Submitted:
28 April 2023
Posted:
02 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
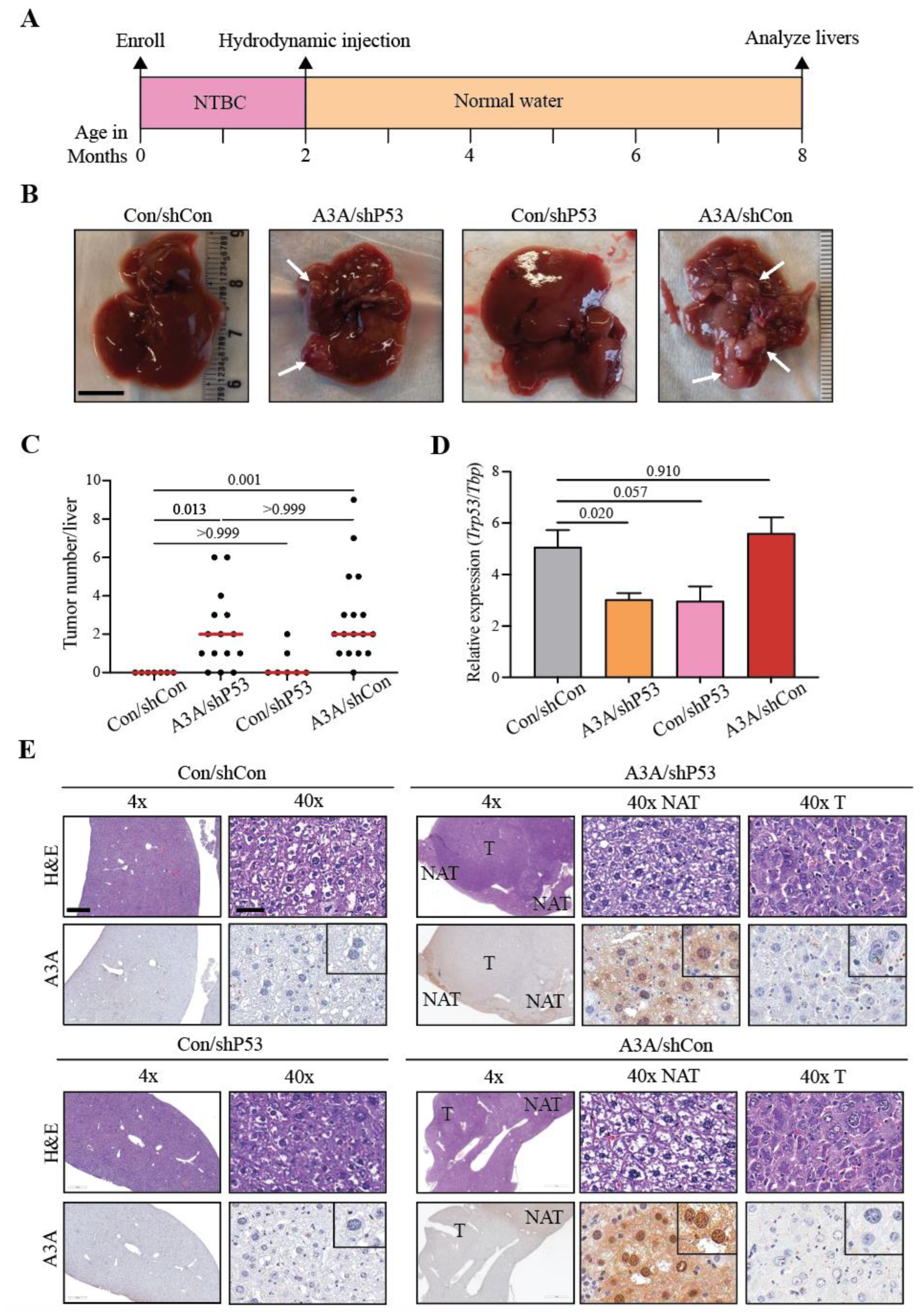
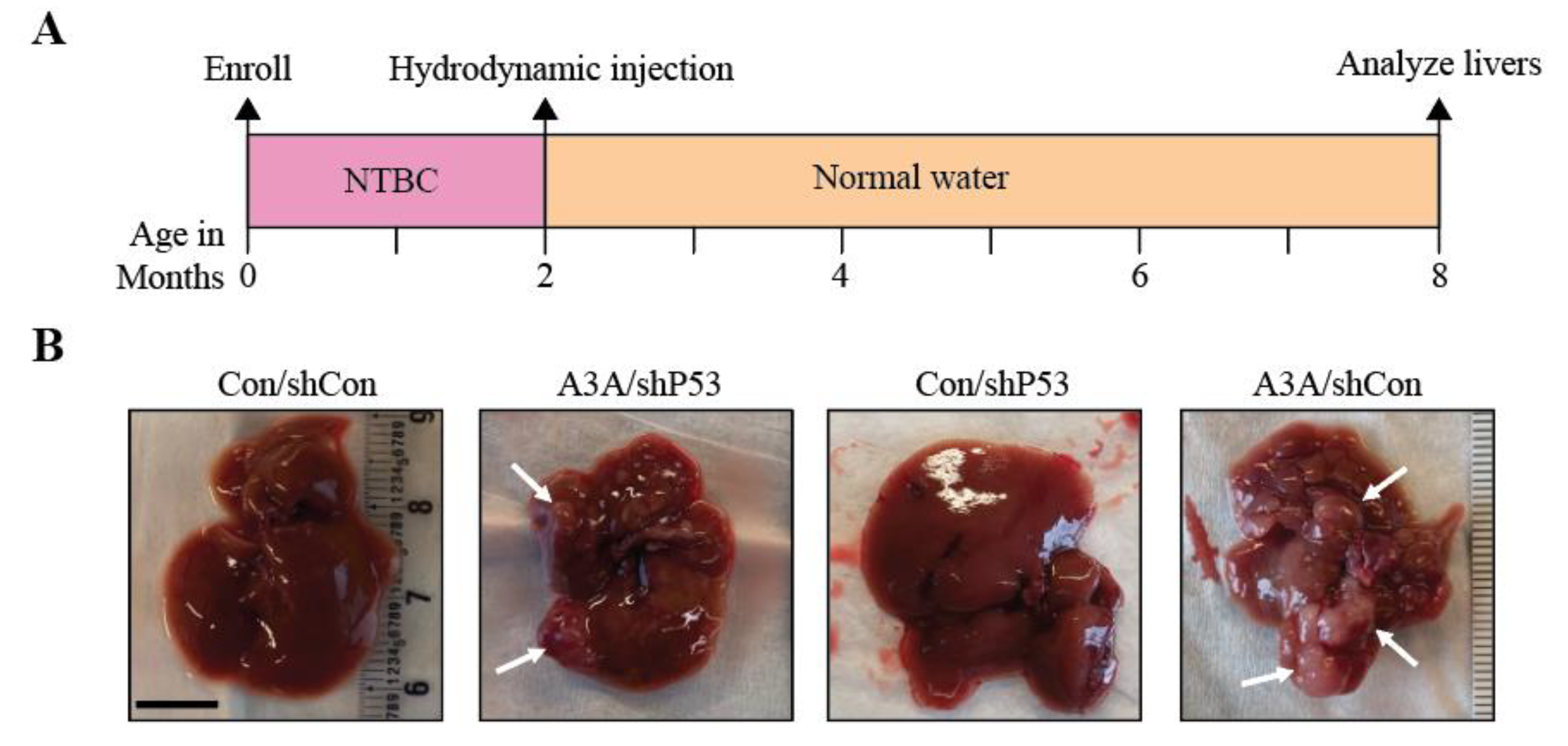
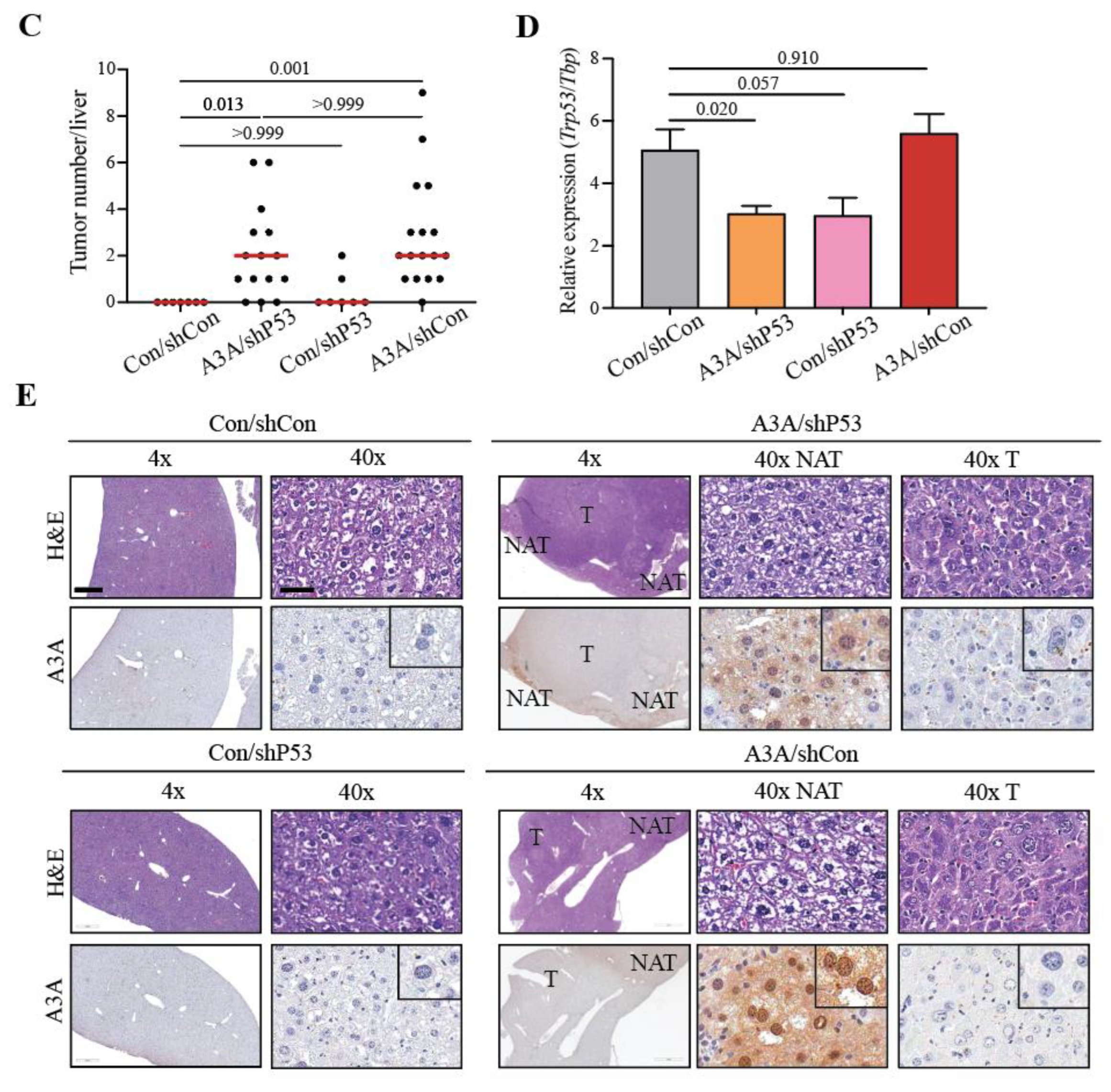
2.1. APOBEC3A alone is sufficient to promote tumorigenesis
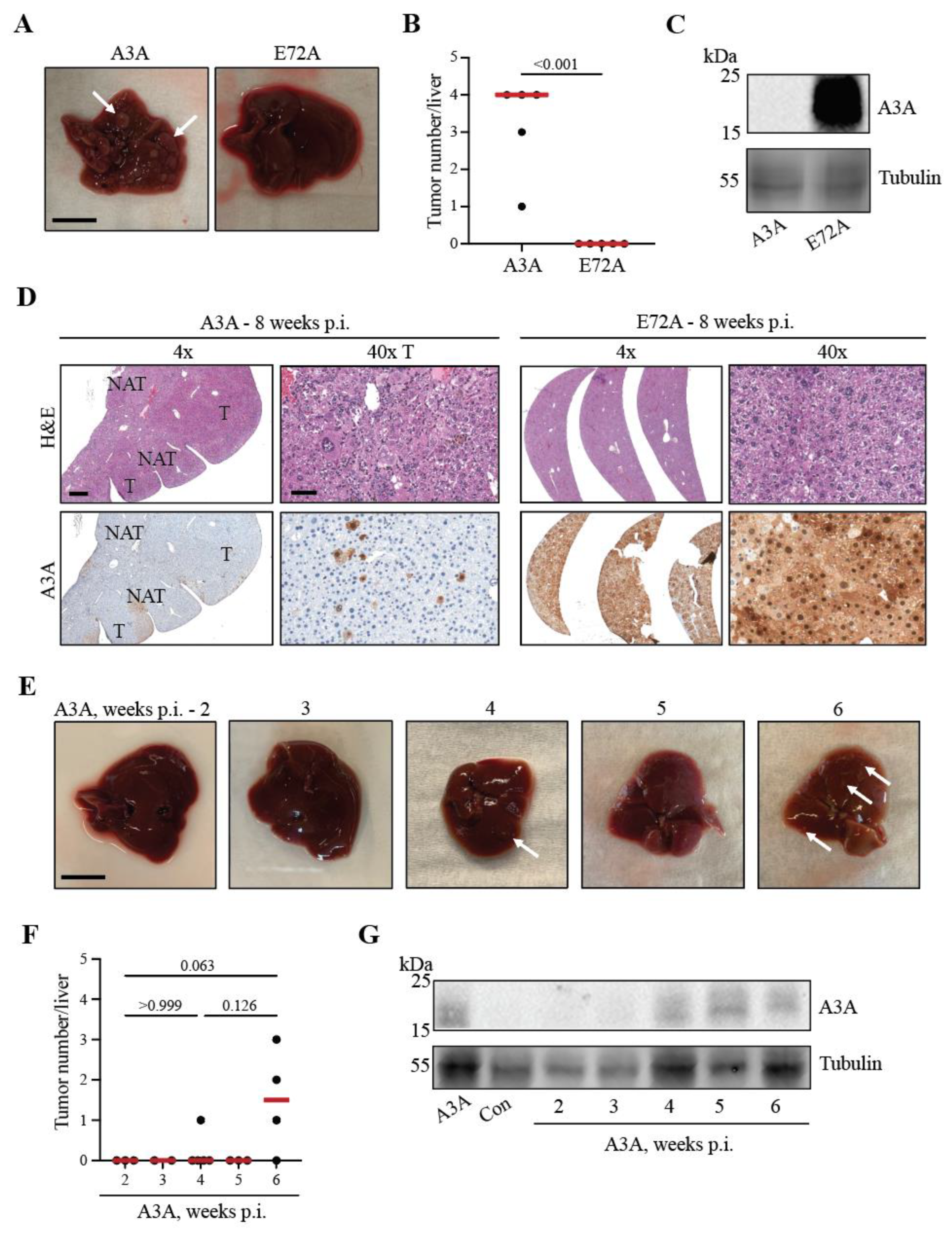
2.2. A3A catalytic activity is essential for tumor formation
2.3. Kinetics of A3A-catalyzed tumor formation
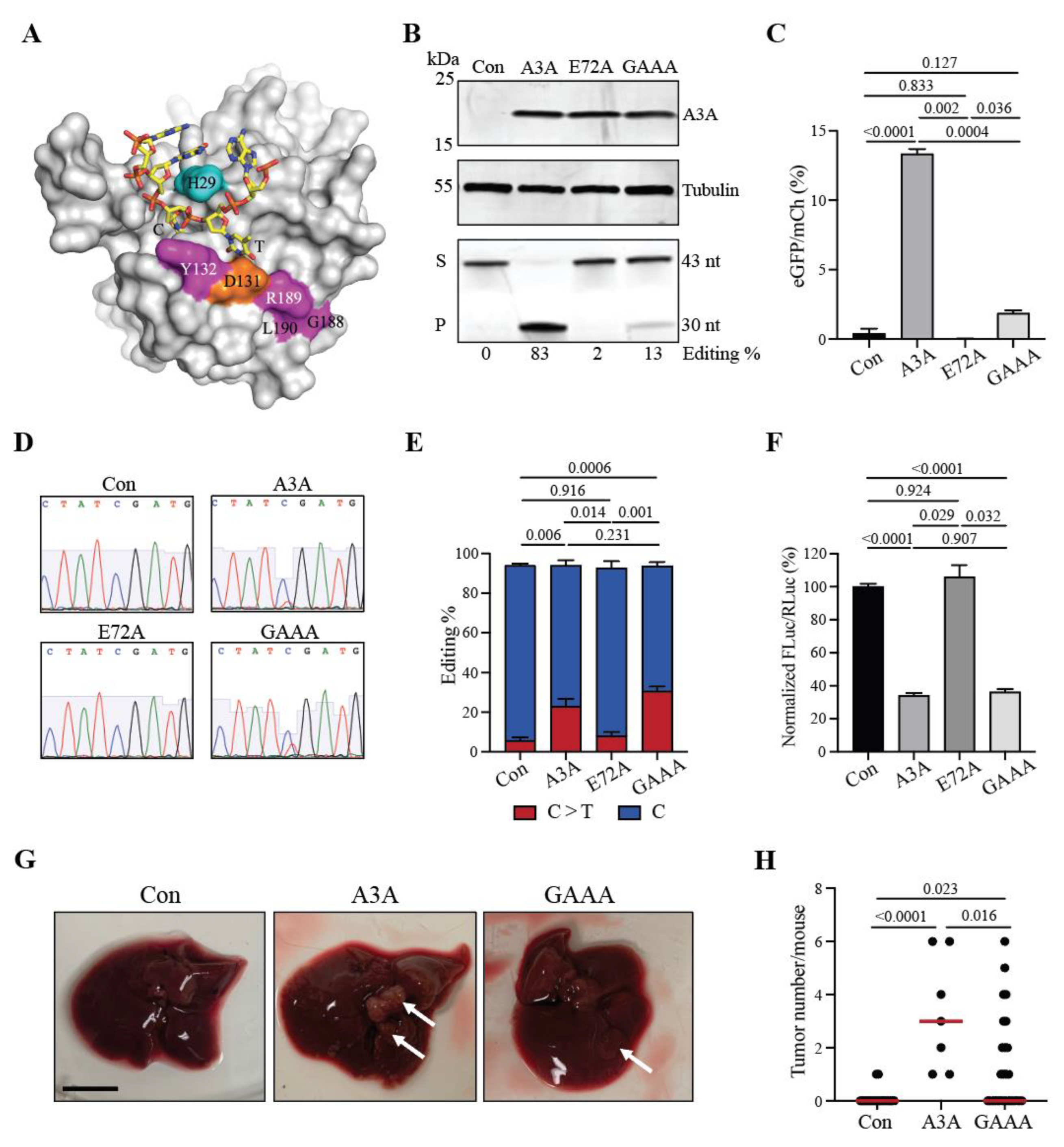
2.4. A3A-catalyzed tumorigenesis is driven by DNA editing
3. Materials and Methods
3.1. Animal care
3.2. Cell culture and reagents
3.3. Cloning
3.4. Liver regeneration experiments
3.5. Immunoblotting
3.6. Deaminase activity assays
3.7. RT-qPCR for P53 knockdown
3.8. RNA editing experiments
3.9. Histology
3.10. Immunohistochemistry
3.11. Immunofluorescence microscopy
3.12. Real-time DNA editing assays
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burns, M.B.; Lackey, L.; Carpenter, M.A.; Rathore, A.; Land, A.M.; Leonard, B.; Refsland, E.W.; Kotandeniya, D.; Tretyakova, N.; Nikas, J.B.; Yee, D.; Temiz, N.A.; Donohue, D.E.; Mcdougle, R.M.; Brown, W.L.; Law, E.K.; Harris, R.S. APOBEC3B Is an Enzymatic Source of Mutation in Breast Cancer. Nature 2013, 494. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; Boyault, S.; Burkhardt, B.; Butler, A.P.; Caldas, C.; Davies, H.R.; Desmedt, C.; Eils, R.; Eyfjörd, J.E.; Foekens, J.A.; Greaves, M.; Hosoda, F.; Hutter, B.; Ilicic, T.; Imbeaud, S.; Imielinsk, M.; Jäger, N.; Jones, D.T.W.; Jonas, D.; Knappskog, S.; Koo, M.; Lakhani, S.R.; López-Otín, C.; Martin, S.; Munshi, N.C.; Nakamura, H.; Northcott, P.A.; Pajic, M.; Papaemmanuil, E.; Paradiso, A.; Pearson, J.V.; Puente, X.S.; Raine, K.; Ramakrishna, M.; Richardson, A.L.; Richter, J.; Rosenstiel, P.; Schlesner, M.; Schumacher, T.N.; Span, P.N.; Teague, J.W.; Totoki, Y.; Tutt, A.N.J.; Valdés-Mas, R.; Van Buuren, M.M.; Van ’T Veer, L.; Vincent-Salomon, A.; Waddell, N.; Yates, L.R.; Zucman-Rossi, J.; Andrew Futreal, P.; McDermott, U.; Lichter, P.; Meyerson, M.; Grimmond, S.M.; Siebert, R.; Campo, E.; Shibata, T.; Pfister, S.M.; Campbell, P.J.; Stratton, M.R. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500. [Google Scholar] [CrossRef] [PubMed]
