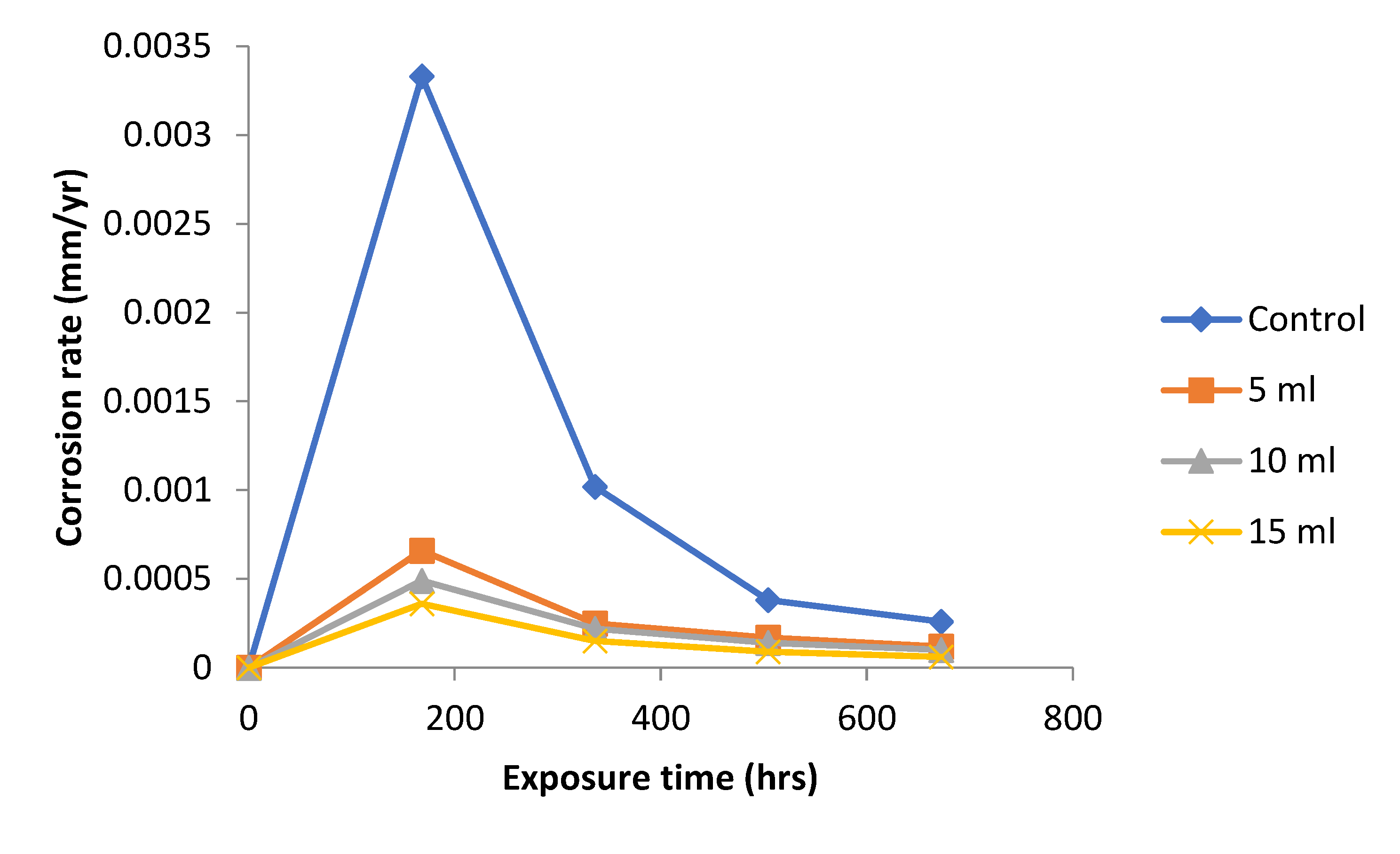
The results obtained for the variation of corrosion rates with exposure time for the mild steel specimens immersed in 0.5 M NaOH with varied concentrations of added
Dioscorea spp. leaf extract is presented
Figure 1. The result obtained show a great value of corrosion rate for the test media without
Dioscorea spp. leaf extract. The addition of
Dioscorea spp. extract to the test media resulted in reduction of corrosion rate. The difference in corrosion rate for the test media with and without
Dioscorea spp. extract was much for the 168 hour interval, but from 336 to 672 hours there was a decrease in corrosion rate for the media without
Dioscorea spp. extract (control experiment). The
Dioscorea spp. extract shows a good inhibition behavior on the corrosion rate of mild steel in 0.5 M NaOH media. This is an agreement with the findings in the literature [
6,
7,
19].
