Submitted:
01 May 2023
Posted:
02 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Uveal Melanoma
3.2. Clinical Presentation – Diagnosis
3.3. Treatment
3.4. Ocular Complications after Radiation Therapy
3.4.1. Ocular surface & Ocular adnexa complications
3.4.2. Sclera
3.4.3. Lens and cataract
3.4.4. Radiation retinopathy
3.4.5. Radiation maculopathy
3.4.6. Retinal detachment
3.4.7. Vitreous hemorrhage:
3.4.8. Choroid
3.4.9. Optic neuropathy
3.4.10. Tumor related lipid exudation
3.4.11. Ocular inflammation
3.4.12. Iris neovascularization – rubeosis iridis
3.4.13. Secondary Glaucoma - Neovascular Glaucoma
3.4.14. Toxic tumor syndrome
3.4.15. Diplopia and strabismus
3.4.16. Sympathetic ophthalmia
3.4.17. Visual acuity
3.4.18. Enucleation due to complications
3.4.19. Recurrences
3.5. Quality of life
4. Conclusion
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Damato, B. Legacy of the Collaborative Ocular Melanoma Study. Arch Ophthalmol 2007, 125, 966–968. [Google Scholar] [CrossRef]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; Caltabiano, R.; Broggi, G.; Puzzo, L.; Russo, A.; Reibaldi, M.; Longo, A.; Vigneri, P.; Avitabile, T.; Ettorre, G.C.; Basile, A. Diagnostic Methods and Therapeutic Options of Uveal Melanoma with Emphasis on MR Imaging—Part II: Treatment Indications and Complications. Insights Imaging 2021, 12. [Google Scholar] [CrossRef]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; Damato, B.E. Uveal Melanoma. Nat Rev Dis Primers 2020, 6. [Google Scholar] [CrossRef]
- Kamrava, M.; Lamb, J.; Soberón, V.; McCannel, T.A. Ocular Complications of Radiotherapy. In Clinical Ophthalmic Oncology; Springer International Publishing, 2019; pp. 117–128. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Piulats, J.M.; Goebeler, M.; Galloway, I.; Lugowska, I.; Becker, J.C.; Vihinen, P.; Van Calster, J.; Hadjistilianou, T.; Proença, R.; Caminal, J.M.; Rogasik, M.; Blay, J.Y.; Kapiteijn, E. Uveal Melanoma: A European Network to Face the Many Challenges of a Rare Cancer. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Garg, G.; Kivelä, T.; Finger, P. Patients Presenting with Stage IV Uveal Melanoma: Lessons Learned. Indian J Ophthalmol 2022, 70, 271–274. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L.; Shields, J.A. Uveal Melanoma: Estimating Prognosis. Indian J Ophthalmol 2015, 63, 93–102. [Google Scholar] [CrossRef]
- Naseripour, M.; Azimi, F.; Mirshahi, R.; Khakpour, G.; Pourhoseingholi, A.; Chaibakhsh, S. Global Incidence and Trend of Uveal Melanoma from 1943-2015: A Meta-Analysis. Asian Pacific Journal of Cancer Prevention 2022, 23, 1791–1801. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal Melanoma: Relatively Rare but Deadly Cancer. Eye (Basingstoke) 2017, 31, 241–257. [Google Scholar] [CrossRef]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of Uveal Melanoma: Where Are We Now? Ther Adv Med Oncol 2018, 10. [Google Scholar] [CrossRef]
- Rodrigues, M.; de Koning, L.; Coupland, S.E.; Jochemsen, A.G.; Marais, R.; Stern, M.H.; Valente, A.; Barnhill, R.; Cassoux, N.; Evans, A.; Galloway, I.; Jager, M.J.; Kapiteijn, E.; Romanowska-Dixon, B.; Ryll, B.; Roman-Roman, S.; Piperno-Neumann, S. So Close, yet so Far: Discrepancies between Uveal and Other Melanomas. a Position Paper from UM Cure 2020. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A. V.; Wakade, O.A.; Mehta, S.; Forte, L.; Long, A.; Dellacava, E.F.; Kaplan, B.; Shields, J.A. Metastasis of Uveal Melanoma Millimeter-by-Millimeter in 8033 Consecutive Eyes. Arch Ophthalmol 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; Patel, S.P. Uveal Melanoma: From Diagnosis to Treatment and the Science in Between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Raffaele, L.; Salamone, V.; Caltabiano, R.; Broggi, G.; Puzzo, L.; Russo, A.; Reibaldi, M.; Longo, A.; Vigneri, P.; Avitabile, T.; Ettorre, G.C.; Basile, A. Diagnostic Methods and Therapeutic Options of Uveal Melanoma with Emphasis on MR Imaging—Part I: MR Imaging with Pathologic Correlation and Technical Considerations. Insights Imaging 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W. The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma V. Twelve-Year Mortality Rates and Prognostic Factors: COMS Report No. 28. Arch Ophthalmol 2006, 124, 1684–1693. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int J Med Sci 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, D.A.; Brock, A.L. Radiation Therapy for Uveal Melanoma: A Review of Treatment Methods Available in 2021. Curr Opin Ophthalmol 2021, 32, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Rusňák, Š.; Hecová, L.; Kasl, Z.; Sobotová, M.; Hauer, L. Therapy of Uveal Melanoma. A Review. Czech and Slovak Ophthalmology 2021, 77, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.E.; Grewal, D.S.; Mruthyunjaya, P. Conjunctival Dehiscence and Scleral Necrosis Following Iodine-125 Plaque Brachytherapy for Uveal Melanoma: A Report of 3 Cases. Ocul Oncol Pathol 2018, 4, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Bernabei, F.; Angi, M.; Pellegrini, M.; Maestri, A.; Romano, V.; Scorcia, V.; Rothschild, P.-R. Iatrogenic Ocular Surface Diseases Occurring during and/or after Different Treatments for Ocular Tumours. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Versura, P.; Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Campos, E.C. Neurotrophic Keratitis: Current Challenges and Future Prospects. Eye Brain 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L.; Rojanaporn, D.; Badal, J.; Devisetty, L.; Emrich, J.; Komarnicky, L.; Shields, J.A. Scleral Necrosis after Plaque Radiotherapy of Uveal Melanoma: A Case-Control Study. Ophthalmology 2013, 120, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Radin, P.P.; Lumbroso-Le Rouic, L.; Levy-Gabriel, C.; Dendale, R.; Sastre, X.; Desjardins, L. Scleral Necrosis after Radiation Therapy for Uveal Melanomas: Report of 23 Cases. Graefe’s Archive for Clinical and Experimental Ophthalmology 2008, 246, 1731–1736. [Google Scholar] [CrossRef]
