Submitted:
26 April 2023
Posted:
27 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
2.1. Materials and Methods.
2.2. Synthesis of Compounds
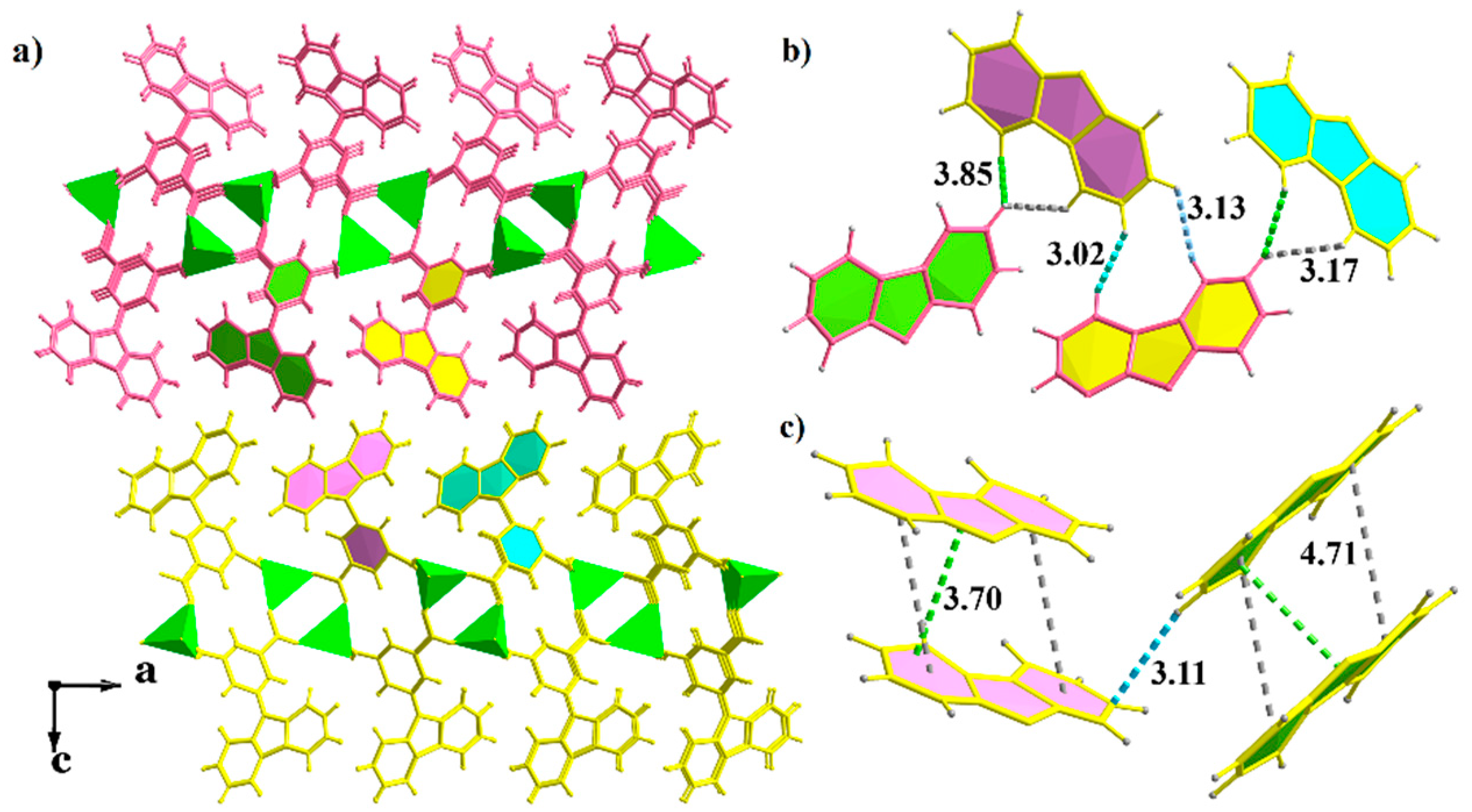
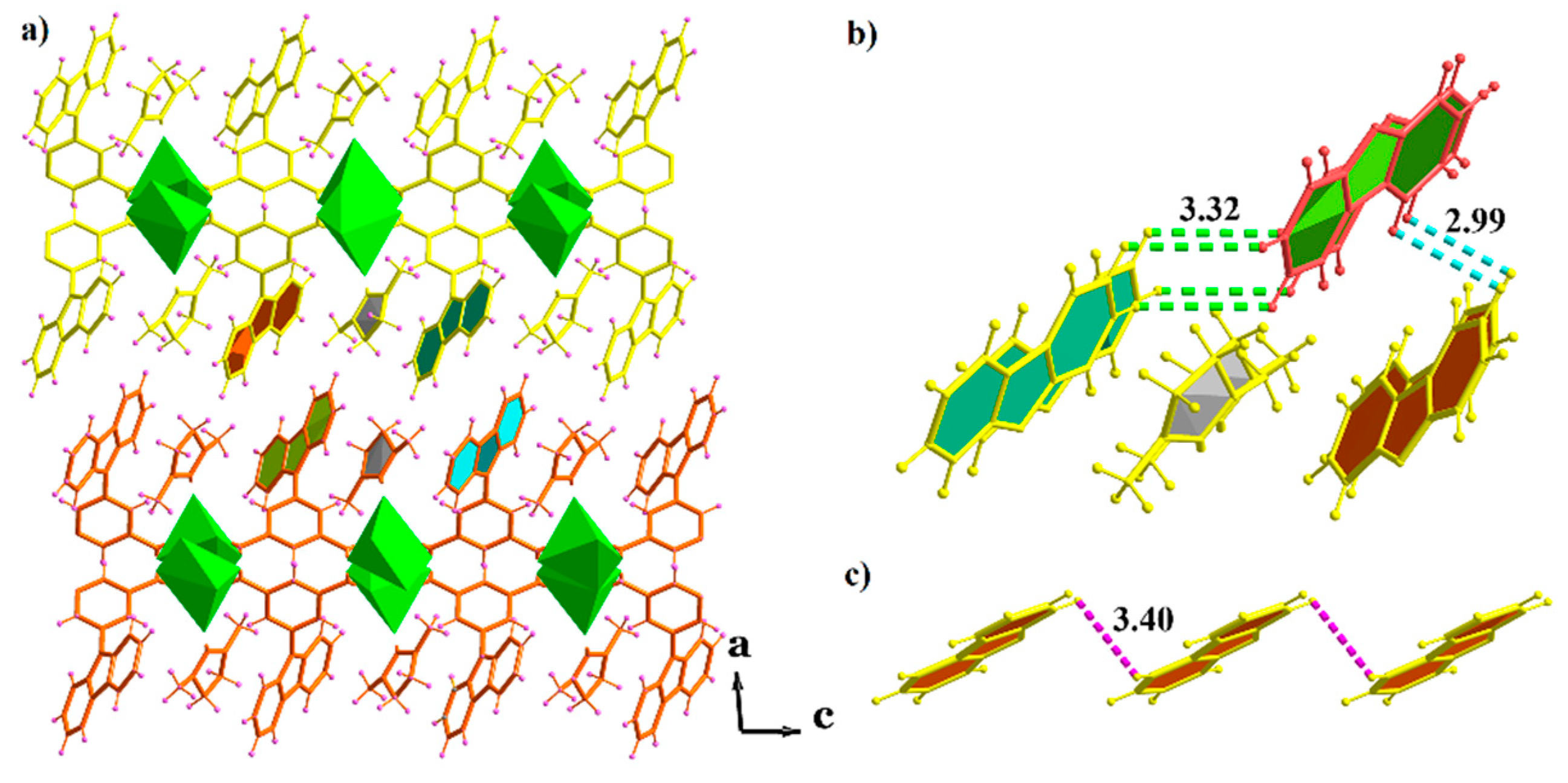
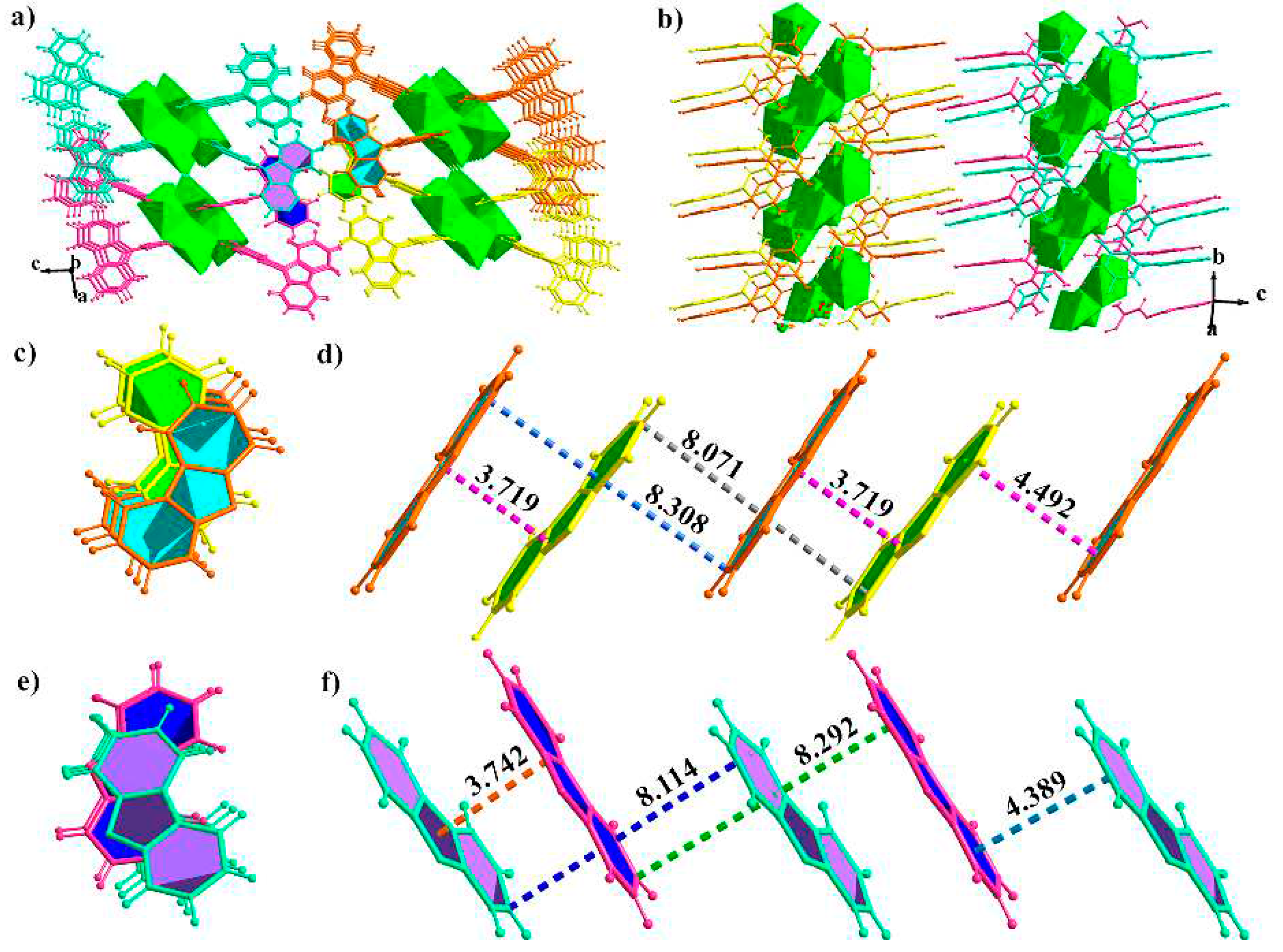
2.3. X-ray Crystal Structures
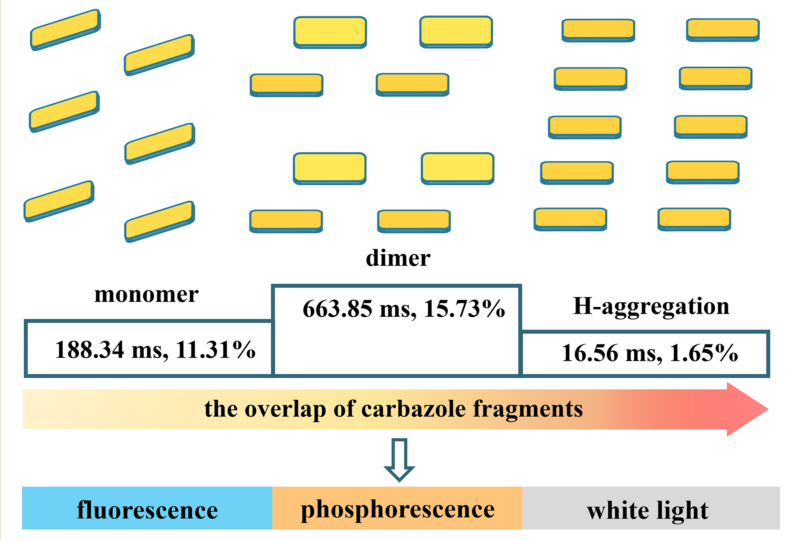
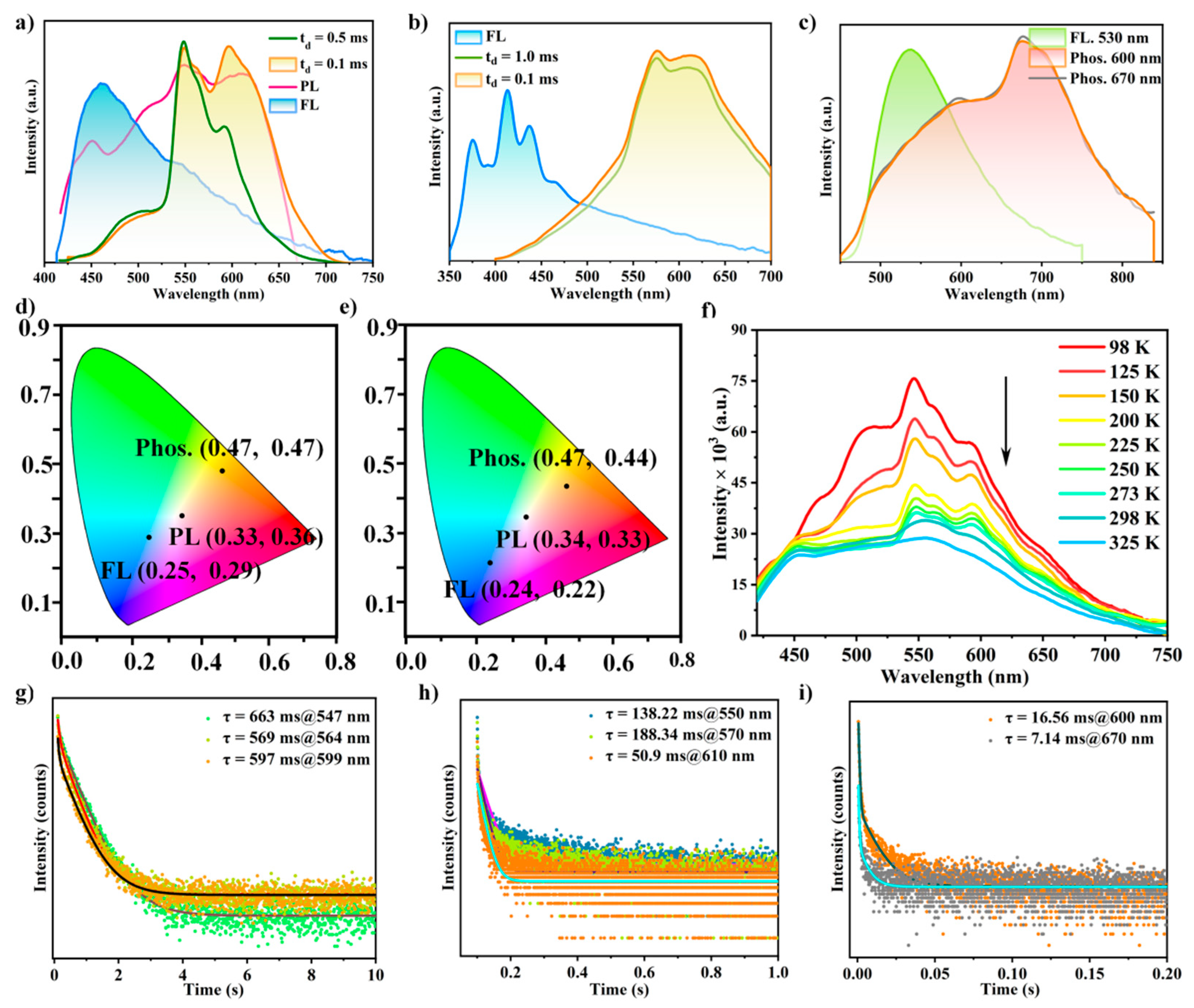
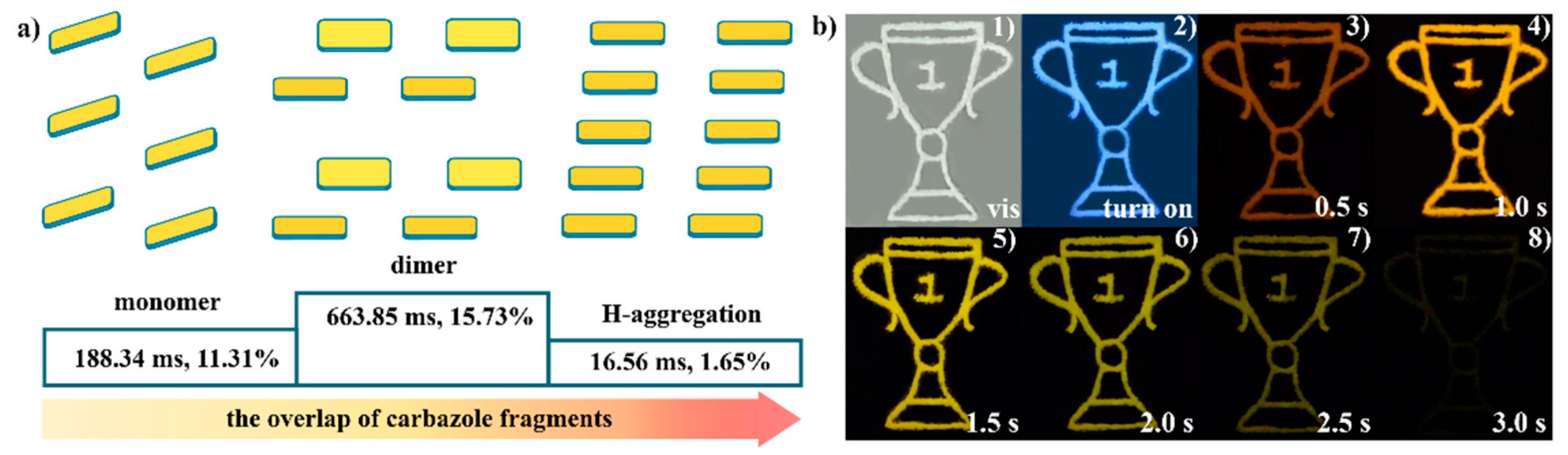
2.4. Structures and Optical properties
3. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nidhankar, A.D; Gouda, G.; Wakchaure, V.C. and Babu, S.S.; Efficient metal-free organic room temperature phosphors. Chem. Sci., 2021, 12, 4216−4236. [CrossRef]
- Hirata, S.; Recent advances in materials with room-temperature phosphorescence: photophysics for triplet exciton stabilization. Adv. Opt. Mater., 2017, 5, 1700116. [CrossRef]
- Yan, X.; Peng, H.; Xiang, Y.; Wang, J.; Yu, L.; Tao, Y.; Li, H.; Huang, W and Chen, R.; Recent Advances on Host-Guest Material Systems toward Organic Room Temperature Phosphorescence. Small, 2022, 18, 2104073. [CrossRef]
- Yang, X.; Dong, Y.; Ma, S.; Ren, J.; Li, N.; Lü, S.; Humidity-resistant organic room-temperature phosphorescence materials synthesized using catalyst-free click reaction. Chem. Eng. J., 2023, 462, 142198. [CrossRef]
- Kursunlu, A. N.; Porphyrin–Bodipy combination: synthesis, characterization and antenna effect, RSC Adv., 2014, 4, 47690-47696. [CrossRef]
