1. Introduction
Pygoscelis penguin populations in the Antarctic Peninsula have experienced steep declines in the last 50 years [
1,
2,
3]. This trend has been attributed to shifts in Antarctic krill (
Euphausia superba) availability due to environmental changes, such as warming, sea ice melting and competition with recovering populations of marine mammals [
4,
5,
6]. However, the trend varies depending on the penguin species, for instance Adélie (
Pygoscelis adeliae) and Chinstrap (
Pygoscelis antarcticus) penguins seem to be experiencing more concerning declines [
1,
2,
6] than Gentoo penguins
Pygoscelis papua [
7] which are more flexible on their feeding requirements compared to the former two species whose diet is comprised almost entirely on krill [
8,
9,
10,
11]. Therefore supporting the view of shifts in krill density and distribution, as claimed by many studies [
12,
13,
14].
Changes in krill density and distribution due to climate change might not be the only factors influencing penguin populations. Not so long ago, studies pointed to a possible interference of the krill fishing over penguins in Western Antarctic Peninsula (WAP), indicating that reduced availability of krill in synergy with increasing fishing catches would affect penguin recruitment [
5]. More recent studies confirmed that population parameters had been affected by increasing krill fishing catches under warming conditions [
15,
16], particularly during the non-breeding as in a recent past, overlap between penguins and the krill fishery during late summer throughout early winter has increased [
16,
17,
18]. Catches in areas close to colonies have increased substantially in some sectors during the chick-rearing after the year 2000 [
16]. Recently, it has been reported that low krill availability increased spatial overlap between foraging penguins and the krill fishery [
19], which might become a concern if the frequency of years with low krill abundance increases over time.
Based on that, it is possible to hypothesize that krill catch increases could be detrimental for the successful rearing of chicks if availability of krill is reduced nearby the colonies. In average, penguin foraging is limited to 30 to 50 km from their nest during chick-rearing in order to allow adults to feed the chicks in short intervals and optimize growth, an adaptation that synchronizes the chicks high energy demands with increasing productivity in the coastal areas of Antarctica [
20,
21]. Unsurprisingly, when food availability near the colony is reduced, breeding success can drop dramatically as foraging trip distances increase, as reported for populations of several seabird species [
22,
23,
24,
25], penguins included [
21,
26]. While since 1982, the management of krill fishing in Antarctica has been successfully conducted by the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR), currently there is an ongoing effort for the development of a revised management strategy that adequately reduces the potential impacts of fishing over krill-predator populations [
27,
28]. In this regard, it is also important to assess whether any impact had existed in the past to provide advice for management actions in the future. Therefore, this study aimed to evaluate the relation between
Pygoscelis penguin breeding success (measured as a rate of chicks raised per nest), Antarctic krill biomass and fishery variability in the WAP. By using data from the Mapping Application for Penguin Populations [
29,
30], data from krill Acoustic Surveys [
14] and data on the krill fishery [
31], it was hypothesized that increasing fishing catches reduced breeding success when krill availability was low.