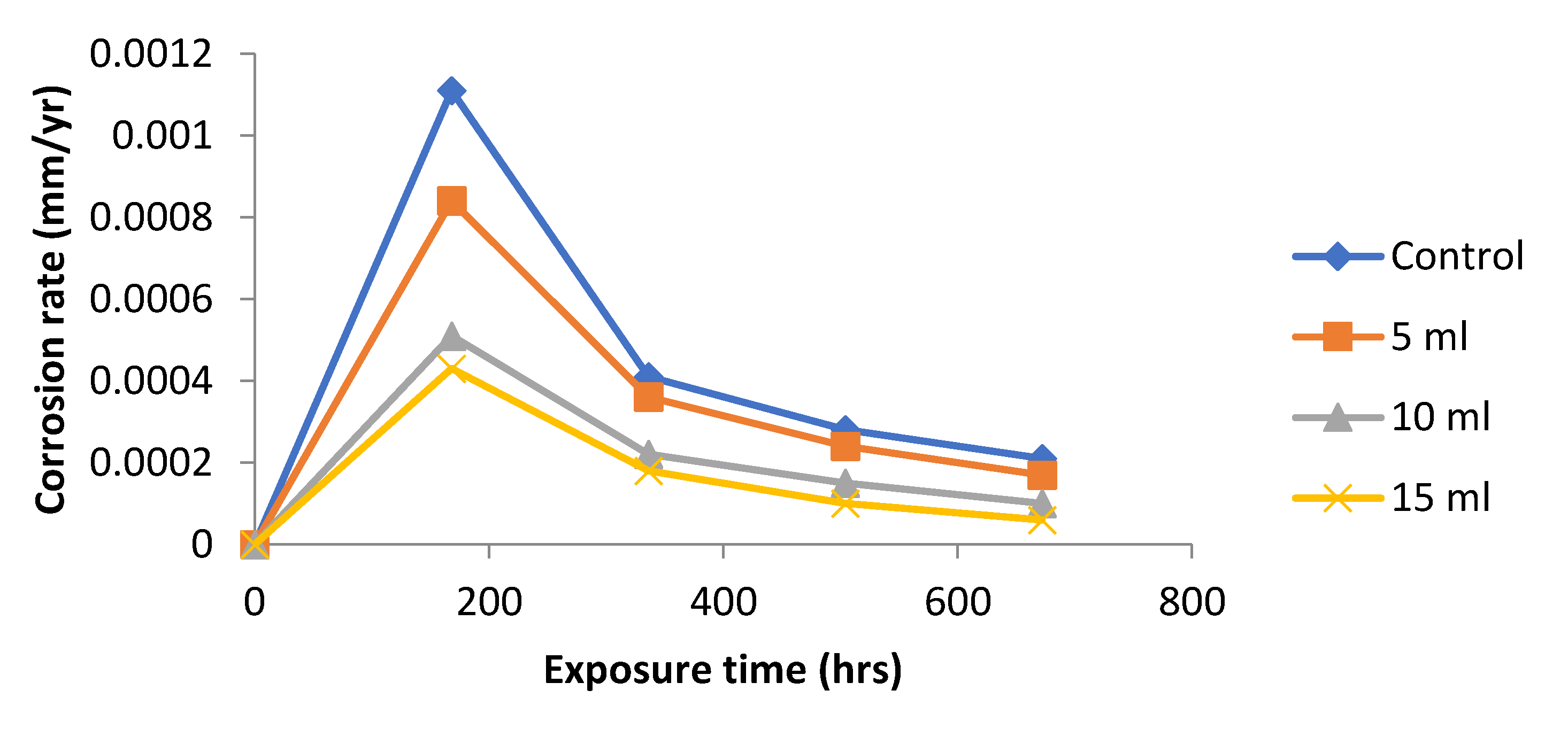
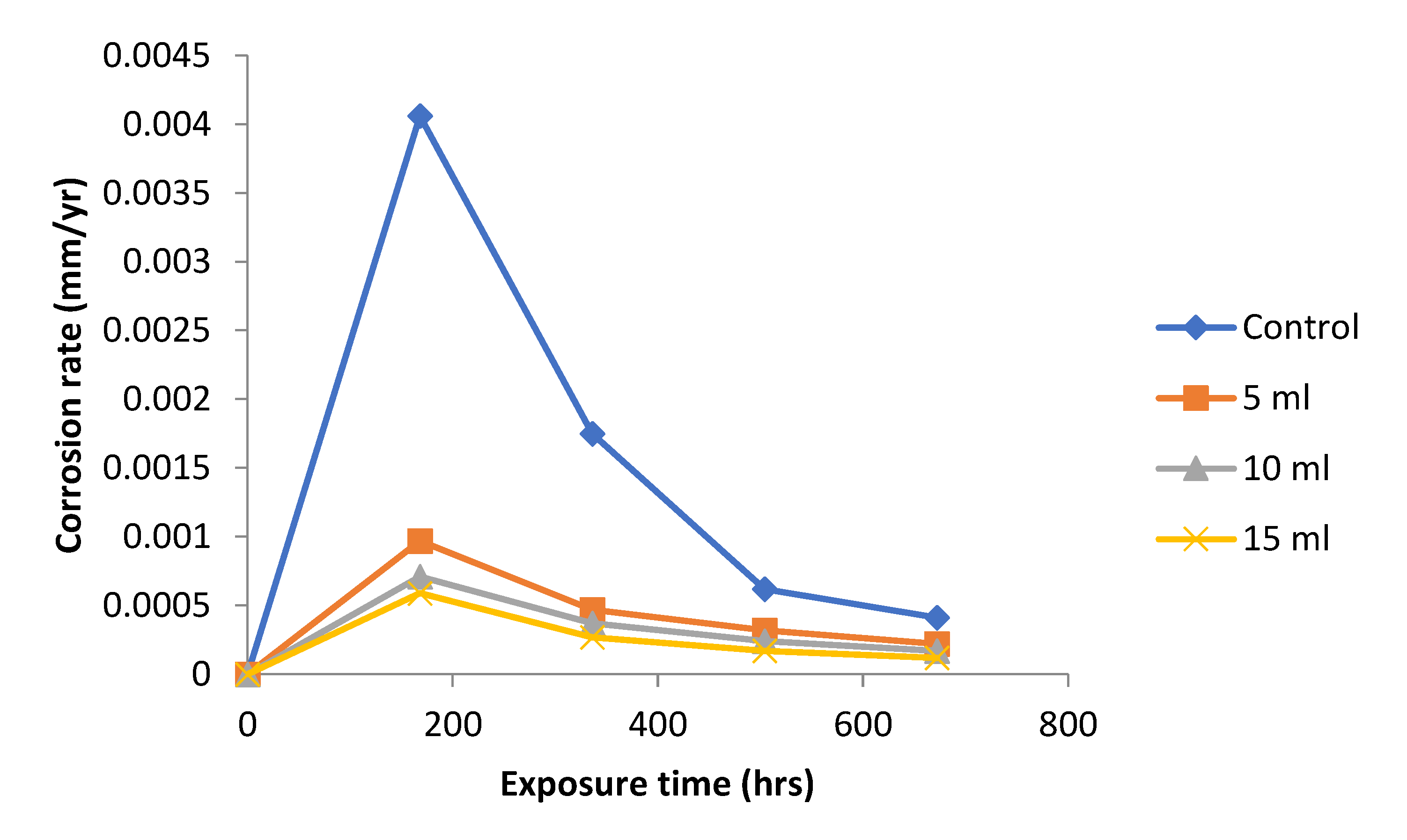
Figure 2 shows the variation of corrosion rate with exposure time for mild steel immersed in 1.0 M NaOH and addition of different concentrations of
Dioscorea spp. leaf extract. Corrosion rates were very slow with the three different
Dioscorea spp. extract concentrations. The control experiment gave higher corrosion rate values throughout the experimental period. These results confirm that plant extract of the
Dioscorea spp. possesses corrosion inhibiting property. It is not certain, however, whether the optimum concentration needed for more effective corrosion inhibition have been reached with any of the three concentrations used. This suggests that the leaf extracts of
Dioscorea spp. is good and efficient inhibitor of corrosion of mild steel within the environment.