- Li, Y.; Li, Q.; Meng, S.; Qin, Y.; Cheng, D.; Gu, H.; Wang, Z.; Ye, Y.; Tan J.; Ultrabroad-band, white light emission from carbon dot-based materials with hybrid fluorescence/phosphorescence for single component white light-emitting diodes. Chinese Chem. Lett., 2023, 34, 107794. [CrossRef]
- Kursunlu, A. N.; Guler, E.; The sensitivity and selectivity properties of a fluorescence sensor based on quinoline-Bodipy. J. lumin., 2014, 145, 608-614. [CrossRef]
- Ma, X. and Liu, Y.; Supramolecular Purely Organic Room-Temperature Phosphorescence. Acc. Chem. Res., 2021, 54, 3403–3414. [CrossRef]
- Wang, H.; Yang, X.; Qin, J. and Ma, L.; Long-lived room temperature phosphorescence of organic–inorganic hybrid systems. Inorg. Chem. Front., 2021, 8, 1942–1950. [CrossRef]
- Li, D.; Lu, F.; Wang, J.; Hu, W.; Cao, X.; Ma, X. and Tian, H.; Amorphous Metal-Free Room-Temperature Phosphorescent Small Molecules with Multicolor Photoluminescence via a Host-Guest and Dual-Emission Strategy. J. Am. Chem. Soc., 2018, 140, 1916–1923. [CrossRef]
- Zhao, W.; He, Z.; Jacky W. Y. Lam, Peng, Q.; Ma, H.; Shuai, Z.; Bai, G.; Hao, J. and Tang, B.; Rational Molecular Design for Achieving Persistent and Efficient Pure Organic Room-Temperature Phosphorescence. Chem, 2016, 1, 592–602. [CrossRef]
- Garain, S.; Ansari, S.N.; Kongasseri, A.A.; Chandra G.B.; Pati, S.K. and George, S.J.; Room temperature charge-transfer phosphorescence from organic donor–acceptor Co-crystals. Chem. Sci., 2022, 13, 10011–10019. [CrossRef]