2. Materials and Methods
2.1. Krill Acoustic Biomass Data
Biomass estimates from acoustic surveys of krill during summer months between 1995-2018 [
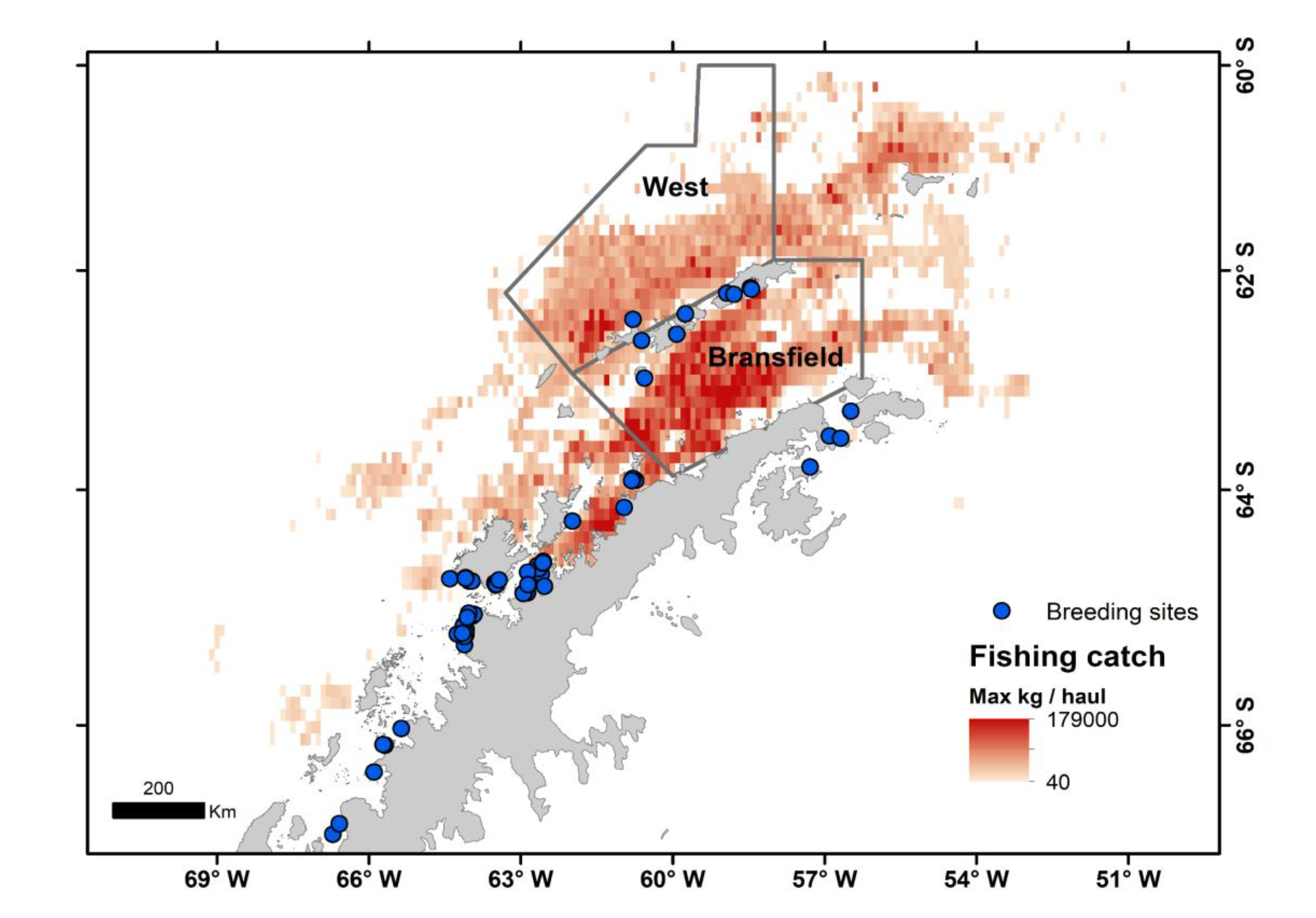
14] were analysed. Acoustic surveys followed transects in 4 different geographical strata representing main fishing hotspots. The two main fishing grounds in subarea 48.1 (
Figure 1) are currently placed in the West and Bransfield Strata [
31], as most of the catch comes from those two areas [
32]. Bayesian linear models with flexible priors (MCMCglmm R package [
33,
34]) and 10999 permutations for calculation of significance were applied to test whether there is time variability of the krill biomass at both strata. For this analysis, missing values were replaced by the first quantile of the biomass for the whole period, following a similar approach proposed as precautionary (Paragraph 2.31 [
35], par. 2.25 [
32]).
2.2. Krill Fishery Data
Catch data [
31] is available at haul by haul aggregation level between 1980 and 2018 for the subarea 48.1, which was subsetted from 1995 onwards to match the start of krill biomass acoustic data. Krill catches (t per haul) were accumulated for each year and each geographical stratum. The percentage of krill removed by the fishery was calculated by dividing the accumulated summer (December to March) catch by the estimated acoustic krill biomass per strata. Bayesian linear models (MCMCglmm R package [
33,
34]) with 10,999 permutations for calculation of significance were applied to test whether there is time variability of the caught krill biomass at each of the two main fishing grounds (West and Bransfield Strata) and whether the amount of caught krill biomass corresponded with the krill availability.