- Nik-Zainal, S.; Wedge, D.C.; Alexandrov, L.B.; Petljak, M.; Butler, A.P.; Bolli, N.; Davies, H.R.; Knappskog, S.; Martin, S.; Papaemmanuil, E.; Ramakrishna, M.; Shlien, A.; Simonic, I.; Xue, Y.; Tyler-Smith, C.; Campbell, P.J.; Stratton, M.R. Association of a Germline Copy Number Polymorphism of APOBEC3A and APOBEC3B with Burden of Putative APOBEC-Dependent Mutations in Breast Cancer. Nature Genetics 2014, 46. [Google Scholar] [CrossRef] [PubMed]
- Nik-Zainal, S.; Davies, H.; Staaf, J.; Ramakrishna, M.; Glodzik, D.; Zou, X.; Martincorena, I.; Alexandrov, L.B.; Martin, S.; Wedge, D.C.; Van Loo, P.; Ju, Y.S.; Smid, M.; Brinkman, A.B.; Morganella, S.; Aure, M.R.; Lingjærde, O.C.; Langerød, A.; Ringnér, M.; Ahn, S.M.; Boyault, S.; Brock, J.E.; Broeks, A.; Butler, A.; Desmedt, C.; Dirix, L.; Dronov, S.; Fatima, A.; Foekens, J.A.; Gerstung, M.; Hooijer, G.K.J.; Jang, S.J.; Jones, D.R.; Kim, H.Y.; King, T.A.; Krishnamurthy, S.; Lee, H.J.; Lee, J.Y.; Li, Y.; McLaren, S.; Menzies, A.; Mustonen, V.; O’Meara, S.; Pauporté, I.; Pivot, X.; Purdie, C.A.; Raine, K.; Ramakrishnan, K.; Rodríguez-González, F.G.; Romieu, G.; Sieuwerts, A.M.; Simpson, P.T.; Shepherd, R.; Stebbings, L.; Stefansson, O.A.; Teague, J.; Tommasi, S.; Treilleux, I.; Van Den Eynden, G.G.; Vermeulen, P.; Vincent-Salomon, A.; Yates, L.; Caldas, C.; Veer, L.V. t.; Tutt, A.; Knappskog, S.; Tan, B.K.T.; Jonkers, J.; Borg, Å.; Ueno, N.T.; Sotiriou, C.; Viari, A.; Futreal, P.A.; Campbell, P.J.; Span, P.N.; Van Laere, S.; Lakhani, S.R.; Eyfjord, J.E.; Thompson, A.M.; Birney, E.; Stunnenberg, H.G.; Van De Vijver, M.J.; Martens, J.W.M.; Børresen-Dale, A.L.; Richardson, A.L.; Kong, G.; Thomas, G.; Stratton, M.R. Landscape of Somatic Mutations in 560 Breast Cancer Whole-Genome Sequences. Nature 2016, 534. [Google Scholar] [CrossRef] [PubMed]
- Burrell, R.A.; McClelland, S.E.; Endesfelder, D.; Groth, P.; Weller, M.-C.; Shaikh, N.; Domingo, E.; Kanu, N.; Dewhurst, S.M.; Gronroos, E.; Chew, S.K.; Rowan, A.J.; Schenk, A.; Sheffer, M.; Howell, M.; Kschischo, M.; Behrens, A.; Helleday, T.; Bartek, J.; Tomlinson, I.P.; Swanton, C. Replication Stress Links Structural and Numerical Cancer Chromosomal Instability. Nature 2013, 494, 492–496. [Google Scholar] [CrossRef]
- Zeman, M.K.; Cimprich, K.A. Causes and Consequences of Replication Stress. Nat Cell Biol 2014, 16, 2–9. [Google Scholar] [CrossRef]