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lussem, B. and Leo, K.; White organic light-emitting diodes with fluorescent tube efficiency. Nature, 2009, 459, 234–238. [CrossRef]
- Sinha, S.; Chowdhury, B.; Ghorai, U.K. and Ghosh, P.; Multitasking behaviour of a small organic compound: solid state bright white-light emission, mechanochromism and ratiometric sensing of Al(iii) and pyrophosphate. Chem. Commun., 2019, 55, 5127–5130. [CrossRef]
- Karmakar, A. and Li, J.; Luminescent MOFs (LMOFs): recent advancement towards a greener WLED technology. Chem. Commun., 2022, 58, 10768–10788. [CrossRef]
- Chen, Y.; Tang, K.; Chen, Y.; Shen, J.; Wu, Y.; Liu, S.; Lee, C.; Chen, C.; Lai, T.; Tung, S.; Jeng, R.; Hung, W.; Jiao, M.; Wu, C. and Chou, P.; Insight into the mechanism and outcoupling enhancement of excimer-associated white light generation. Chem. Sci., 2016, 7, 3556–3563. [CrossRef]
- Zhou, C.; Zhang, S.; Gao, Y.; Liu, H.; Shan, T.; Liang, X.; Yang, B. and Ma, Y.; Ternary Emission of Fluorescence and Dual Phosphorescence at Room Temperature: A Single-Molecule White Light Emitter Based on Pure Organic Aza-Aromatic Material. Adv. Funct. Mater., 2018, 1802407. [CrossRef]
- Xu, B.; Mu, Y.; Mao, Z.; Xie, Z.; Wu, H.; Zhang, Y.; Jin, C.; Chi, Z.; Liu, S.; Xu, J.; Wu, Y.; Lu, P.; Lien, A. and Bryce, M.; Achieving remarkable mechanochromism and white-light emission with thermally activated delayed fluorescence through the molecular heredity principle. Chem. Sci., 2016, 7, 2201–2206. [CrossRef]