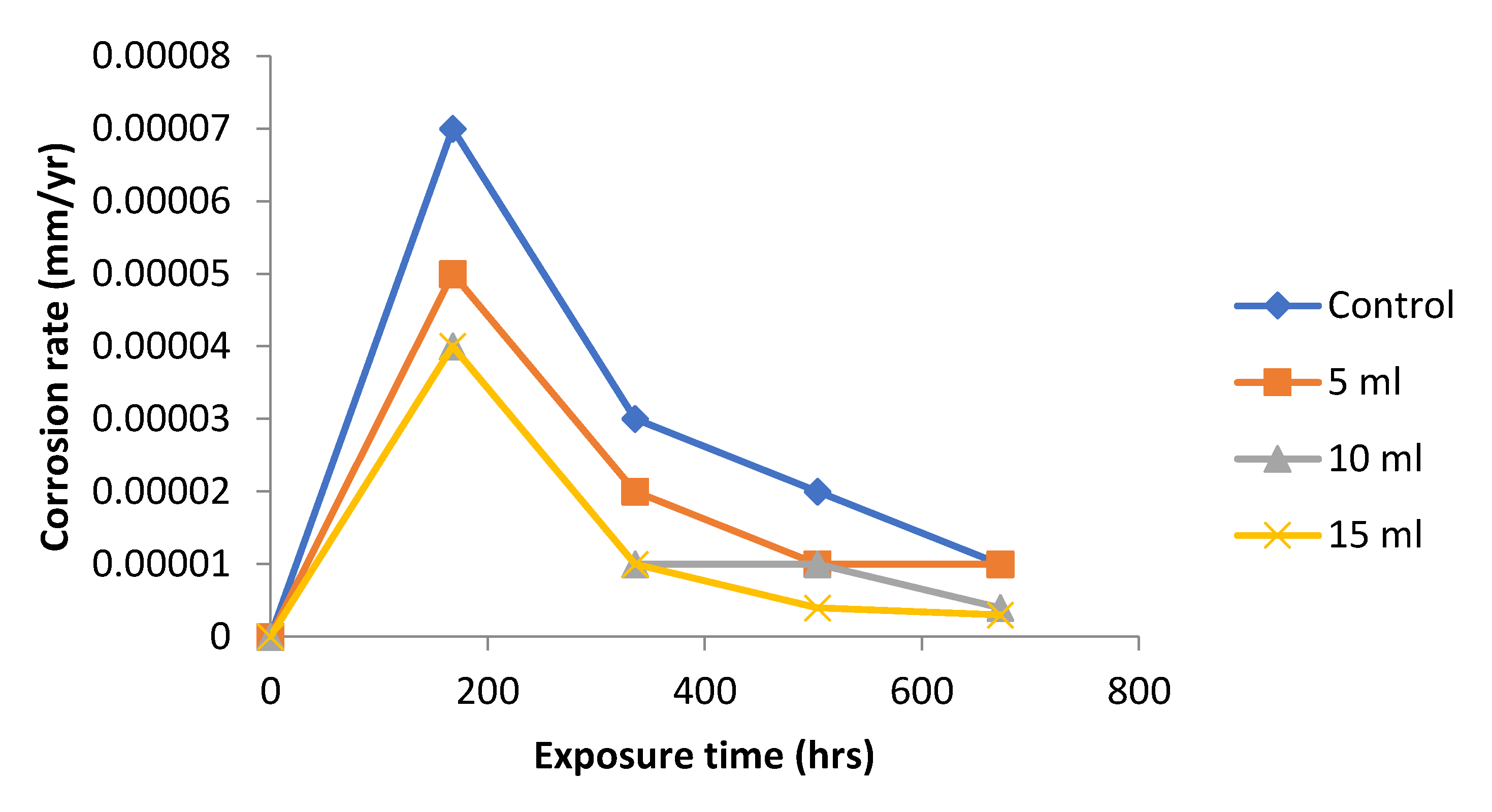
The results obtained for the variation of corrosion rate 0.5 M NaOH is presented
Figure 3. From the graph, the control experiment has the highest magnitude of corrosion rate because
Dioscorea spp. extract was not added. The addition of
Dioscorea spp. extracts to the test medium reduced corrosion significantly throughout the experimental period. The results obtained for the 5, 10 and 15 ml respectively
Dioscorea spp. extract addition to the test medium all similar trend in corrosion rate, indicating that little extract concentration have inhibition effect. The results confirmed the very good effect of the
Dioscorea spp. extract on the corrosion inhibition of mild steel in 0.5 M NaOH.