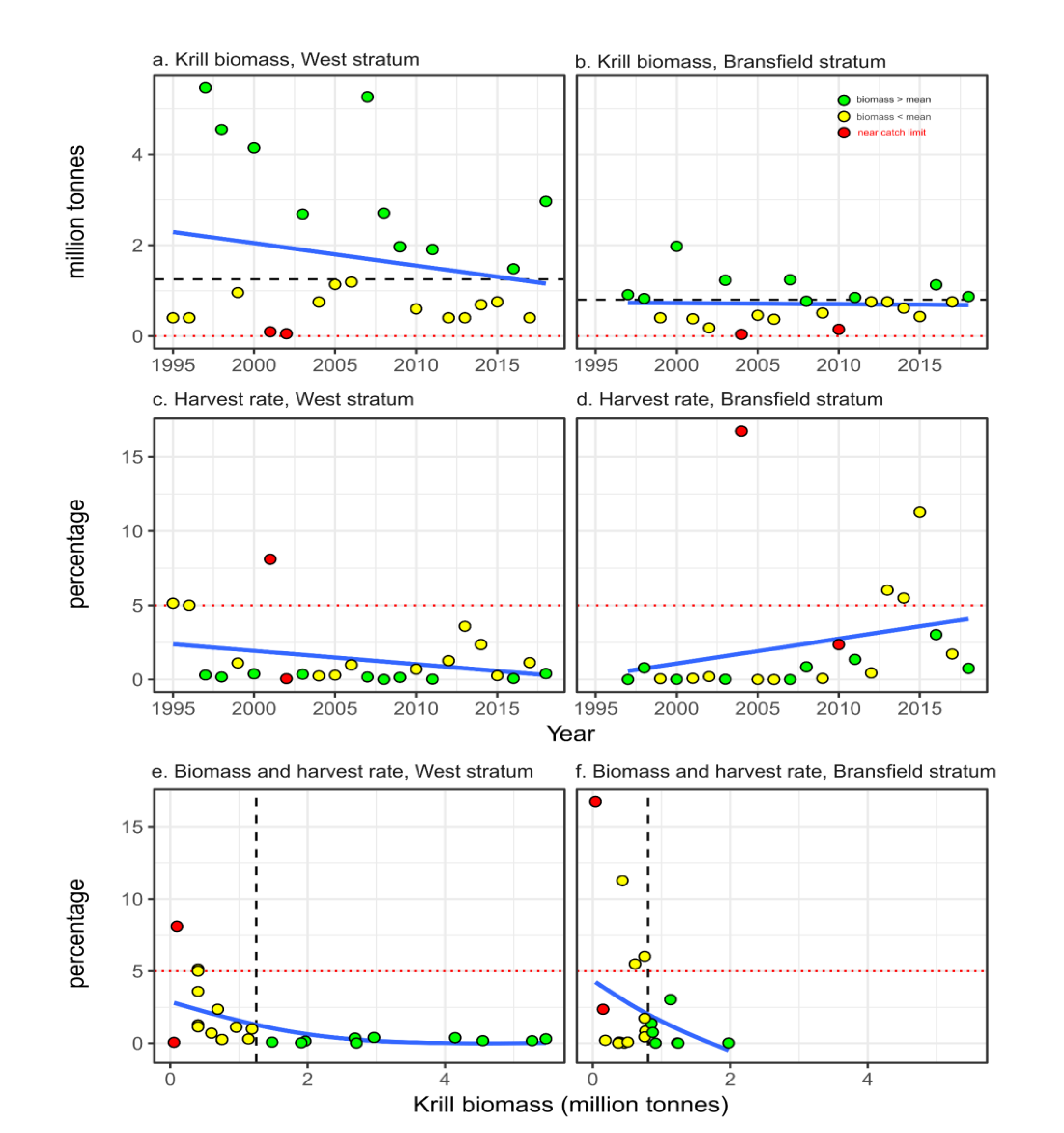
Conservation Measure 51-07 sets the individual catch limit for subarea 48.1 in 155,000 tons (corresponding to 25% of the trigger level which is 620,000 tons). This was established in order to prevent fishing catches from going over an amount that could trigger impacts to the ecosystem [
32,
36] Therefore, we identified periods when the estimated biomass reached low values near the catch limit.