- Wang, H.; Wang, J.; Zhang, T.; Xie, Z.; Zhang, X.; Sun, H.; Xiao, Y.; Yu, T. and Huang, W.; Breaching Kasha’s rule for dual emission: mechanisms, materials and applications. J. Mater. Chem. C, 2021, 9, 10154–10172. [CrossRef]
- Ono, T. and Hisaeda, Y.; Flexible-color tuning and white-light emission in three-, four-, and five-component host/guest co-crystals by charge-transfer emissions as well as effective energy transfers. J. Mater. Chem. C, 2019, 7, 2829–2842. [CrossRef]
- Li, Q. and Li, Z.; Molecular Packing: Another Key Point for the Performance of Organic and Polymeric Optoelectronic Materials. Acc. Chem. Res., 2020, 53, 962–973. [CrossRef]
- Zhang, J.; Huang, X. and Chen, X.; Supramolecular isomerism in coordination polymers. Chem. Soc. Rev., 2009, 38, 2385–2396. [CrossRef]
- Dang,L.L.; Li, T.T.; Zhang, T.T.; Zhao, Y.; Chen, T.; Gao, X.; Ma, L.F. and Jin, G.X.; Highly selective synthesis and near-infrared photothermal conversion of metalla-Borromean ring and [2]catenane assemblies. Chem. Sci., 2022, 13, 5130–5140. [CrossRef]
- Jiang, Y.; Zhang, K.; Zhou, M.; Gao, P. and Fu, H.; A fluorescence/phosphorescence dual-emitting metal-organic framework exhibiting two approaches for single-phase white-light emission. J. Solid State Chem., 2021, 304, 122563. [CrossRef]
- Fu, H.R.; Jiang, Y.Y..; Luo, J.H.; Li, T.; A Robust Heterometallic Cd(II)/Ba(II)-Organic Framework with Exposed Amino Group and Active Sites Exhibiting Excellent CO2/CH4 and C2H2/CH4 Separation. Chin. J. Struct. Chem., 2022, 41, 2203287–2203292. [CrossRef]