- Talluri, S.; Samur, M.K.; Buon, L.; Kumar, S.; Potluri, L.B.; Shi, J.; Prabhala, R.H.; Shammas, M.A.; Munshi, N.C. Dysregulated APOBEC3G Causes DNA Damage and Promotes Genomic Instability in Multiple Myeloma. Blood Cancer J. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Burns, M.B.; Lackey, L.; Carpenter, M.A.; Rathore, A.; Land, A.M.; Leonard, B.; Refsland, E.W.; Kotandeniya, D.; Tretyakova, N.; Nikas, J.B.; Yee, D.; Temiz, N.A.; Donohue, D.E.; McDougle, R.M.; Brown, W.L.; Law, E.K.; Harris, R.S. Apobec3b Is an Enzymatic Source of Mutation in Breast Cancer. Nature 2013, 494, 366–370. [Google Scholar] [CrossRef]
- Burns, M.B.; Temiz, N.A.; Harris, R.S. Evidence for APOBEC3B Mutagenesis in Multiple Human Cancers. Nat Genet 2013, 45, 977–983. [Google Scholar] [CrossRef]
- Swanton, C.; McGranahan, N.; Starrett, G.J.; Harris, R.S. APOBEC Enzymes: Mutagenic Fuel for Cancer Evolution and Heterogeneity. Cancer Discov 2015, 5, 704–712. [Google Scholar] [CrossRef]
- Roberts, S.A.; Gordenin, D.A. Hypermutation in Human Cancer Genomes: Footprints and Mechanisms. Nat Rev Cancer 2014, 14, 786–800. [Google Scholar] [CrossRef]
- Suspène, R.; Mussil, B.; Laude, H.; Caval, V.; Berry, N.; Bouzidi, M.S.; Thiers, V.; Wain-Hobson, S.; Vartanian, J.-P. Self-Cytoplasmic DNA Upregulates the Mutator Enzyme APOBEC3A Leading to Chromosomal DNA Damage. Nucleic Acids Res 2017, 45, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Starrett, G.J.; Luengas, E.M.; McCann, J.L.; Ebrahimi, D.; Temiz, N.A.; Love, R.P.; Feng, Y.; Adolph, M.B.; Chelico, L.; Law, E.K.; Carpenter, M.A.; Harris, R.S. The DNA Cytosine Deaminase APOBEC3H Haplotype I Likely Contributes to Breast and Lung Cancer Mutagenesis. Nat Commun 2016, 7, 12918. [Google Scholar] [CrossRef] [PubMed]
- Sieuwerts, A.M.; Schrijver, W.A.M.E.; Dalm, S.U.; de Weerd, V.; Moelans, C.B.; Ter Hoeve, N.; van Diest, P.J.; Martens, J.W.M.; van Deurzen, C.H.M. Progressive APOBEC3B MRNA Expression in Distant Breast Cancer Metastases. PLoS One 2017, 12, e0171343. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, E.; Yamashita, N.; Tanaka, K.; Inoue, Y.; Akiyoshi, S.; Saeki, H.; Oki, E.; Kitao, H.; Maehara, Y. Expression of APOBEC3B MRNA in Primary Breast Cancer of Japanese Women. PLOS ONE 2016, 11, e0168090. [Google Scholar] [CrossRef] [PubMed]
- Cortez, L.M.; Brown, A.L.; Dennis, M.A.; Collins, C.D.; Brown, A.J.; Mitchell, D.; Mertz, T.M.; Roberts, S.A. APOBEC3A Is a Prominent Cytidine Deaminase in Breast Cancer. PLOS Genetics 2019, 15, e1008545. [Google Scholar] [CrossRef] [PubMed]