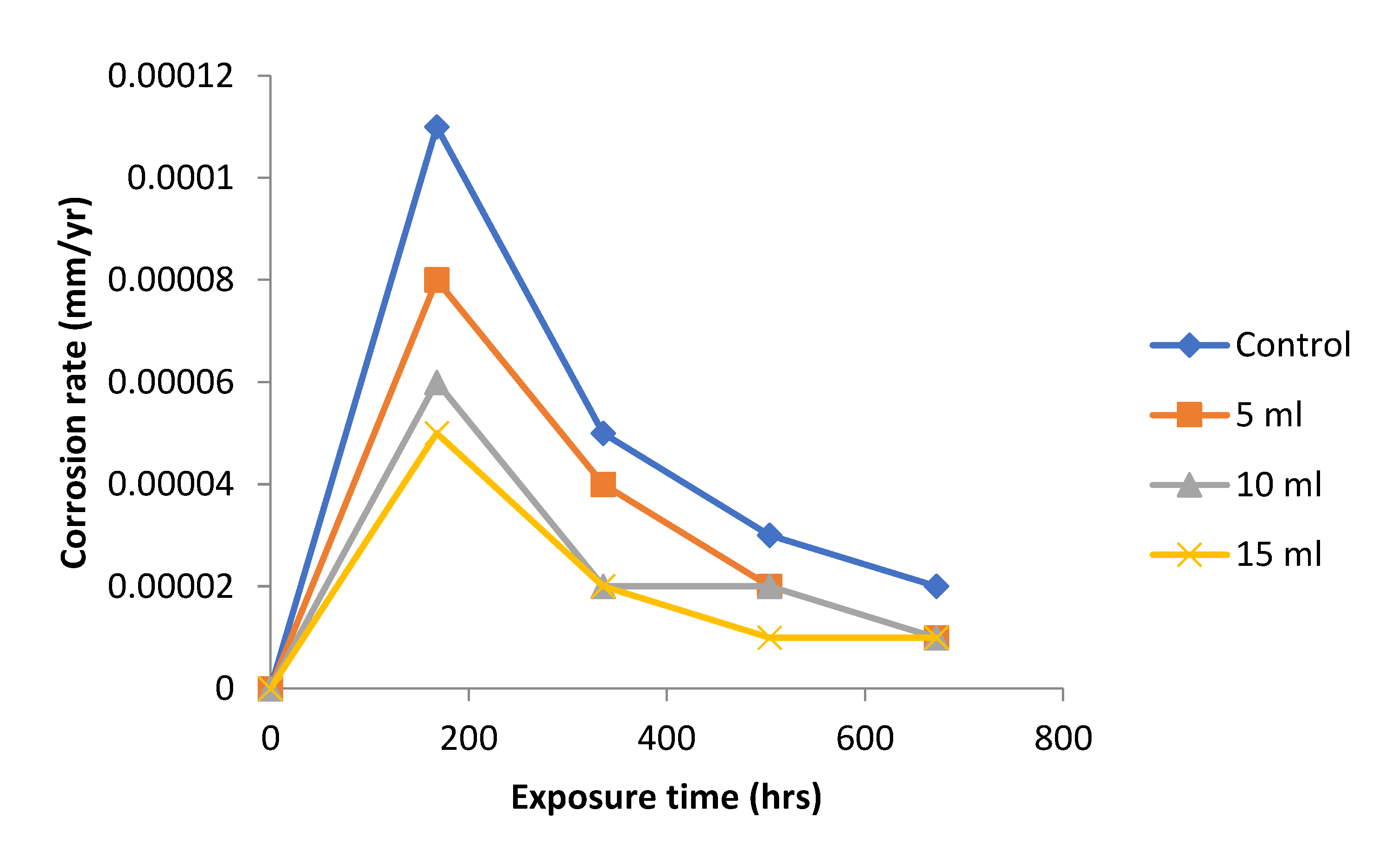
Figure 4 shows the graph of variation of corrosion rate. The
Dioscorea spp. extracts addition to the test medium reduced corrosion rate significantly throughout the experimental period compared to the control experiment. The results obtained for the 5, 10 and 15 ml respectively
Dioscorea spp. extract addition to the test medium have close corrosion rate value. The results confirmed the effectiveness of the
Dioscorea spp. extract on the corrosion rate of mild steel in 1.0 M NaOH media. The extract concentration of 15 ml addition appeared to be the best, having a corrosion rate value of 0.00005, 0.00002, 0.00001 and .00001 mm/yr respectively for 168 to 672 hours, respectively, followed by 10 and 5 ml, respectively concentration of
Dioscorea spp. [
13]. This is explained from purview of the chemical constituents of the leaf extract appears to contain more species [
13].