- Gündüz, K. Plaque Radiotherapy of Uveal Melanoma With Predominant Ciliary Body Involvement. Archives of Ophthalmology 1999, 117. [Google Scholar] [CrossRef]
- Shields, C.L.; Naseripour, M.; Cater, J.; Shields, J.A.; Demirci, H.; Youseff, A.; Freire, J. Plaque Radiotherapy for Large Posterior Uveal Melanomas (≥8-Mm Thick) in 354 Consecutive Patients 11Presented in Part at the Annual Meeting of the American Academy of Ophthalmology, October 2002. Ophthalmology 2002, 109, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, Z.M. Early-Onset Scleral Necrosis After Iodine I 125 Plaque Radiotherapy for Ciliochoroidal Melanoma. Archives of Ophthalmology 1999, 117. [Google Scholar] [CrossRef]
- Chaudhry, I.A.; Liu, M.; Shamsi, F.A.; Arat, Y.O.; Shetlar, D.J.; Boniuk, M. Corneoscleral Necrosis after Episcleral Au-198 Brachytherapy of Uveal Melanoma. Retina 2009, 29, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, A.P. Severe Corneoscleral Infection. Archives of Ophthalmology 1993, 111. [Google Scholar] [CrossRef]
- Caminal Mitjana, J.M.; Quintana Casany, M.; Pera Fábregas, J.; Cinos Cope, C.; Guedea, F. Results of Iodine-125 Radiotherapy in the Treatment of Uveal Melanoma. Arch Soc Esp Oftalmol 2002, 77, 29–37. [Google Scholar]
- Passarin, O.; Zografos, L.; Schalenbourg, A.; Moulin, A.; Guex-Crosier, Y. Scleritis after Proton Therapy in Uveal Melanoma. Klin Monbl Augenheilkd 2012, 229, 395–398. [Google Scholar] [CrossRef]
- Jager, M.J.; Desjardins, L.; Kivelä, T.; Damato, B.E. Treatment of Uveal Melanoma by Accelerated Proton Beam. Dev Ophthalmol. Basel, Karger 2012, 49, 41–57. [Google Scholar] [CrossRef]
- Peddada, K.V.; Sangani, R.; Menon, H.; Verma, V. Complications and Adverse Events of Plaque Brachytherapy for Ocular Melanoma. J Contemp Brachytherapy 2019, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Böker, A.; Pilger, D.; Cordini, D.; Seibel, I.; Riechardt, A.I.; Joussen, A.M.; Bechrakis, N.E. Neoadjuvant Proton Beam Irradiation vs. Adjuvant Ruthenium Brachytherapy in Transscleral Resection of Uveal Melanoma. Graefe’s Archive for Clinical and Experimental Ophthalmology 2018, 256, 1767–1775. [Google Scholar] [CrossRef]
- Gündüz, K.; Shields, C.L.; Shields, J.A.; Cater, J.; Freire, J.E.; Brady, L.W. Radiation Complications and Tumor Control after Plaque Radiotherapy of Choroidal Melanoma with Macular Involvement. Am J Ophthalmol 1999, 127, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Espensen, C.A.; Kiilgaard, J.F.; Appelt, A.L.; Fog, L.S.; Herault, J.; Maschi, C.; Caujolle, J.P.; Thariat, J. Dose-Response and Normal Tissue Complication Probabilities after Proton Therapy for Choroidal Melanoma. Ophthalmology 2021, 128, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.K.; Ranjan, R.; Tyagi, M.; Agrawal, H.; Reddy, S. Radiation Retinopathy: Detection and Management Strategies. Clinical Ophthalmology 2021, 15, 3797–3809. [Google Scholar] [CrossRef]
- McCannel, T.A.; Kim, E.; Kamrava, M.; Lamb, J.; Caprioli, J.; Yang, D.; McCannel, C.A. New Ultra–Wide-Field Angiographic Grading Scheme for Radiation Retinopathy after Iodine-125 Brachytherapy for Uveal Melanoma. Retina 2018, 38, 2415–2421. [Google Scholar] [CrossRef]
- Horgan, N.; Shields, C.L.; Mashayekhi, A.; Teixeira, L.F.; Materin, M.A.; Shields, J.A. Early Macular Morphological Changes Following Plaque Radiotherapy for Uveal Melanoma. Retina 2008, 28, 263–273. [Google Scholar] [CrossRef]
- Srivastava, O.; Weis, E. Outcomes of Second-Line Intravitreal Anti-VEGF Switch Therapy in Radiation Retinopathy Secondary to Uveal Melanoma: Moving from Bevacizumab to Aflibercept. Ocul Oncol Pathol 2022, 8, (4–6). [Google Scholar] [CrossRef]
- Miguel, D.; De Frutos-Baraja, J.M.; López-Lara, F.; Saornil, M.A.; García-Álvarez, C.; Alonso, P.; Diezhandino, P. Radiobiological Doses, Tumor, and Treatment Features Influence on Outcomes after Epiescleral Brachytherapy. A 20-Year Retrospective Analysis from a Single-Institution: Part II. J Contemp Brachytherapy 2018, 10, 347–359. [Google Scholar] [CrossRef]
- Beykin, G.; Pe’er, J.; Hemo, Y.; Frenkel, S.; Chowers, I. Pars Plana Vitrectomy to Repair Retinal Detachment Following Brachytherapy for Uveal Melanoma. British Journal of Ophthalmology 2013, 97, 1534–1537. [Google Scholar] [CrossRef]
- Chia, S.N.; Smith, H.B.; Hammer, H.M.; Kemp, E.G. Incidence and Indications for Pars Plana Vitrectomy Following the Treatment of Posterior Uveal Melanomas in Scotland. Eye 2015, 29, 748–756. [Google Scholar] [CrossRef]