- Buisson, R.; Lawrence, M.S.; Benes, C.H.; Zou, L. APOBEC3A and APOBEC3B Activities Render Cancer Cells Susceptible to ATR Inhibition. Cancer Research 2017, 77, 4567–4578. [Google Scholar] [CrossRef]
- Green, A.M.; Landry, S.; Budagyan, K.; Avgousti, D.C.; Shalhout, S.; Bhagwat, A.S.; Weitzman, M.D. APOBEC3A Damages the Cellular Genome during DNA Replication. Cell Cycle 2016, 15, 998–1008. [Google Scholar] [CrossRef]
- Pecori, R.; Di Giorgio, S.; Paulo Lorenzo, J.; Nina Papavasiliou, F. Functions and Consequences of AID/APOBEC-Mediated DNA and RNA Deamination. Nat Rev Genet 2022, 23, 505–518. [Google Scholar] [CrossRef]
- Koh, G.; Degasperi, A.; Zou, X.; Momen, S.; Nik-Zainal, S. Mutational Signatures: Emerging Concepts, Caveats and Clinical Applications. Nat Rev Cancer 2021, 21, 619–637. [Google Scholar] [CrossRef]
- Lackey, L.; Demorest, Z.L.; Land, A.M.; Hultquist, J.F.; Brown, W.L.; Harris, R.S. APOBEC3B and AID Have Similar Nuclear Import Mechanisms. Journal of Molecular Biology 2012, 419. [Google Scholar] [CrossRef]
- Jónsson, S.R.; Andrésdóttir, V. Host Restriction of Lentiviruses and Viral Countermeasures: APOBEC3 and Vif. Viruses 2013, 5, 1934–1947. [Google Scholar] [CrossRef]
- Stavrou, S.; Zhao, W.; Blouch, K.; Ross, S.R. Deaminase-Dead Mouse APOBEC3 Is an In Vivo Retroviral Restriction Factor. J Virol 2018, 92, e00168-18. [Google Scholar] [CrossRef]
- Boumelha, J.; de Carné Trécesson, S.; Law, E.K.; Romero-Clavijo, P.; Coelho, M.A.; Ng, K.W.; Mugarza, E.; Moore, C.; Rana, S.; Caswell, D.R.; Murillo, M.; Hancock, D.C.; Argyris, P.P.; Brown, W.L.; Durfee, C.; Larson, L.K.; Vogel, R.I.; Suárez-Bonnet, A.; Priestnall, S.L.; East, P.; Ross, S.J.; Kassiotis, G.; Molina-Arcas, M.; Swanton, C.; Harris, R.; Downward, J. An Immunogenic Model of KRAS-Mutant Lung Cancer Enables Evaluation of Targeted Therapy and Immunotherapy Combinations. Cancer Research 2022, 82, 3435–3448. [Google Scholar] [CrossRef]
- Law, E.K.; Levin-Klein, R.; Jarvis, M.C.; Kim, H.; Argyris, P.P.; Carpenter, M.A.; Starrett, G.J.; Temiz, N.A.; Larson, L.K.; Durfee, C.; Burns, M.B.; Vogel, R.I.; Stavrou, S.; Aguilera, A.N.; Wagner, S.; Largaespada, D.A.; Starr, T.K.; Ross, S.R.; Harris, R.S. APOBEC3A Catalyzes Mutation and Drives Carcinogenesis in Vivo. Journal of Experimental Medicine 2020, 217. [Google Scholar] [CrossRef]