- Yuan, J.; Dong, J.; Lei, S. and Hu, W.; Long afterglow MOFs: a frontier study on synthesis and applications. Mater. Chem. Front., 2021, 5, 6824–6849. [CrossRef]
- Jiang, Y.; Liu, H.; Li, T.; Zhang, K.; Gao, P.; M. Zhou, Zhang L. and Fu, H.; Coordination-Induced Approach of a Carbazole-Based Molecule to Modulate Packing Modes for Ultralong Room-Temperature Phosphorescence. Cryst. Growth Des., 2022, 22, 2725–2732. [CrossRef]
- Zhou, H.; Han, J.; Cuan, J. and Zhou, Y.; Responsive luminescent MOF materials for advanced anticounterfeiting. Chem. Eng. J., 2022, 431, 134170. [CrossRef]
- Wen, Y.; Liu, H.; Zhang, S.; Gao, Y.; Yan, Y. and Yang, B.; One-dimensional π–π stacking induces highly efficient pure organic room-temperature phosphorescence and ternary-emission single-molecule white light. J. Mater. Chem. C, 2019, 7, 12502–12508. [CrossRef]
- Yang, Y.; Yang, X.; Fang, X.; Wang, K. and Yan, D.; Reversible Mechanochromic Delayed Fluorescence in 2D Metal-Organic Micro/Nanosheets: Switching Singlet-Triplet States through Transformation between Exciplex and Excimer. Adv. Sci., 2018, 5, 1801187. [CrossRef]
- Wang, Z.; Zhu, C.; Wei, Z.; Fan, Y. and Pan, M.; Breathing-Ignited Long Persistent Luminescence in a Resilient Metal–Organic Framework. Chem. Mater., 2020, 32, 841–848. [CrossRef]
- Liu, J.; Chen, Z.; Hu, J.; Sun, H.; Liu, Y.; Liu, Z.; Li, J.; Time-resolved color-changing long-afterglow for security systems based on metal–organic hybrids. Inorg. Chem. Front., 2022, 9, 584–591. [CrossRef]