2.3. Penguin Data
Data of
Pygoscelis penguin colonies (Adélie
P. adeliae, Chinstrap
P. antarcticus and Gentoo
P. papua penguins) for the CCAMLR Subarea 48.1 was downloaded from the Mapping Application for Penguins Populations and Projected Dynamics MAPPPD [
29,
30]. All the data that had counts of both nests (between November and January) and chicks (January to February) were selected for the calculation of breeding success. A total of 72 counts of nests and chicks in the same season were available for 21 colonies of Adélie penguins; a total of 81 counts of nests and chicks in the same season were available for 18 colonies of Chinstrap penguins and; a total of 163 counts of nests and chicks in the same season were available for 41 colonies of Gentoo penguins.
When there were successive counts in the same season, the highest number of nests (later counts with less nests would mean nests started to fail) and the lowest number of chicks were used. Breeding success was calculated as a proportion of successfully raised chicks per nest by simply dividing the number of chicks late in the season per number of nests early in the season. This resulted in an index that varies from 0 (all chicks died in that colony) to 2 (all chicks survived), where values equal to 1 indicates that, on average, nests were able to raise one of the two chicks, whereas values below 1 indicate a proportion of nests that failed entirely (i.e. a value of 0.6 indicates 0.4 - 40% - of nests had the two chicks deceived). Values of 2 mean a 100% of survival, which was only found in two cases when nest counts were 1 and 2. Those two cases were excluded as the low number of nests is not a good representation of penguin colonies; a single count of 80,000 pairs was also excluded (
Appendix A line 340).
Frequency distribution of chicks per nest was not normal (Shapiro Wilk’s W = 0.967, P<0.001) nor independence between samples could be expected. Therefore, general trends of colonies were tested using Bayesian mixed models (MCMCglmm R package [
33,
34]) with sites and species as random terms. Random intercepts were extracted from the mixed models and a Bayesian linear model was used to test whether there was latitudinal coherence of the random intercept.
2.4. Responses of Penguins Breeding Success
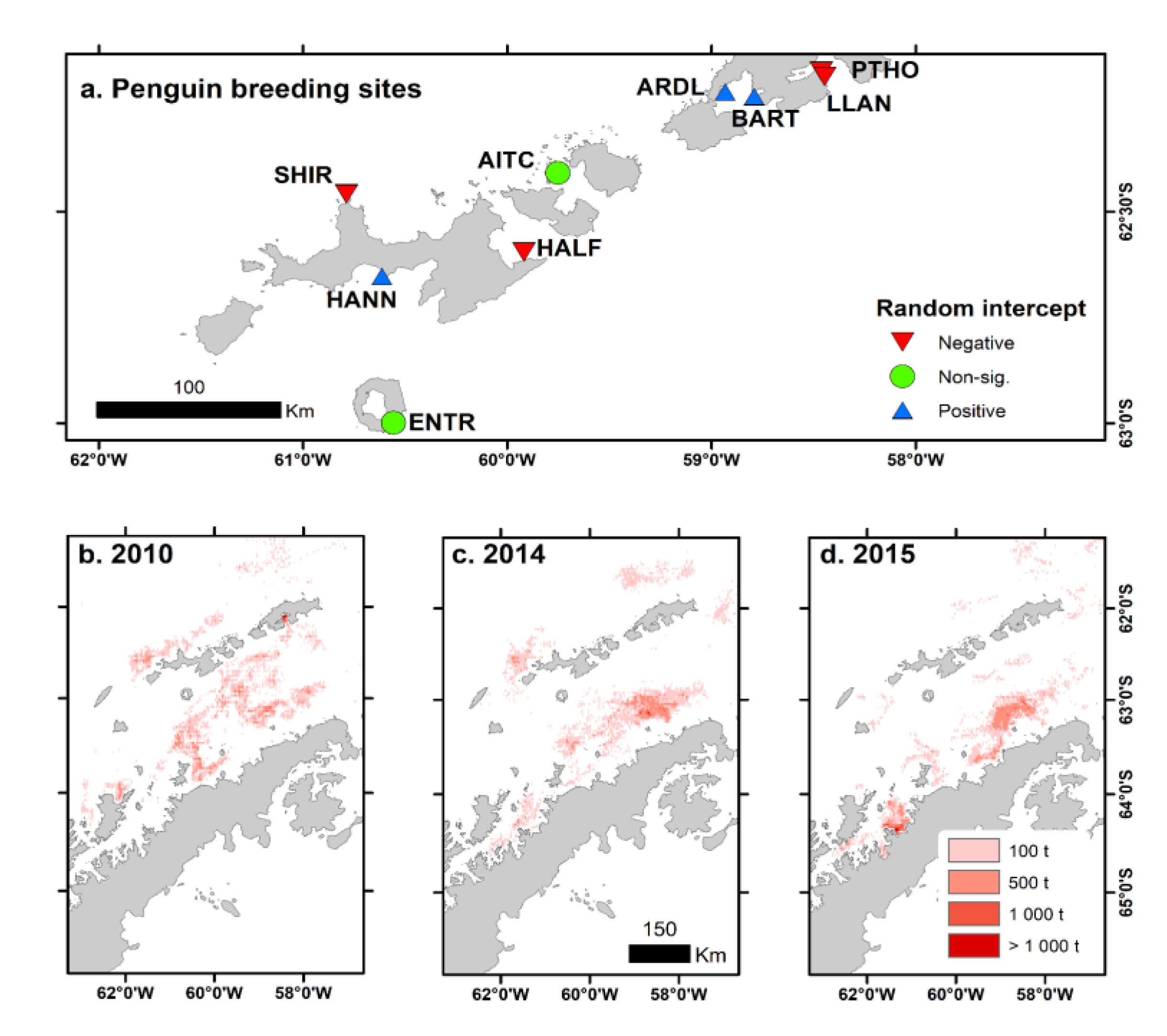
For testing effects of fishery, each penguin colony was assigned to one stratum, colonies outside the strata were excluded for this analysis, therefore limiting data to 9 sites in the South Shetland Islands (