The corrosion rate of the mild steel in the absence of the
Dioscorea spp. leaf extract and the presence of the
Dioscorea spp. leaves extract were determined at various concentrations of leaf extract in 0.5 M H
2SO
4 media is shown in
Figure 5. From the plot, it can be seen that the normal corrosion profile for passivating metals were noticed. This involves a sharp rise in corrosion rate followed by a gentle decrease as duration time increased. The rate of decrease of corrosion rate was very high in the first seven days of the experiments and then slowed down subsequently. This is as a result of the formation of thin film oxide on the surface of the coupon that acted as barrier between the coupon surface and the media itself. The figure also confirmed that the loss in the corrosion rate of the coupons decreases as the concentration of
Dioscorea spp. extract increases, indicating good corrosion inhibition performance of leaves extract in the acidic environment. This is in agreement with the findings in the current research [
16,
26].
Figure 6 is a graph of corrosion rate versus exposure time in the different concentrations of
Dioscorea spp. extract in 1.0 M H
2SO
4 solution medium just discussed. As expected, the graphs display higher corrosion rates for the coupons subjected in control experiment (without
Dioscorea spp. extract). As with the
Dioscorea spp. extract media, the trend of a very high initial corrosion rate which drops very rapidly in the first seven days and then decreased less rapidly afterwards, may be noticed. These graphs also show that in general, the coupons subjected in these media experienced higher corrosion rates as compared to 0.5 M H
2SO
4 media. This result is consistent with the findings of [
2,
8].
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
4. Conclusions
In this study, weight loss analysis of leaf extract was used to study the ability of Dioscorea spp. leaf extract to retard the corrosion of mild steel in different concentrations of acidic, alkaline and salt environments. The following conclusions are drawn:,
The results indicated that the corrosion rates of mild steel increased with increasing concentration of leaves extract; at the highest inhibitor concentration of 15 ml, the inhibition efficiency is increased markedly and reached ≥92 %.
Dioscorea spp. extracts act as good inhibitor for corrosion of mild steel in both acidic and alkaline environments.
The corrosion rates of mild steel strongly depends on the concentration of Dioscorea spp. leaf extract.
that the Dioscorea spp. leaf extracts act as good green corrosion inhibitor and can be used to retards the corrosion rate of mild steel if the appropriate concentration is used.
Dioscorea spp. leaf extracts have proved to be a promising natural source material as an alternative non-toxic, low cost and eco-friendly inhibitors that can replace the synthetic chemicals which are currently used in various biomedical, biostructural, metallurgical, nanomaterials, and in manufacturing industries.
The leaf extract (used as one of the corrosion inhibitors) grows and survives easily in all parts of Nigeria. The leaf extract has enormous economic potentials. It should therefore be cultivated in large quantities throughout the country; to be used as one of the corrosion inhibitors of plant origin. This will reduce our dependence on imported toxic, non-environmentally friendly and expensive corrosion inhibitors.
vii. From the results obtained in this study, it is necessary to carry out regular corrosion inhibition studies using this leaf extract (Dioscorea spp. ) on other metals to evaluate their efficiencies on such metals at different concentrations.