- Wang, Z.; Zhu, C. Y.; Mo, J. T.; Xu, X. Y.; Ruan, J.; Pan, M.; Su, C. Y.; Multi-mode Color-tunable Long Persistent Luminescence in Single Component Coordination Polymers. Angew Chem., Int Ed, 2021, 60, 2526–2533. [CrossRef]
- Shi, Y.; Ma, H.; Sun, Z.; Zhao, W.; Sun, G.; Peng, Q.; Optimal Dihedral Angle in Twisted Donor–Acceptor Organic Emitters for Maximized Thermally Activated Delayed Fluorescence. Angew. Chem., Int. Ed., 2022, 61, e202213463. [CrossRef]
- Qian,C.; Ma, Z.; Fu, X.; Zhang, X.; Li, Z.; Jin, H.; Chen, M.; Jiang, H.; Jia X. and Ma, Z.; More than Carbazole Derivatives Activate Room Temperature Ultralong Organic Phosphorescence of Benzoindole Derivatives. Adv. Mater., 2022, 34, 2200544. [CrossRef]
- Ling, K.; Shi, H.; Wang, H.; Fu, L.; Lv, A.; Huang, K.; Ye, W.; Gu, M.; Ma, C.; Yao, X.; Jia, W.; Zhi, J.; Yao, W.; An, Z.; Ma, H. and Huang, W.; Prolonging Ultralong Organic Phosphorescence Lifetime to 2.5 s through Confining Rotation in Molecular Rotor. Adv. Opt. Mater., 2019, 7, 1800820. [CrossRef]
- Zhou,M.; Gao, P.; Jiang, Y.; Zhou, Y.; Wu, J.; Zhu, X. and Fu, H.; Multiemission tunability with ultralong and time–dependent room-temperature phosphorescence from isophthalic acid-decorated carbazole by coordination–induced crystallization. Dyes Pigm., 2021, 195, 109715. [CrossRef]
- Sheldrick,G.M.; Crystal structure refinement with SHELXL. Acta Crystallogr. C, 2015, 71, 3–8. [CrossRef]