- Gibran, S.K.; Kapoor, K.G. Management of Exudative Retinal Detachment in Choroidal Melanoma. Clin Exp Ophthalmol 2009, 37, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.; Samuel Houston, S.; Shah, N.; Decatur, C.; Lonngi, M; Feuer, W. Markoe. Intravitreal Bevacizumab Combined with Plaque Brachytherapy Reduces Melanoma Tumor Volume and Enhances Resolution of Exudative Detachment. Clinical Ophthalmology 2013, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Lumbroso, L.; Desjardins, L.; Levy, C.; Plancher, C.; Frau, E.; D’hermies, F.; Schlienger, P.; Mammar, H.; Delacroix, S.; Nauraye, C.; Ferrand, R.; Desblancs, C.; Mazal, A.; Asselain, B. Intraocular Inflammation after Proton Beam Irradiation for Uveal Melanoma. Br J Ophthalmol 2001, 85, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.R.; Gittos, A.; Richter, M.; Hungerford, J.L.; Errington, R.D.; Cree, I.A. Proton Beam Therapy and Iris Neovascularisation in Uveal Melanoma. Eye 2006, 20, 832–836. [Google Scholar] [CrossRef]
- Detorakis, E.T.; Engstrom, R.E.; Wallace, R.; Straatsma, B.R. Iris and Anterior Chamber Angle Neovascularization after Iodine 125 Brachytherapy for Uveal Melanoma. Ophthalmology 2005, 112, 505–510. [Google Scholar] [CrossRef]
- Mantel, I.; Schalenbourg, A.; Bergin, C.; Petrovic, A.; Weber, D.C.; Zografos, L. Prophylactic Use of Bevacizumab to Avoid Anterior Segment Neovascularization Following Proton Therapy for Uveal Melanoma. Am J Ophthalmol 2014, 158, 693–701e2. [Google Scholar] [CrossRef]
- Mazzini, C.; Pieretti, G.; Vicini, G.; Nicolosi, C.; Scoccianti, S.; Pertici, M.; Greto, D.; Desideri, I.; Bordi, L.; Pecchioli, G.; Virgili, G. Clinical Outcomes and Secondary Glaucoma after Gamma-Knife Radiosurgery and Ruthenium-106 Brachytherapy for Uveal Melanoma: A Single Institution Experience. Melanoma Res 2021, 31, 38–48. [Google Scholar] [CrossRef]
- Zahorjanová, P.; Sekáč, J.; Babál, P.; Štubňa, M. Enucleation after Stereotactic Radiosurgery in Patients with Uveal Melanoma. Cesk Slov Oftalmol 2020, 76, 46–51. [Google Scholar] [CrossRef]
- Siedlecki, J.; Reiterer, V.; Leicht, S.; Foerster, P.; Kortüm, K.; Schaller, U.; Priglinger, S.; Fuerweger, C.; Muacevic, A.; Eibl-Lindner, K. Incidence of Secondary Glaucoma after Treatment of Uveal Melanoma with Robotic Radiosurgery versus Brachytherapy. Acta Ophthalmol 2017, 95, e734–e739. [Google Scholar] [CrossRef]
- Sharkawi, E.; Oleszczuk, J.D.; Bergin, C.; Zografos, L. Baerveldt Shunts in the Treatment of Glaucoma Secondary to Anterior Uveal Melanoma and Proton Beam Radiotherapy. British Journal of Ophthalmology 2012, 96, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, L.M.; Somani, S.; Altomare, F.; Simpson, E.R. Intracameral Bevacizumab in the Treatment of Neovascular Glaucoma and Exudative Retinal Detachment after Brachytherapy in Choroidal Melanoma. Canadian Journal of Ophthalmology 2009, 44, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, C.; Konstantinidis, L.; Damato, B. Effects of Radiotherapy on Uveal Melanomas and Adjacent Tissues. Eye (Basingstoke) 2013, 27, 163–171. [Google Scholar] [CrossRef]
- Mahdjoubi, A.; Najean, M.; Lemaitre, S.; Dureau, S.; Dendale, R.; Levy, C.; Rouic, L.L. Le; Desjardins, L.; Cassoux, N. Intravitreal Bevacizumab for Neovascular Glaucoma in Uveal Melanoma Treated by Proton Beam Therapy. Graefes Arch Clin Exp Ophthalmol 2018, 256, 411–420. [Google Scholar] [CrossRef]
- Dunavoelgyi, R.; Zehetmayer, M.; Gleiss, A.; Geitzenauer, W.; Kircher, K.; Georg, D.; Schmidt-Erfurth, U.; Poetter, R.; Dieckmann, K. Hypofractionated Stereotactic Photon Radiotherapy of Posteriorly Located Choroidal Melanoma with Five Fractions at Ten Gy - Clinical Results after Six Years of Experience. Radiotherapy and Oncology 2013, 108, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Cassoux, N.; Cayette, S.; Plancher, C.; Lumbroso-Le Rouic, L.; Levy-Gabriel, C.; Asselain, B.; Sastre, X.; Couturier, J.; Arrufat, S.; Piperno-Neumann, S.; Dendale, R.; Lehoang, P.; Desjardins, L. Does Endoresection Prevent Neovascular Glaucoma in Patient Treated with Proton Beam Irradiation? Retina 2013, 33, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Seibel, I.; Riechardt, A.I.; Heufelder, J.; Cordini, D.; Joussen, A.M. Adjuvant Ab Interno Tumor Treatment After Proton Beam Irradiation. Am J Ophthalmol 2017, 178, 94–100. [Google Scholar] [CrossRef]
- Gündüz, A.K.; Mirzayev, I. Surgical Approach in Intraocular Tumors. Turk J Ophthalmol 2022, 52, 125–138. [Google Scholar] [CrossRef]
- Romano, M.R.; Catania, F.; Confalonieri, F.; Zollet, P.; Allegrini, D.; Sergenti, J.; Lanza, F.B.; Ferrara, M.; Angi, M. Vitreoretinal Surgery in the Prevention and Treatment of Toxic Tumour Syndrome in Uveal Melanoma: A Systematic Review. Int J Mol Sci 2021, 22, 10066. [Google Scholar] [CrossRef]
- Konstantinidis, L.; Groenewald, C.; Coupland, S.E.; Damato, B. Trans-Scleral Local Resection of Toxic Choroidal Melanoma after Proton Beam Radiotherapy. British Journal of Ophthalmology 2014, 98, 775–779. [Google Scholar] [CrossRef]
- Bensoussan, E.; Thariat, J.; Maschi, C.; Delas, J.; Schouver, E.D.; Hérault, J.; Baillif, S.; Caujolle, J.P. Outcomes after Proton Beam Therapy for Large Choroidal Melanomas in 492 Patients. Am J Ophthalmol 2016, 165, 78–87. [Google Scholar] [CrossRef]
- Damato, B.; Kacperek, A.; Errington, D.; Heimann, H. Proton Beam Radiotherapy of Uveal Melanoma. Saudi Journal of Ophthalmology 2013, 27, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, A.; Tuncer, S.; Shields, C.L.; Shields, J.A. Tumor-Related Lipid Exudation and Associated Tumor-Related Complications after Plaque Radiotherapy of Posterior Uveal Melanoma. Eur J Ophthalmol 2013, 23, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, A.; Tuncer, S.; Shields, C.L.; Shields, J.A. Tumor-Related Lipid Exudation after Plaque Radiotherapy of Choroidal Melanoma: The Role of Bruch’s Membrane Rupture. Ophthalmology 2010, 117, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Abri Aghdam, K.; Soltan Sanjari, M.; Naseripour, M.; Manafi, N.; Sedaghat, A.; Bakhti, S. The Impacts of Episcleral Plaque Brachytherapy on Ocular Motility. J Binocul Vis Ocul Motil 2021, 71, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Dawson, E.; Sagoo, M.S.; Mehta, J.S.; Comer, R.; Hungerford, J.; Lee, J. Strabismus in Adults with Uveal Melanoma Following Episcleral Plaque Brachytherapy. J AAPOS 2007, 11, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Sener, E.C.; Kiratli, H.; Gedik, S.; Sanac, A.S. Ocular Motility Disturbances after Episcleral Plaque Brachytherapy for Uveal Melanoma. J AAPOS 2004, 8, 38–45. [Google Scholar] [CrossRef]