- Durfee, C.; Temiz, N.A.; Levin-Klein, R.; Argyris, P.P.; Alsøe, L.; Carracedo, S.; Vega, A.A. de la; Proehl, J.; Holzhauer, A.M.; Seeman, Z.J.; Lin, Y.-H. T.; Vogel, R.I.; Sotillo, R.; Nilsen, H.; Harris, R.S. Human APOBEC3B Promotes Tumor Heterogeneity in Vivo Including Signature Mutations and Metastases. bioRxiv 2023. [Google Scholar] [CrossRef]
- Vega, A.A. de la; Temiz, N.A.; Tasakis, R.; Somogyi, K.; Reuveni, E.; Ben-David, U.; Stenzinger, A.; Poth, T.; Papavasiliou, N.; Harris, R.S.; Sotillo, R. Acute Expression of Human APOBEC3B in Mice Causes Lethality Associated with RNA Editing. bioRxiv 2022. [Google Scholar] [CrossRef]
- Grompe, M.; Overturf, K.; Al-Dhalimy, M.; Finegold, M. Therapeutic Trials in the Murine Model of Hereditary Tyrosinaemia Type I: A Progress Report. Journal of Inherited Metabolic Disease 1998, 21. [Google Scholar] [CrossRef]
- Wangensteen, K.J.; Wilber, A.; Keng, V.W.; Chen, Y.; Matise, I.; Wangensteen, L.; Steer, C.J.; McIvor, R.S.; Largaespada, D.A.; Wang, X.; Ekker, S.C. A Method for Lifelong Genetic Manipulation of Regenerating Hepatocytes in Mouse. Cell Research 2008, 18. [Google Scholar] [CrossRef]
- Keng, V.W.; Tschida, B.R.; Bell, J.B.; Largaespada, D.A. Modeling Hepatitis B Virus X-Induced Hepatocellular Carcinoma in Mice with the Sleeping Beauty Transposon System. Hepatology 2011, 53. [Google Scholar] [CrossRef]
- Keng, V.W.; Villanueva, A.; Chiang, D.Y.; Dupuy, A.J.; Ryan, B.J.; Matise, I.; Silverstein, K.A.T.; Sarver, A.; Starr, T.K.; Akagi, K.; Tessarollo, L.; Collier, L.S.; Powers, S.; Lowe, S.W.; Jenkins, N.A.; Copeland, N.G.; Llovet, J.M.; Largaespada, D.A. A Conditional Transposon-Based Insertional Mutagenesis Screen for Genes Associated with Mouse Hepatocellular Carcinoma. Nature Biotechnology 2009, 27. [Google Scholar] [CrossRef]
- Burns, M.B.; Temiz, N.A.; Harris, R.S. Evidence for APOBEC3B Mutagenesis in Multiple Human Cancers. Nature Genetics 2013, 45. [Google Scholar] [CrossRef]
- Harjes, S.; Kurup, H.M.; Rieffer, A.E.; Bayarjargal, M.; Filitcheva, J.; Su, Y.; Hale, T.K.; Filichev, V.V.; Harjes, E.; Harris, R.S.; Jameson, G.B. Structure-Guided Inhibition of the Cancer DNA-Mutating Enzyme APOBEC3A. bioRxiv 2023. [Google Scholar] [CrossRef]
- Sharma, S.; Patnaik, S.K.; Thomas Taggart, R.; Kannisto, E.D.; Enriquez, S.M.; Gollnick, P.; Baysal, B.E. APOBEC3A Cytidine Deaminase Induces RNA Editing in Monocytes and Macrophages. Nat Commun 2015, 6, 6881. [Google Scholar] [CrossRef]