- Bourhis,L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K. and Puschmann, H.; SHELXT-integrated space-group and crystal-structure determination. Acta Cryst. 2015. A71, 3-8. [CrossRef]
- Janiak,C.; A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc., Dalton Trans., 2000, 3885–3896. [CrossRef]
- Jena, S.; Munthasir, A.T.M. and Thilagar, P.; Ultralong room temperature phosphorescence and ultraviolet fluorescence from simple triarylphosphine oxides. J. Mater. Chem. C, 2022, 10, 9124−9131. [CrossRef]
- Liu, H.; Zhang, K.; Gao, P.; Luo, J.; Jiang, Y.; Zhou, M.; Li, T.; Zhu, X. and Fu, H.; Realization of Single-Phase White-Light-Emitting Materials with Time-Evolution Ultralong Room-Temperature Phosphorescence by Coordination Assemblies. Inorg. Chem., 2022, 61, 1636−1643. [CrossRef]
- Liu, Y.; Ma, Z.; Liu, J.; Chen, M.; Ma, Z. and Jia, X.; Robust White-Light Emitting and Multi-Responsive Luminescence of a Dual-Mode Phosphorescence Molecule. Adv. Opt. Mater., 2021, 9, 2001685. [CrossRef]
- Wei, Q.; Kleine, P.; Karpov, Y.; Qiu, X.; Komber, H.; Sahre, K.; Kiriy, A.; Lygaitis, R.; Lenk, S.; Reineke, S.; Voit, B.; Conjugation-Induced Thermally Activated Delayed Fluorescence (TADF): From Conventional Non-TADF Units to TADF-Active Polymers. Adv. Funct. Mater., 2017, 27, 1605051. [CrossRef]
- Gužauskas, M.; Narbutaitis, E.; Volyniuk, D.; Baryshnikov, G.; Minaev, B.; Ågren, H.; Chao, Y.; Chang, C.; Rutkis, M.; Grazulevicius, J.; Polymorph acceptor-based triads with photoinduced TADF for UV sensing. Chem. Eng. J., 2021, 425, 131549. [CrossRef]