Author Contributions
Conceptualization, Hyacinth Idu, Blessing Ifeanyichukwu and Ndubuisi Idenyi; Data curation, Blessing Ifeanyichukwu; Funding acquisition, Ndubuisi Idenyi; Investigation, Hyacinth Idu and Blessing Ifeanyichukwu; Supervision, Ndubuisi Idenyi; Writing – original draft, Hyacinth Idu an Blessing Ifeanyichukwu; Writing – review & editing, Hyacinth Idu and Ndubuisi Idenyi. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Source of Data: From experimental results from corrosion tests carried out by researchers (Idu, Idenyi and Idenyi, 2023).
Acknowledgments
The author are grateful to Prof. D. U. Onah from the Department of Industrial Physics, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria, for his technical support. The authors acknowledge the contributions of the technical staff of the Centre for Energy Research and Development, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria, in performing the characterisations.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Compliance with Ethical Standards
This article does not contain any studies involving human or animal subjects.
References
- N.E. Idenyi, P.A. Nwofe, and H.K. Idu, Influence of moringa oleifera and psidium guavaja leaves extract on the corrosion susceptibility of mild steel in an alkaline medium. Journal of applied science research, 11(2015): 158-163.
- N.E. Idenyi, P.A. Nwofe, P.E. Agbo, and Idu, H.K.(2015). Effects of psidium guavaja (guava) and moringa oleifera leaves extract on the corrosion susceptibility of mild steel in an acidic medium, Australian journal of basic and applied sciences, 9(35): 245-250.
- H.K. Idu, N.E. Idenyi, and P.A. Nwofe, Investigations on the inhibitory properties of moringa oleifera and psidium guajava leaves extract on the corrosion susceptibility of mild steel. Middle-east journal of scientific research, 23, (2015), 2862-2867.
- K.O. Jamiu and M.A. Olorunfemi, Corrosion inhibition of mild steel in acidic medium by jathro phacurcas leaves extract, Journal of electrochemical science and technology, 4, (2013), 81-87.
- G. Ji, A. Shadma, S. Shanthi and P. Rajiv, Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution, Corrosion Science 90, (2015), 107-117.
- E. Kowsari, M. Payami, R. Amini, B. Ramezanzadeh, and M. Javanbakht, Task-specific ionic liquid as a new green inhibitor of mild steel corrosion, Applied Surface Science, 289, (2014), 478-486. [CrossRef]
- S.I. Anyanwu, O. Eseonu, H.U. Nwosu, Studies on corrosion characteristics of carbon steel exposed to Na2CO3, Na2SO4 and NaCl solutions of different concentrations, The International Journal Of Engineering And Science (IJES), 3, (2014), 48-60.
- K.K. Anupama, K.M. Shainy, K. M. and A. Joseph, Excellent anticorrosion behavior of ruta graveolens extract (RGE) for mild steel in hydrochloric acid: electro analytical studies on the effect of time temperature and inhibitor concentration, J Bio Tribo Corros, 2 (2016), 56-72. [CrossRef]
- Benali, H. Benmehdi, O. Hasnaoui, C. Selles, and R. Salghi, Green corrosion inhibitor: inhibitive action of tannin extract of chamaerops humilis plant for the corrosion of mild steel in 0.5 M H2SO4. J. Mater. Environ. Sci, 4, ( 2013), 127-138.
- L.Y.S. Helen, A.A. Rahim, B. Saad, M.I. Saleh, M. I., and P.B. Raja, Aquilaria crassna leaves extracts: A green corrosion inhibitor for mild steel in 1 M HCl medium. Int. J. Electrochem. Sci, 9, 2014), 830-846. [CrossRef]
- E.A. Mohsin, M.K. Husam. N.A. Rasha. Inhibition of copper corrosion in H2SO4, Nacl and NaOH solutions by citrullus colocynthis fruits extract, Journal of natural sciences research, 4, (2014), 60-73.
- P. Mourya, S. Banerjee, and M.M. Singh, Corrosion inhibition of mild steel in acidic solution by tagetes erecta (marigold flower) extract as a green inhibitor. Corrosion Science, 85, (2014), 352-363. [CrossRef]
- L.A. Nnanna1, G. Nnanna, J. Nnakaife, N. Ekekwe, and P. Eti, Aqueous extracts of pentaclethra macrophylla bentham roots as eco-friendly corrosion inhibition for mild steel in 0.5M KOH medium. 2016; 6.