- Jalili, P.; Bowen, D.; Langenbucher, A.; Park, S.; Aguirre, K.; Corcoran, R.B.; Fleischman, A.G.; Lawrence, M.S.; Zou, L.; Buisson, R. Quantification of Ongoing APOBEC3A Activity in Tumor Cells by Monitoring RNA Editing at Hotspots. Nat Commun 2020, 11, 2971. [Google Scholar] [CrossRef]
- Tang, G.; Xie, B.; Hong, X.; Qin, H.; Wang, J.; Huang, H.; Hao, P.; Li, X. Creating RNA Specific C-to-U Editase from APOBEC3A by Separation of Its Activities on DNA and RNA Substrates. ACS Synth. Biol. 2021, 10, 1106–1115. [Google Scholar] [CrossRef]
- Wangensteen, K.J.; Wilber, A.; Keng, V.W.; He, Z.; Matise, I.; Wangensteen, L.; Carson, C.M.; Chen, Y.; Steer, C.J.; McIvor, R.S.; Largaespada, D.A.; Wang, X.; Ekker, S.C. A Facile Method for Somatic, Lifelong Manipulation of Multiple Genes in the Mouse Liver. Hepatology 2008, 47, 1714–1724. [Google Scholar] [CrossRef]
- Keng, V.W.; Villanueva, A.; Chiang, D.Y.; Dupuy, A.J.; Ryan, B.J.; Matise, I.; Silverstein, K.A.T.; Sarver, A.; Starr, T.K.; Akagi, K.; Tessarollo, L.; Collier, L.S.; Powers, S.; Lowe, S.W.; Jenkins, N.A.; Copeland, N.G.; Llovet, J.M.; Largaespada, D.A. A Conditional Transposon-Based Insertional Mutagenesis Screen for Genes Associated with Mouse Hepatocellular Carcinoma. Nat Biotechnol 2009, 27, 264–274. [Google Scholar] [CrossRef]
- Brown, W.L.; Law, E.K.; Argyris, P.P.; Carpenter, M.A.; Levin-Klein, R.; Ranum, A.N.; Molan, A.M.; Forster, C.L.; Anderson, B.D.; Lackey, L.; Harris, R.S. A Rabbit Monoclonal Antibody against the Antiviral and Cancer Genomic DNA Mutating Enzyme APOBEC3B. Antibodies (Basel) 2019, 8, 47. [Google Scholar] [CrossRef]
- Kluesner, M.G.; Nedveck, D.A.; Lahr, W.S.; Garbe, J.R.; Abrahante, J.E.; Webber, B.R.; Moriarity, B.S. EditR: A Method to Quantify Base Editing from Sanger Sequencing. The CRISPR Journal 2018, 1, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Argyris, P.P.; Naumann, J.; Jarvis, M.C.; Wilkinson, P.E.; Ho, D.P.; Islam, M.N.; Bhattacharyya, I.; Gopalakrishnan, R.; Li, F.; Koutlas, I.G.; Giubellino, A.; Harris, R.S. Primary Mucosal Melanomas of the Head and Neck Are Characterised by Overexpression of the DNA Mutating Enzyme APOBEC3B. Histopathology 2023, 82, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Argyris, P.P.; Wilkinson, P.E.; Jarvis, M.C.; Magliocca, K.R.; Patel, M.R.; Vogel, R.I.; Gopalakrishnan, R.; Koutlas, I.G.; Harris, R.S. Endogenous APOBEC3B Overexpression Characterizes HPV-Positive and HPV-Negative Oral Epithelial Dysplasias and Head and Neck Cancers. Mod Pathol 2021, 34, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Serebrenik, A.A.; Argyris, P.P.; Jarvis, M.C.; Brown, W.L.; Bazzaro, M.; Vogel, R.I.; Erickson, B.K.; Lee, S.