- Dou,X.; Zhu, T.; Wang, Z.; Sun, W.; Lai, Y.; Sui, K.; Tan, Y.; Zhang, Y. and Yuan, W.; Color-Tunable, Excitation-Dependent, and Time-Dependent Afterglows from Pure Organic Amorphous Polymers. Adv. Mater., 2020, 32, 2004768. [CrossRef]
- Li, H.; Gu, J.; Wang, Z.; Wang, J.; He, F.; Li, P.; Tao, Y.; Li, H.; Xie, G.; Huang, W.; Zheng, C. and Chen, R.; Single-component color-tunable circularly polarized organic afterglow through chiral clusterization. Nat. Commun., 2022, 13, 429. [CrossRef]
- Chen, J.; Yu, T.; Ubba, E.; Xie, Z.; Yang, Z.; Zhang, Y.; Liu, S.; Xu, J.; Aldred, M.P. and Chi, Z.; Achieving Dual-Emissive and Time-Dependent Evolutive Organic Afterglow by Bridging Molecules with Weak Intermolecular Hydrogen Bonding. Adv. Opt. Mater. 2019, 7, 1801593. [CrossRef]
- Forni, A.; Lucenti, E.; Botta, C. and Cariati, E.; Metal free room temperature phosphorescence from molecular self-interactions in the solid state. J. Mater. Chem. C, 2018, 6, 4603–4626. [CrossRef]
- Hamzehpoor, E. and Perepichka, D.F.; Crystal Engineering of Room Temperature Phosphorescence in Organic Solids. Angew. Chem. Int. Ed., 2020, 59, 9977–9981. [CrossRef]
- Wang,Y.; Yang, J.; Tian, Y.; Fang, M.; Liao, Q.; Wang, L.; Hu, W.; Tang, B. and Li, Z.; Persistent organic room temperature phosphorescence: what is the role of molecular dimers? Chem. Sci., 2020, 11, 833−838. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