- Nagendran, S.T.; Finger, P.T.; Campolattaro, B.N. Extraocular Muscle Repositioning and Diplopia. Ophthalmology 2014, 121, 2268–2274. [Google Scholar] [CrossRef]
- Langmann, A.; Langmann, G.; Unlücerci, C.; Haller, E. Motility Disorders in Brachytherapy of Choroid Melanomas with Ru106 Applicators. Ophthalmologe 1995, 92, 76–78. [Google Scholar]
- Shields, C.L.; Demirci, H.; Marr, B.P.; Mashayekhi, A.; Dai, V.V.; Materin, M.A.; Shields, J.A. Intravitreal Triamcinolone Acetonide for Acute Radiation Papillopathy. Retina 2006, 26, 537–544. [Google Scholar] [CrossRef]
- Brour, J.; Desjardins, L.; Lehoang, P.; Bodaghi, B.; Lumbroso-Lerouic, L.; Dendale, R.; Cassoux, N. Sympathetic Ophthalmia after Proton Beam Irradiation for Choroïdal Melanoma. Ocul Immunol Inflamm 2012, 20, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Easom, H.A. Sympathetic Ophthalmia Associated With Malignant Melanoma. Arch Ophthalmol 1963, 70, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T.; Chin, K.J.; Duvall, G. Palladium-103 Ophthalmic Plaque Radiation Therapy for Choroidal Melanoma: 400 Treated Patients. Ophthalmology 2009, 116, 790–796.e1. [Google Scholar] [CrossRef]
- Konstantinidis, L.; Roberts, D.; Errington, R.D.; Kacperek, A.; Heimann, H.; Damato, B. Transpalpebral Proton Beam Radiotherapy of Choroidal Melanoma. Br J Ophthalmol 2015, 99, 232–235. [Google Scholar] [CrossRef]
- Abrams, M.J.; Gagne, N.L.; Melhus, C.S.; Mignano, J.E. Brachytherapy vs. External Beam Radiotherapy for Choroidal Melanoma: Survival and Patterns-of-Care Analyses. Brachytherapy 2016, 15, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.; Harper, C.; McAllister, I.; Perry, A. Iodine-125 Episcleral Plaque Therapy in Uveal Melanoma. Clin Exp Ophthalmol 2000, 28, 409–413. [Google Scholar] [CrossRef]
- Sikuade, M.J.; Salvi, S.; Rundle, P.A.; Errington, D.G.; Kacperek, A.; Rennie, I.G. Outcomes of Treatment with Stereotactic Radiosurgery or Proton Beam Therapy for Choroidal Melanoma. Eye 2015, 29, 1194–1198. [Google Scholar] [CrossRef]
- Muacevic, A.; Nentwich, M.; Wowra, B.; Staerk, S.; Kampik, A.; Schaller, U. Development of a Streamlined, Non-Invasive Robotic Radiosurgery Method for Treatment of Uveal Melanoma. Technol Cancer Res Treat 2008, 7, 369–373. [Google Scholar] [CrossRef]
- Akbaba, S.; Foerster, R.; Nicolay, N.H.; Arians, N.; Bostel, T.; Debus, J.; Hauswald, H. Linear Accelerator-Based Stereotactic Fractionated Photon Radiotherapy as an Eye-Conserving Treatment for Uveal Melanoma. Radiat Oncol 2018, 13, 140. [Google Scholar] [CrossRef]
- Zorlu, F.; Selek, U.; Kiratli, H. Initial Results of Fractionated CyberKnife Radiosurgery for Uveal Melanoma. J Neurooncol 2009, 94, 111–117. [Google Scholar] [CrossRef]
- Lipman, R.M.; Tripathi, B.J.; Tripathi, R.C. Cataracts Induced by Microwave and Ionizing Radiation. Surv Ophthalmol 1988, 33, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, M.M.; Tagliaferri, L.; Azario, L.; Lenkowicz, J.; Lanza, A.; Autorino, R.; Caputo, C.G.; Gambacorta, M.A.; Valentini, V.; Blasi, M.A. Ruthenium Brachytherapy for Uveal Melanomas: Factors Affecting the Development of Radiation Complications. Brachytherapy 2018, 17, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Arabi, A.; Shahraki, T. Plaque Brachytherapy in Iris and Iridociliary Melanoma: A Systematic Review of Efficacy and Complications. J Contemp Brachytherapy 2021, 13, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Incidence of Cataract and Outcomes after Cataract Surgery in the First 5 Years after Iodine 125 Brachytherapy in the Collaborative Ocular Melanoma Study. COMS Report No. 27. Ophthalmology 2007, 114. [Google Scholar] [CrossRef]
- Weber, B.; Paton, K.; Ma, R.; Pickles, T. Outcomes of Proton Beam Radiotherapy for Large Non-Peripapillary Choroidal and Ciliary Body Melanoma at TRIUMF and the BC Cancer Agency. Ocul Oncol Pathol 2016, 2, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Tseng, V.L.; Coleman, A.L.; Zhang, Z.-F.; McCannel, T.A. Complications from Plaque versus Proton Beam Therapy for Choroidal Melanoma: A Qualitative Systematic Review. J Cancer Ther 2016, 07, 169–185. [Google Scholar] [CrossRef]
- Thariat, J.; Jacob, S.; Caujolle, J.P.; Maschi, C.; Baillif, S.; Angellier, G.; Mathis, T.; Rosier, L.; Carnicer, A.; Hérault, J.; Salleron, J. Cataract Avoidance with Proton Therapy in Ocular Melanomas. Invest Ophthalmol Vis Sci 2017, 58, 5378–5386. [Google Scholar] [CrossRef]
- Maheshwari, A.; Finger, P.T. Regression Patterns of Choroidal Melanoma: After Palladium-103 (103Pd) Plaque Brachytherapy. Eur J Ophthalmol 2018, 28, 722–730. [Google Scholar] [CrossRef]
- Finger, P.T. Laser Photocoagulation for Radiation Retinopathy after Ophthalmic Plaque Radiation Therapy. British Journal of Ophthalmology 2005, 89, 730–738. [Google Scholar] [CrossRef]