-H.; Goergen, K.M.; Maurer, M.J.; Heinzen, E.P.; Oberg, A.L.; Huang, Y.; Hou, X.; Weroha, S.J.; Kaufmann, S.H.; Harris, R.S. The DNA Cytosine Deaminase APOBEC3B Is a Molecular Determinant of Platinum Responsiveness in Clear Cell Ovarian Cancer. Clin Cancer Res 2020, 26, 3397–3407. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.C.; Carpenter, M.A.; Temiz, N.A.; Brown, M.R.; Richards, K.A.; Argyris, P.P.; Brown, W.L.; Yee, D.; Harris, R.S. Mutational Impact of APOBEC3B and APOBEC3A in a Human Cell Line. bioRxiv 2022. [Google Scholar] [CrossRef]
- St Martin, A.; Salamango, D.; Serebrenik, A.; Shaban, N.; Brown, W.L.; Donati, F.; Munagala, U.; Conticello, S.G.; Harris, R.S. A Fluorescent Reporter for Quantification and Enrichment of DNA Editing by APOBEC–Cas9 or Cleavage by Cas9 in Living Cells. Nucleic Acids Research 2018, 46, e84. [CrossRef] [PubMed]
- St Martin, A.; Salamango, D.J.; Serebrenik, A.A.; Shaban, N.M.; Brown, W.L.; Harris, R.S. A Panel of EGFP Reporters for Single Base Editing by APOBEC-Cas9 Editosome Complexes. Sci Rep 2019, 9, 497. [CrossRef]
- Jiang, Q.; Crews, L.A.; Holm, F.; Jamieson, C.H.M. RNA Editing-Dependent Epitranscriptome Diversity in Cancer Stem Cells. Nat Rev Cancer 2017, 17, 381–392. [Google Scholar] [CrossRef]
- Kurkowiak, M.; Arcimowicz, Ł.; Chruściel, E.; Urban-Wójciuk, Z.; Papak, I.; Keegan, L.; O’Connell, M.; Kowalski, J.; Hupp, T.; Marek-Trzonkowska, N. The Effects of RNA Editing in Cancer Tissue at Different Stages in Carcinogenesis. RNA Biol 2021, 18, 1524–1539. [Google Scholar] [CrossRef]
- Eisenberg, E.; Levanon, E.Y. A-to-I RNA Editing — Immune Protector and Transcriptome Diversifier. Nat Rev Genet 2018, 19, 473–490. [Google Scholar] [CrossRef]
- Anadón, C.; Guil, S.; Simó-Riudalbas, L.; Moutinho, C.; Setien, F.; Martínez-Cardús, A.; Moran, S.; Villanueva, A.; Calaf, M.; Vidal, A.; Lazo, P.A.; Zondervan, I.; Savola, S.; Kohno, T.; Yokota, J.; Ribas de Pouplana, L.; Esteller, M. Gene Amplification-Associated Overexpression of the RNA Editing Enzyme ADAR1 Enhances Human Lung Tumorigenesis. Oncogene 2016, 35, 4407–4413. [Google Scholar] [CrossRef]