- L.A. Nnanna, O.I. Owate, and E.E. Oguzie, Inhibition of mild steel corrosion in HCl solution by pentaclethra macrophylla bentham extract. International journal of materials engineering, 4, (2014), 171-179.
- N.A. Odewunmi, S.A. Umoren, and Z.M. Gasem, Utilization of watermelon rind extract as a green corrosion inhibitor for mild steel in acidic media. Journal of Industrial and Engineering Chemistry, 21, (2015). 239-247. [CrossRef]
- H.K Idu, P.A. Nwofe, P.N. Kalu, and N.E. Idenyi, Moringa oleifera and psidium guajava leaves extract as low-cost, eco-friendly inhibitors of corrosion on mild steel in an acidic media. American-Eurasian journal of scientific research, 11, (2016), 177-182.
- M.H. Hussin, A.A. Rahim, M.N.M. Ibrahim, and N. Brosse, N. The capability of ultra filtrated alkaline and organosolv oil palm (Elaeis guineensis) fronds lignin as green corrosion inhibitor for mild steel in 0.5 M HCl solution, Measurement, 78, (2016), 90-103.
- T.U. Onuegbu, E.T. Umoh, and C.N. Ehiedu, Emilia sonchifolia extract as green corrosion inhibitor for mild steel in acid medium using weight loss method, Journal of natural sciences research, 3, (2013), 52-55.
- Osita, O. Ignatius, and U.G. Lawan, Corrosion inhibition of mild steel by various plant extracts in acid media. Research journal of applied sciences, engineering and technology 10, (2015), 1197-1205.
- P.B. Raja, A.K. Qureshi, A.A. Rahim, H. Osman, and K. Awang, Neolamarckia cadamba alkaloids as eco-friendly corrosion inhibitors for mild steel in 1M HCl media. Corrosion Science, 69, (2013), 292-301. [CrossRef]
- K.M. Shainy, P.A. Rugmini, K.N. Unni, S. Benjamin, and A. Joseph, surface interaction and corrosion inhibition of mild steel in hydrochloric acid using pyoverdine, an eco-friendly bio- molecule, J Bio TriboCorros 2, (2016), 20-24. [CrossRef]
- W.D. Callister, Materials science and engineering: New York, John Wiley and Sons Inc., (2007), 579-582.
- P. Dhaundiyal, S. Bashir, V. Sharma, A. Kumar, An investigation on mitigation of corrosion of mild steel by Origanum vulgare in acidic medium, Bull. Chem. Soc. Ethiop 33, (2019), 159-168.
- D, Onukwuli, V. C. Anadebe and C. S. Okafor, Optimum prediction for inhibition efficiency of sapium ellipticum leaf extract as corrosion inhibitor of aluminum alloy (AA3003) in hydrochloric acid solution using electrochemical impedance spectroscopy and response surface methodology. Bull. Chem. Soc. Ethiop 34(1), (2020), 175-191.
- E. Alibakhshi, M. E. Alibakhshi, M. Ramezanzaeh, S. A. Haddadi, G. Bahlakeh, B. Ramezanzadeh, and M. Mahdavia. Persian liquorice extract as a highly efficient sustainable corrosion inhibitor for mild steel in sodium chloride solution, J. Clean. Prod. 210, (2019), 660-672. [CrossRef]
- Z. Sanaei, G. Bahlakeh, B. Ramezanzadeh, and M. Ramezanzadeh, Application of green molecules from chicory aqueous extract for steel corrosion mitigation against chloride ions attack, the experimental examinations and electronic / atomic level computational studies. J. Mol. Liq. 290 (2019), Article ID 111176. [CrossRef]
- Moha, B. Said, K. Younes, H. Abdelhakim, T. Saida , S. Issam , W. Ismail , G. Abdallah, B. Abdelkbir, T. Mohamed, Z. Abdelkader. Green approach to corrosion inhibition of carbon steel by fucus spiralis extract in 1 m hcl medium . Biointerface research in Applied Chemistry 12 (5), (2022), 7075 - 7091.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).