- Le, B.H.A.; Kim, J.W.; Deng, H.; Rayess, N.; Jennelle, R.L.; Zhou, S.Y.; Astrahan, M.A.; Berry, J.L. Outcomes of Choroidal Melanomas Treated with Eye Physics Plaques: A 25-Year Review. Brachytherapy 2018, 17, 981–989. [Google Scholar] [CrossRef]
- Singaravelu, J.; Oakey, Z.B.; Wrenn, J.M.; Singh, A.D. Intravitreal Fluocinolone Acetonide Implant for Radiation Retinopathy: Report of Preliminary Findings. Retina 2023, 8, 230–235. [Google Scholar] [CrossRef] [PubMed]
- HORGAN, N.; SHIELDS, C.L.; MASHAYEKHI, A.; TEIXEIRA, L.F.; MATERIN, M.A.; SHIELDS, J.A. EARLY MACULAR MORPHOLOGICAL CHANGES FOLLOWING PLAQUE RADIOTHERAPY FOR UVEAL MELANOMA. Retina 2008, 28, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Chronopoulos, A.; Schutz, J.S.; Reibaldi, M. Treatment of Radiation Maculopathy and Radiation-Induced Macular Edema: A Systematic Review. Surv Ophthalmol 2021, 66, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Chinskey, N.D.; Zheng, Q.-D.; Zacks, D.N. Control of Photoreceptor Autophagy After Retinal Detachment: The Switch From Survival to Death. Investigative Opthalmology & Visual Science 2014, 55, 688. [Google Scholar] [CrossRef]
- McCannel, T.A.; McCannel, C.A. External Drainage for Primary Surgical Management of Uveal Melanoma Exudative Retinal Detachment. Retina 2017, 37, 1006–1007. [Google Scholar] [CrossRef]
- Kowal, J.; Markiewicz, A.; Debicka-Kumela, M.; Bogdali, A.; Jakubowska, B.; Karska-Basta, I.; Romanowska-Dixon, B. Analysis of Local Recurrence Causes in Uveal Melanoma Patients Treated with 125I Brachytherapy - A Single Institution Study. J Contemp Brachytherapy 2019, 11, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, A.; Bergin, C.; Schalenbourg, A.; Goitein, G.; Zografos, L. Proton Therapy for Uveal Melanoma in 43 Juvenile Patients: Long-Term Results. Ophthalmology 2014, 121, 898–904. [Google Scholar] [CrossRef]
- Parrozzani, R.; Pilotto, E.; Dario, A.; Miglionico, G.; Midena, E. Intravitreal Triamcinolone Versus Intravitreal Bevacizumab in the Treatment of Exudative Retinal Detachment Secondary to Posterior Uveal Melanoma. Am J Ophthalmol 2013, 155, 127–133.e2. [Google Scholar] [CrossRef]
- Zhou, X.; Ishikawa, H.; Gomi, F. Macular Hole and Vitreous Hemorrhage Subsequent to Stereotactic Hypofractionated Radiotherapy for Choroidal Melanoma: A Case Report and Review of the Literature. Front Oncol 2022, 12. [Google Scholar] [CrossRef]
- Papakostas, T.D.; Lane, A.M.; Morrison, M.; Gragoudas, E.S.; Kim, I.K. Long-Term Outcomes After Proton Beam Irradiation in Patients With Large Choroidal Melanomas. JAMA Ophthalmol 2017, 135, 1191. [Google Scholar] [CrossRef]
- Marinkovic, M.; Horeweg, N.; Laman, M.S.; Bleeker, J.C.; Ketelaars, M.; Peters, F.P.; Luyten, G.P.M.; Creutzberg, C.L. Ruthenium-106 Brachytherapy for Iris and Iridociliary Melanomas. Br J Ophthalmol 2018, 102, 1154–1159. [Google Scholar] [CrossRef]
- Semenova, E.; Finger, P.T. Palladium-103 Plaque Radiation Therapy for American Joint Committee on Cancer T3- and T4-Staged Choroidal Melanomas. JAMA Ophthalmol 2014, 132, 205–213. [Google Scholar] [CrossRef]
- Sagoo, M.S.; Shields, C.L.; Emrich, J.; Mashayekhi, A.; Komarnicky, L.; Shields, J.A. Plaque Radiotherapy for Juxtapapillary Choroidal Melanoma: Treatment Complications and Visual Outcomes in 650 Consecutive Cases. JAMA Ophthalmol 2014, 132, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Lane, A.M.; Egan, K.M.; Munzenrider, J.; Gragoudas, E.S. Natural History of Radiation Papillopathy after Proton Beam Irradiation of Parapapillary Melanoma. Ophthalmology 2010, 117, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Riechardt, A.I.; Cordini, D.; Willerding, G.D.; Georgieva, I.; Weber, A.; Seibel, I.; Lakotka, N.; Bechrakis, N.E.; Foerster, M.H.; Moser, L.; Joussen, A.M. Proton Beam Therapy of Parapapillary Choroidal Melanoma. Am J Ophthalmol 2014, 157, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Modorati, G.M.; Dagan, R.; Mikkelsen, L.H.; Andreasen, S.; Ferlito, A.; Bandello, F. Gamma Knife Radiosurgery for Uveal Melanoma: A Retrospective Review of Clinical Complications in a Tertiary Referral Center. Ocul Oncol Pathol 2020, 6, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sarici, A.M.; Pazarli, H. Gamma-Knife-Based Stereotactic Radiosurgery for Medium- and Large-Sized Posterior Uveal Melanoma. Graefe’s Archive for Clinical and Experimental Ophthalmology 2013, 251, 285–294. [Google Scholar] [CrossRef]
- Mills, M.D.; Harbour, J.W. Lipid Exudation Following Plaque Radiotherapy for Posterior Uveal Melanoma. Am J Ophthalmol 2006, 141. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.; Meissner, F.; Riechardt, A.I.; Bonaventura, T.; Löwen, J.; Heufelder, J.; Joussen, A.M. Breakdown of the Blood-Eye Barrier in Choroidal Melanoma after Proton Beam Radiotherapy. Graefes Arch Clin Exp Ophthalmol 2019, 257, 2323–2328. [Google Scholar] [CrossRef]
- Wen, J.C.; Oliver, S.C.; McCannel, T.A. Ocular Complications Following I-125 Brachytherapy for Choroidal Melanoma. Eye 2009, 23, 1254–1268. [Google Scholar] [CrossRef]