- Teoh, P.J.; An, O.; Chung, T.-H.; Chooi, J.Y.; Toh, S.H.M.; Fan, S.; Wang, W.; Koh, B.T.H.; Fullwood, M.J.; Ooi, M.G.; de Mel, S.; Soekojo, C.Y.; Chen, L.; Ng, S.B.; Yang, H.; Chng, W.J. Aberrant Hyperediting of the Myeloma Transcriptome by ADAR1 Confers Oncogenicity and Is a Marker of Poor Prognosis. Blood 2018, 132, 1304–1317. [Google Scholar] [CrossRef]
- Lazzari, E.; Mondala, P.K.; Santos, N.D.; Miller, A.C.; Pineda, G.; Jiang, Q.; Leu, H.; Ali, S.A.; Ganesan, A.-P.; Wu, C.N.; Costello, C.; Minden, M.; Chiaramonte, R.; Stewart, A.K.; Crews, L.A.; Jamieson, C.H.M. Alu-Dependent RNA Editing of GLI1 Promotes Malignant Regeneration in Multiple Myeloma. Nat Commun 2017, 8, 1922. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Anant, S.; Lee, R.M.; Kennedy, S.; Viskochil, D.; Davidson, N.O. C→U Editing of Neurofibromatosis 1 MRNA Occurs in Tumors That Express Both the Type II Transcript and Apobec-1, the Catalytic Subunit of the Apolipoprotein B MRNA–Editing Enzyme. The American Journal of Human Genetics 2002, 70, 38–50. [Google Scholar] [CrossRef]
- Cappione, A.J.; French, B.L.; Skuse, G.R. A Potential Role for NF1 MRNA Editing in the Pathogenesis of NF1 Tumors. Am J Hum Genet 1997, 60, 305–312. [Google Scholar]
- Skuse, G.R.; Cappione, A.J.; Sowden, M.; Metheny, L.J.; Smith, H.C. The Neurofibromatosis Type I Messenger RNA Undergoes Base-Modification RNA Editing. Nucleic Acids Research 1996, 24, 478–486. [Google Scholar] [CrossRef]
- Yamanaka, S.; Balestra, M.E.; Ferrell, L.D.; Fan, J.; Arnold, K.S.; Taylor, S.; Taylor, J.M.; Innerarity, T.L. Apolipoprotein B MRNA-Editing Protein Induces Hepatocellular Carcinoma and Dysplasia in Transgenic Animals. Proceedings of the National Academy of Sciences of the United States of America 1995, 92, 8483. [Google Scholar] [CrossRef]
- Harris, R.S.; Petersen-Mahrt, S.K.; Neuberger, M.S. RNA Editing Enzyme APOBEC1 and Some of Its Homologs Can Act as DNA Mutators. Molecular Cell 2002, 10, 1247–1253. [Google Scholar] [CrossRef]
- Ikeda, T.; Ong, E.B.B.; Watanabe, N.; Sakaguchi, N.; Maeda, K.; Koito, A. Creation of Chimeric Human/Rabbit APOBEC1 with HIV-1 Restriction and DNA Mutation Activities. Sci Rep 2016, 6, 19035. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Shimoda, M.; Ebrahimi, D.; VandeBerg, J.L.; Harris, R.S.; Koito, A.; Maeda, K. Opossum APOBEC1 Is a DNA Mutator with Retrovirus and Retroelement Restriction Activity. Sci Rep 2017, 7, 46719. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