- Kim, E.A.; Salazar, D.; McCannel, C.A.; Kamrava, M.; Demanes, D.J.; Lamb, J.; Caprioli, J.; McCannel, T.A. Glaucoma after Iodine-125 Brachytherapy for Uveal Melanoma: Incidence and Risk Factors. J Glaucoma 2020, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Riechardt, A.I.; Pilger, D.; Cordini, D.; Seibel, I.; Gundlach, E.; Hager, A.; Joussen, A.M. Neovascular Glaucoma after Proton Beam Therapy of Choroidal Melanoma: Incidence and Risk Factors. Graefes Arch Clin Exp Ophthalmol 2017, 255, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Dalvin, L.A.; Chang, M.; Mazloumi, M.; Fortin, P.; McGarrey, M.; Martin, A.; Yaghy, A.; Yang, X.; Vichitvejpaisal, P.; Mashayekhi, A.; Shields, J.A. Visual Outcome at 4 Years Following Plaque Radiotherapy and Prophylactic Intravitreal Bevacizumab (Every 4 Months for 2 Years) for Uveal Melanoma. JAMA Ophthalmol 2020, 138. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Wang, Y.; Chen, R.; Liu, Y.; Li, Y.; Wei, W. Retrospective Analysis of Secondary Enucleation for Uveal Melanoma after Plaque Radiotherapy. BMC Ophthalmol 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- van Beek, J.G.M.; van Rij, C.M.; Baart, S.J.; Yavuzyigitoglu, S.; Bergmann, M.J.; Paridaens, D.; Naus, N.C.; Kiliç, E. Fractionated Stereotactic Radiotherapy for Uveal Melanoma: Long-Term Outcome and Control Rates. Acta Ophthalmol 2022, 100, 511–519. [Google Scholar] [CrossRef]
- Tran, E.; Ma, R.; Paton, K.; Blackmore, E.; Pickles, T. Outcomes of Proton Radiation Therapy for Peripapillary Choroidal Melanoma at the BC Cancer Agency. Int J Radiat Oncol Biol Phys 2012, 83, 1425–1431. [Google Scholar] [CrossRef]
- Fernandes, B.F.; Weisbrod, D.; Yücel, Y.H.; Follwell, M.; Krema, H.; Heydarian, M.; Xu, W.; Payne, D.; McGowan, H.; Simpson, E.R.; Laperriere, N.; Sahgal, A. Neovascular Glaucoma after Stereotactic Radiotherapy for Juxtapapillary Choroidal Melanoma: Histopathologic and Dosimetric Findings. Int J Radiat Oncol Biol Phys 2011, 80, 377–384. [Google Scholar] [CrossRef]
- Mishra, K.K.; Daftari, I.K.; Weinberg, V.; Cole, T.; Quivey, J.M.; Castro, J.R.; Phillips, T.L.; Char, D.H. Risk Factors for Neovascular Glaucoma after Proton Beam Therapy of Uveal Melanoma: A Detailed Analysis of Tumor and Dose-Volume Parameters. Int J Radiat Oncol Biol Phys 2013, 87, 330–336. [Google Scholar] [CrossRef]
- Vempuluru, V.S.; Jakati, S.; Krishnamurthy, R.; Senthil, S.; Kaliki, S. Glaucoma as the Presenting Sign of Intraocular Tumors: Beware of the Masquerading Sign. Int Ophthalmol 2020, 40, 1789–1795. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Di Nicola, M.; Gigliotti, C.R.; Battista, M.; Miserocchi, E.; del Vecchio, A.; Mortini, P.; Bandello, F.; Modorati, G.M. Predictive Factors of Radio-Induced Complications in 194 Eyes Undergoing Gamma Knife Radiosurgery for Uveal Melanoma. Acta Ophthalmol 2021, 99, e1458–e1466. [Google Scholar] [CrossRef]
- Gigliotti, C.R.; Modorati, G.; Di Nicola, M.; Fiorino, C.; Perna, L.A.; Miserocchi, E.; Franzin, A.; Picozzi, P.; Bolognesi, A.; Mortini, P.; Del Vecchio, A.; Calandrino, R. Predictors of Radio-Induced Visual Impairment after Radiosurgery for Uveal Melanoma. Br J Ophthalmol 2018, 102, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, N.; Tsuji, H.; Ishikawa, H.; Koyama-Ito, H.; Kamada, T.; Mizoe, J.E.; Ito, Y.; Naganawa, S.; Ohnishi, Y.; Tsujii, H. Risk Factors for Neovascular Glaucoma after Carbon Ion Radiotherapy of Choroidal Melanoma Using Dose-Volume Histogram Analysis. Int J Radiat Oncol Biol Phys 2007, 67, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Kosydar, S.; Robertson, J.C.; Woodfin, M.; Mayr, N.A.; Sahgal, A.; Timmerman, R.D.; Lo, S.S. Systematic Review and Meta-Analysis on the Use of Photon-Based Stereotactic Radiosurgery Versus Fractionated Stereotactic Radiotherapy for the Treatment of Uveal Melanoma. Am J Clin Oncol 2021, 44, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Al-Wassia, R.; Dal Pra, A.; Shun, K.; Shaban, A.; Corriveau, C.; Edelstein, C.; Deschenes, J.; Ruo, R.; Patrocinio, H.; Cury, F.L.B.; Deblois, F.; Shenouda, G. Stereotactic Fractionated Radiotherapy in the Treatment of Juxtapapillary Choroidal Melanoma: The Mcgill University Experience. Int J Radiat Oncol Biol Phys 2011, 81. [Google Scholar] [CrossRef] [PubMed]
- Krema, H.; Somani, S.; Sahgal, A.; Xu, W.; Heydarian, M.; Payne, D.; McGowan, H.; Michaels, H.; Simpson, E.R.; Laperriere, N. Stereotactic Radiotherapy for Treatment of Juxtapapillary Choroidal Melanoma: 3-Year Follow-Up. Br J Ophthalmol 2009, 93, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Krema, H.; Heydarian, M.; Beiki-Ardakani, A.; Weisbrod, D.; Xu, W.; Simpson, E.R.; Sahgal, A. A Comparison between 125Iodine Brachytherapy and Stereotactic Radiotherapy in the Management of Juxtapapillary Choroidal Melanoma. Br J Ophthalmol 2013, 97, 327–332. [Google Scholar] [CrossRef]
- Caminal, J.M.; Padrón-Pérez, N.; Arias, L.; Masuet-Aumatell, C.; Gutiérrez, C.; Piulats, J.M.; Pera, J.; Català, J.; Rubio, M.J.; Arruga, J. Transscleral Resection without Hypotensive Anaesthesia vs Iodine-125 Plaque Brachytherapy in the Treatment of Choroidal Melanoma. Eye (Basingstoke) 2016, 30, 833–842. [Google Scholar] [CrossRef]
- Damato, B. Developments in the Management of Uveal Melanoma. Clin Exp Ophthalmol 2004, 32, 639–647. [Google Scholar] [CrossRef]
- Finger, P.T. Radiation Therapy for Choroidal Melanoma. Surv Ophthalmol 1997, 42, 215–232. [Google Scholar] [CrossRef]
- Seddon, J.M.; Gragoudas, E.S.; Egan, K.M.; Glynn, R.J.; Munzenrider, J.E.; Austin-Seymour, M.; Goitein, M.; Verhey, L.; Urie, M.; Koehler, A. Uveal Melanomas Near the Optic Disc or Fovea. Ophthalmology 1987, 94, 354–361. [Google Scholar] [CrossRef]
- Damato, B.; Patel, I.; Campbell, I.R.; Mayles, H.M.; Errington, R.D. Local Tumor Control after 106Ru Brachytherapy of Choroidal Melanoma. Int J Radiat Oncol Biol Phys 2005, 63, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Montorio, D.; D’ Andrea, L.; Farella, A.; Matano, E.; Giuliano, M.; Liuzzi, R.; Breve, M.A.; De Placido, S.; Cennamo, G. Long-Term Outcomes in Uveal Melanoma After Ruthenium-106 Brachytherapy. Front Oncol 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, T.K.; Tang, Q.; Grunstein, L.L.; McCannel, T.A.; Straatsma, B.R.; Miller, K.M. Outcomes of Cataract Surgery in Eyes with Ocular Melanoma Treated with Iodine-125 Brachytherapy. J Cataract Refract Surg 2018, 44, 287–294. [Google Scholar] [CrossRef] [PubMed]
- McCannel, T.A. Post-Brachytherapy Tumor Endoresection for Treatment of Toxic Maculopathy in Choroidal Melanoma. Eye 2013, 27, 984–988. [Google Scholar] [CrossRef]
- Jung, S.-K.; Park, Y.-H.; Shin, D.; Kim, H.-S.; Jung, J.-H.; Kim, T.-H.; Moon, S.H. Visual Outcomes of Proton Beam Therapy for Choroidal Melanoma at a Single Institute in the Republic of Korea. PLoS One 2020, 15. [Google Scholar] [CrossRef]
- Verma, V.; Mehta, M.P. Clinical Outcomes of Proton Radiotherapy for Uveal Melanoma. Clin Oncol 2016, 28, e17–e27. [Google Scholar] [CrossRef]
- Wackernagel, W.; Holl, E.; Tarmann, L.; Avian, A.; Schneider, M.R.; Kapp, K.; Langmann, G. Visual Acuity after Gamma-Knife Radiosurgery of Choroidal Melanomas. Br J Ophthalmol 2013, 97, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Egger, E.; Zografos, L.; Schalenbourg, A.; Beati, D.; Bhringer, T.; Chamot, L.; Goitein, G. Eye Retention after Proton Beam Radiotherapy for Uveal Melanoma. Int J Radiat Oncol Biol Phys 2003, 55, 867–880. [Google Scholar] [CrossRef]
- Verschueren, K.M.S.; Creutzberg, C.L.; Schalij-Delfos, N.E.; Ketelaars, M.; Klijsen, F.L.L.; Haeseker, B.I.; Ligtenberg, S.M.B.; Keunen, J.E.E.; Marijnen, C.A.M. Long-Term Outcomes of Eye-Conserving Treatment with Ruthenium106 Brachytherapy for Choroidal Melanoma. Radiother Oncol 2010, 95, 332–338. [Google Scholar] [CrossRef]
- Bergman, L.; Nilsson, B.; Lundell, G.; Lundell, M.; Seregard, S. Ruthenium Brachytherapy for Uveal Melanoma, 1979–2003: Survival and Functional Outcomes in the Swedish Population. Ophthalmology 2005, 112, 834–840. [Google Scholar] [CrossRef]
- Macdonald, E.C.A.; Cauchi, P.; Kemp, E.G. Proton Beam Therapy for the Treatment of Uveal Melanoma in Scotland. Br J Ophthalmol 2011, 95, 1691–1695. [Google Scholar] [CrossRef]
- Damato, B.; Kacperek, A.; Chopra, M.; Campbell, I.R.; Errington, R.D. Proton Beam Radiotherapy of Choroidal Melanoma: The Liverpool-Clatterbridge Experience. Int J Radiat Oncol Biol Phys 2005, 62, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.R.; Nanji, A.A.; Galor, A.; Karp, C.L. Management of Conjunctival Malignant Melanoma: A Review and Update. Expert Rev Ophthalmol 2014, 9, 185–204. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Pagliara, M.M.; Fionda, B.; Scupola, A.; Azario, L.; Sammarco, M.G.; Autorino, R.; Lancellotta, V.; Cammelli, S.; Caputo, C.G.; Monge, R.M.; Kovács, G.; Gambacorta, M.A.; Valentini, V.; Blasi, M.A. Personalized Re-Treatment Strategy for Uveal Melanoma Local Recurrences after Interventional Radiotherapy (Brachytherapy): Single Institution Experience and Systematic Literature Review. J Contemp Brachytherapy 2019, 11, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Negretti, G.S.; Harley, U.; Arora, A.K.; Hay, G.; Sagoo, M.S.; Damato, B.E. Detecting Progression of Treated Choroidal Melanomas: Is Ultrasonography Necessary? Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Bagger, M.; Espensen, C.; Rasmussen, K.; Dogrusöz, M.; Jager, M.J.; Appelt, A.; Kiilgaard, J.F. Genetic Status Affects Disease-Specific Mortality but Not the Incidence of Local Recurrence in Patients with Uveal Melanoma. Ophthalmology 2023. [Google Scholar] [CrossRef]
- Reimer, J.; Voigtlaender-Fleiss, A.; Karow, A.; Bornfeld, N.; Esser, J.; Helga Franke, G. The Impact of Diagnosis and Plaque Radiotherapy Treatment of Malignant Choroidal Melanoma on Patients’ Quality of Life. Psychooncology 2006, 15, 1077–1085. [Google Scholar] [CrossRef]
- Chmielowska, K.; Tomaszewski, K.A.; Pogrzebielski, A.; Brandberg, Y.; Romanowska-Dixon, B. Translation and Validation of the Polish Version of the EORTC QLQ-OPT30 Module for the Assessment of Health-Related Quality of Life in Patients with Uveal Melanoma. Eur J Cancer Care 2013, 22, 88–96. [Google Scholar] [CrossRef]
- Brandberg, Y.; Damato, B.; Kivelä, T.; Kock, E.; Seregard, S. The EORTC Ophthalmic Oncology Quality of Life Questionnaire Module (EORTC QLQ-OPT30). Development and Pre-Testing (Phase I-III). Eye (Lond) 2004, 18, 283–289. [Google Scholar] [CrossRef]
- Chabert, S.; Velikay-Parel, M.; Zehetmayer, M. Influence of Uveal Melanoma Therapy on Patients’ Quality of Life: A Psychological Study. Acta Ophthalmol Scand 2004, 82, 25–31. [Google Scholar] [CrossRef]
- Cruickshanks, K.J.; Fryback, DG.; Nondahl, DM.; Robinson, N.; Keesey, U.; Dalton, DS.; Robertson, DM.; Chandra, SR.; Mieler, WF.; Zakov, ZN.; Custer, PL.; Del Priore, LV.; Albert, DM. Treatment Choice and Quality of Life in Patients With Choroidal Melanoma. Arch Ophthalmol 1999, 117, 461–467. [Google Scholar] [CrossRef]
- Suchocka-Capuano, A.; Brédart, A.; Dolbeault, S.; Rouic, L.L.-L.; Lévy-Gabriel, C.; Desjardins, L.; Flahault, C.; Bungener, C. Quality of Life and Psychological State in Patients with Choroidal Melanoma: Longitudinal Study. Bull Cancer 2011, 98, 97–107. [Google Scholar] [CrossRef]
- Klingenstein, A.; Fürweger, C.; Mühlhofer, A.K.; Leicht, S.F.; Schaller, U.C.; Muacevic, A.; Wowra, B.; Hintschich, C.; Eibl, K.H. Quality of Life in the Follow-up of Uveal Melanoma Patients after Enucleation in Comparison to CyberKnife Treatment. Graefes Arch Clin Exp Ophthalmol 2016, 254, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Hope-Stone, L.; Brown, S.L.; Heimann, H.; Damato, B.; Salmon, P. Two-Year Patient-Reported Outcomes Following Treatment of Uveal Melanoma. Eye (Lond) 2016, 30, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.A.; Kozlova, A.; Shoushtari, A.N.; Hay, J.L.; Francis, J.H.; Abramson, D.H. Quality of Life Concerns in Patients with Uveal Melanoma after Initial Diagnosis. Ocul Oncol Pathol 2020, 6, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Brandberg, Y.; Kock, E.; Oskar, K.; Trampe, E.A.; Seregard, S. Psychological Reactions and Quality of Life in Patients with Posterior Uveal Melanoma Treated with Ruthenium Plaque Therapy or Enucleation: A One Year Follow-up Study. Eye (Lond) 2000, 14, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.; Hope-Stone, L.; Cooper, B.; Brown, S.; Heimann, H.; Dunn, L. Patient-Reported Outcomes and Quality of Life after Treatment for Choroidal Melanoma. Ocul Oncol Pathol 2019, 5, 402–411. [Google Scholar] [CrossRef]
- Melia, M.; Moy, CS.; Reynolds, SM.; Cella, D.; Murray, TG.; Hovland, KR.; Hayman, JA.; Mangione, CM. Collaborative Ocular Melanoma Study-Quality Of Life Study Group. Development and Validation of Disease-Specific Measures for Choroidal Melanoma. Arch Ophthalmol 2003, 121. [Google Scholar] [CrossRef]
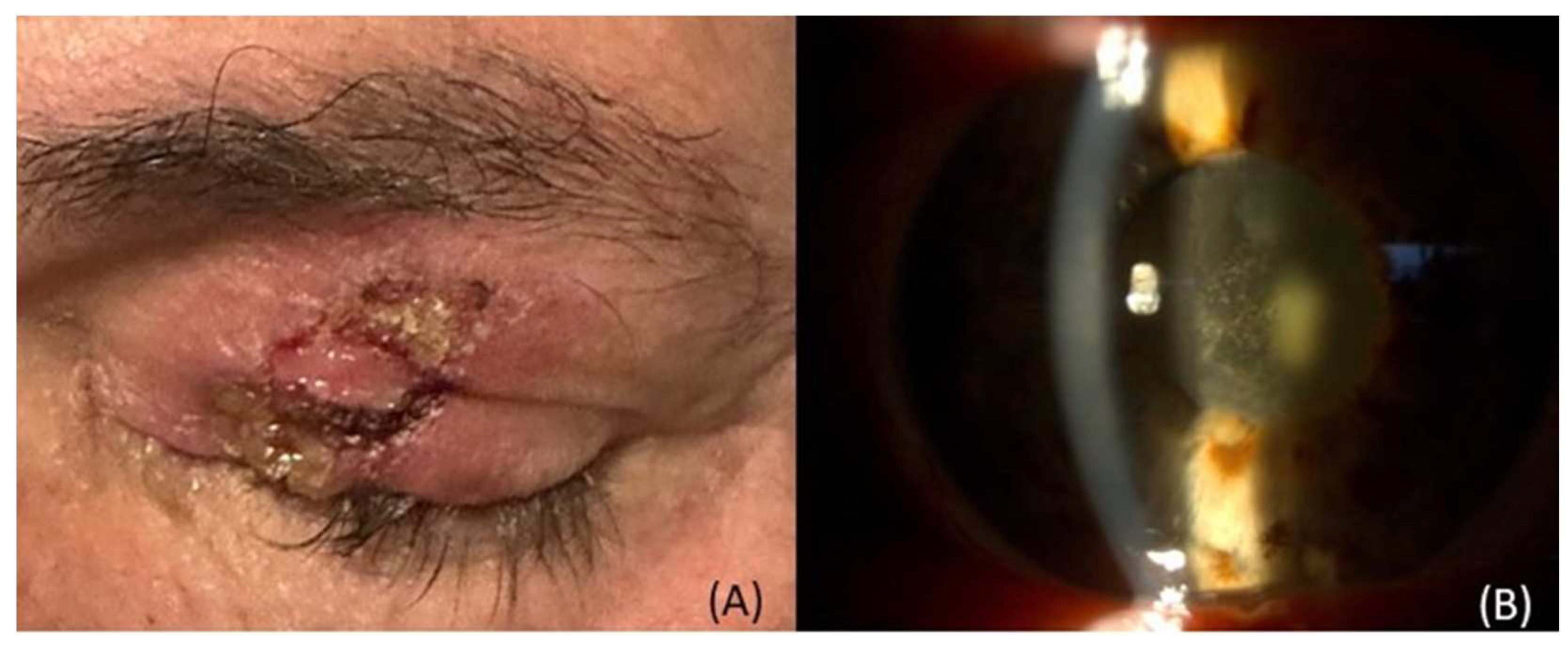
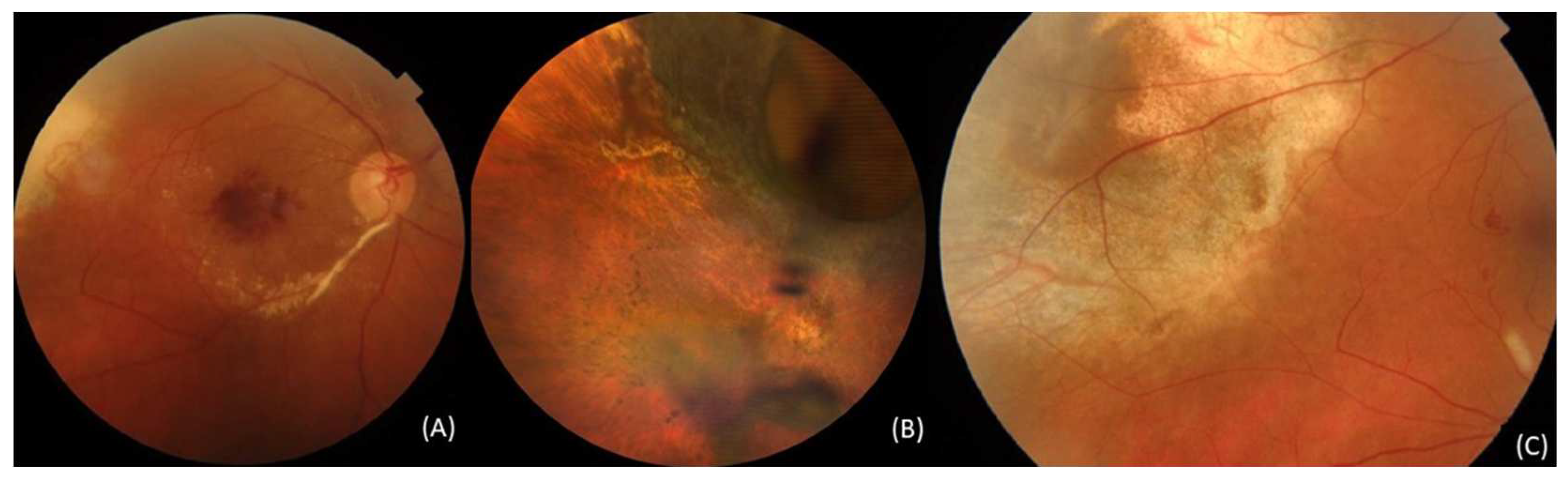
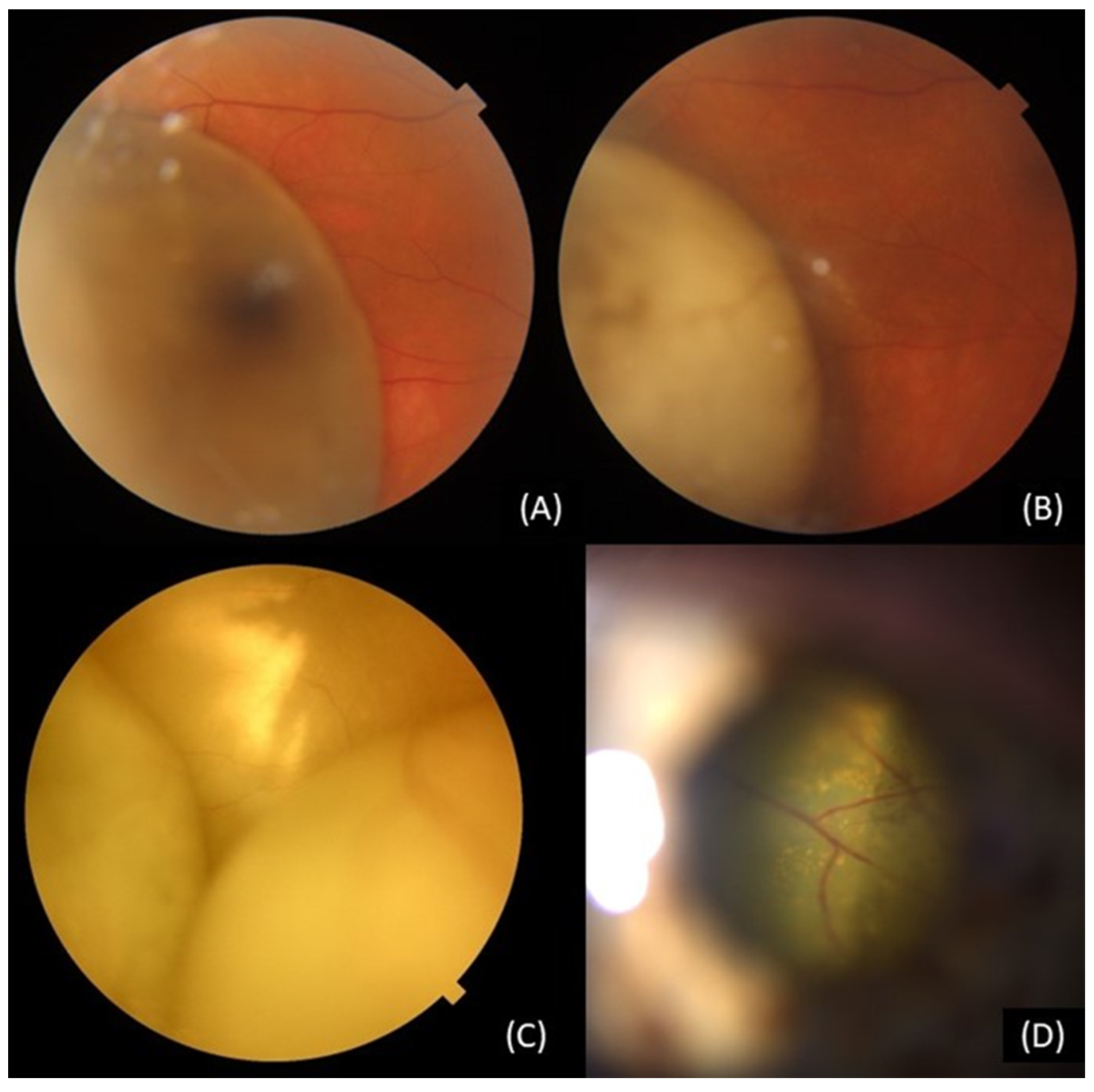
| Complication | Available Therapeutic Approaches |
|
Ocular Surface & Ocular adnexa complications |
Artificial tears, topical corticosteroids and antibiotic treatment [19,20] |
| Reconstructive surgery, tarsorrhaphy or gold weight implantation [19,20] | |
| Contact lenses, autologous serum eye drops, conjunctival flaps, amniotic membrane transplantation, cyanoacrylate gluing [20,21] | |
| Lamellar or penetrating keratoplasty [20,21] | |
| Scleral Necrosis | Observation Artificial lubrication [19,22,23,24,25,26,27,28] •Artificial tears, gels or ointments, like prednisolone acetate 1% |
| Surgical techniques [19,22,23,24,25,26,27,28] •tissue glue •amniotic membrane transplantation •conjunctival graft/flap •scleral patch graft •vital Tenon’s fascia transposition •dermal patch graft •hyperbaric oxygen therapy •Enucleation |
|
| Scleritis | Systematic [28,29,30] •Corticosteroids •NSAIDs |
| Topical [28,29,30] •Cycloplegics •Beta-blockers •Corticosteroids •NSAIDs |
|
| Radiation Induced Cataract | Phacoemulsification [31,32,33] |
| Radiation maculpathy | Intravitreal anti-VEGF therapy (bevacizumab, ranibizumab and aflibercept) [34,35,36,37,38] |
| Intravitreal Corticosteroids (triamcinolone acetonide and dexamethasone implant) [34] | |
| Subtenon triamcinolone acetonide | |
| Intravitreal fluocinolone acetonide implant [39] | |
| Retinal Detachment (RD) | Observation [40] |
| Pars Plana Vitrectomy [41,42] | |
| Scleral buckles [41,42] | |
| Intravitreal triamcinolone, bevacizumab [43,44] | |
| Vitreous Hemorrhage (VH) | Observation [32,42] |
| Pars Plana Vitrectomy (in case of recurrence or complications) [32,42] | |
| Ocular Inflammation | Topical Corticosteroids and Cycloplegics [2,45,46,47] |
| Iris Neovascularization (NVI) | Panretinal Photocoagulation (PRP) [2,46,47] |
| Anti-VEGF therapy [48] | |
| Surgical techniques - Endoresection [4,48] | |
| Secondary Glaucoma (SG) | IOP-lowering medical therapy |
| Laser treatment [49,50,51] •cyclophotocoagulation (CPC) •YAG-iridotomy |
|
| Glaucoma drainage devices [52] | |
| Neovascular Glaucoma (NVG) | IOP-lowering medical therapy and glaucoma drainage devices |
| Intravitreal or intracameral bevacizumab [4,31,53,54,55] | |
| Panretinal Photocoagulation (PRP) [47,49,55,56] | |
| Transpupillary Thermotherapy (TTT) [ 31,48,57] | |
| Surgical techniques [31,57,58,59] •Endoresection •Endodrainage |
|
| Enucleation | |
| Toxic Tumor Syndrome (TTS) | Endoresection [4,54,58,60,61,62] |
| Intravitreal anti-VEGF and steroids [2,31,54,62,63] | |
| Endodrainage [58,60] | |
| Transpupillary Thermotherapy (TTT) [2,31,54,62,63] | |
| Exoresection [62] | |
| Tumor Related Lipid Exudation (TRLE) | Transpupillary thermotherapy (TTT) [64] |
| Anti-VEGF therapy [65] | |
| Local resection [65] | |
| Diplopia and Strabismus | Prisms Botulinum toxin injection Strabismus Surgery [29,66,67,68,69,70] |
| Optic neuropathy | Intravitreal injection of triamcinolone acetonide (4 mg/0.1 mL) [71] |
| Sympathetic ophthalmia | Intravenous corticosteroids, followed by oral administration starting at 1 mg/kg/day with a gradual tapering [72] |
| Visual acuity | The therapy depends on the part of the eye that has received the radiation |
| Trial | Identifier |
|---|---|
| Dexamethasone Implant for Retinal Detachment in Uveal Melanoma | NCT04082962 |
| Influence of Oral Treatment with Citicoline for the Prevention of Radiation Optic Neuropathy in Patients Treated for Uveal Melanomas With Proton Beam Therapy | NCT01338389 |
| Endoresection of the Tumor Scar or Transpupillary Thermotherapy for the Treatment of Large Uveal Melanomas (Endoresection-Laser) | NCT02874040 |
| Prevention of Neovascular Glaucoma by Intravitreal Injections of Anti-VEGF in Patients Treated with Proton Therapy for a Large Choroidal Melanoma (PROTECT) | NCT03172299 |
| Safety and Efficacy of Silicone Oil Tamponade for Surgical Attenuation of Radiation Damage in Choroidal Melanoma | NCT01460810 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