Figure 1) considering the 3 species together. For merging fishing and penguin population data, only catches matching the breeding season were used (December, January, February and March). A measure of how much the catch represented the krill biomass in each strata was calculated by dividing the catch by the biomass of the strata (harvest rate from now on). Based on the distribution of chicks raised per nest in relation to the harvest rate (
Appendix A line 547), catches were classified as above (n=14) or below (n=52) 5% of the available krill biomass during summer (December to March). Years also were classified as those with krill biomass above (n=34) or below (n=32) the median biomass. Within this dataset (
Appendix A line 564), frequency distribution of chicks per nest was not normal (Shapiro Wilk’s W = 0.948, P=0.008) despite there was homogeneity of variances between years of harvest rate above and below 5% (Bartlett’s K
2=0.404, P=0.525) and krill biomass below or above the median (Bartlett’s K
2=2.383, P=0.122). Important to note that in all cases when harvest rate was <5% of the biomass, the krill biomass was above the median, so catching more than 5% of the standing biomass only occurred in periods of low krill biomass. Therefore, data was grouped in 3 categories: (1) Krill biomass > median, Catch < 5%; (2) Krill biomass < median, Catch < 5% and; (3) Krill biomass < median, Catch > 5%. A Bayesian Mixed model (MCMCglmm R package) was applied to test for differences in penguin breeding among those groups using sites as random terms with 10999 permutations
Statistics were done using R v4.2.1 [
37]. Details of the analyses can be found in the full reproducible code available as Supplementary Material in
Appendix A. Data is available as Supplementary Material in
Appendix B.
3. Results
3.1. Krill Biomass and Fishing
Krill biomass at the analysed strata ranged from 39,166 to 5,466,556 tones. 58.33% and 59.10% of the acoustic estimations for West and Bransfield strata respectively, were lower than the within stratum estimate mean. On four occasions krill biomass was near the catch limit at one of the two strata (
Figure 2a,b). Krill biomass showed a significant decrease between 1995 and 2018 in the West (
Figure 2a) stratum (F
1,22=30.30, simulated P<0.001), and a slight decrease in the Bransfield (
Figure 2b) stratum (F
1,22=90.91, simulated P<0.001). Harvest rate decreased in the West stratum (
Figure 2c); however, it was not significant (F
1,22=13.16, simulated P=0.270) while a significant increase was observed in the Bransfield (
Figure 2d) stratum (F1,22=9.40, simulated P<0.001). Harvest rate was inversely proportional to krill biomass (F
1,22=24.09, simulated P=0.052) in the two strata (
Figure 2e,f).
3.2. Penguin Population Trends
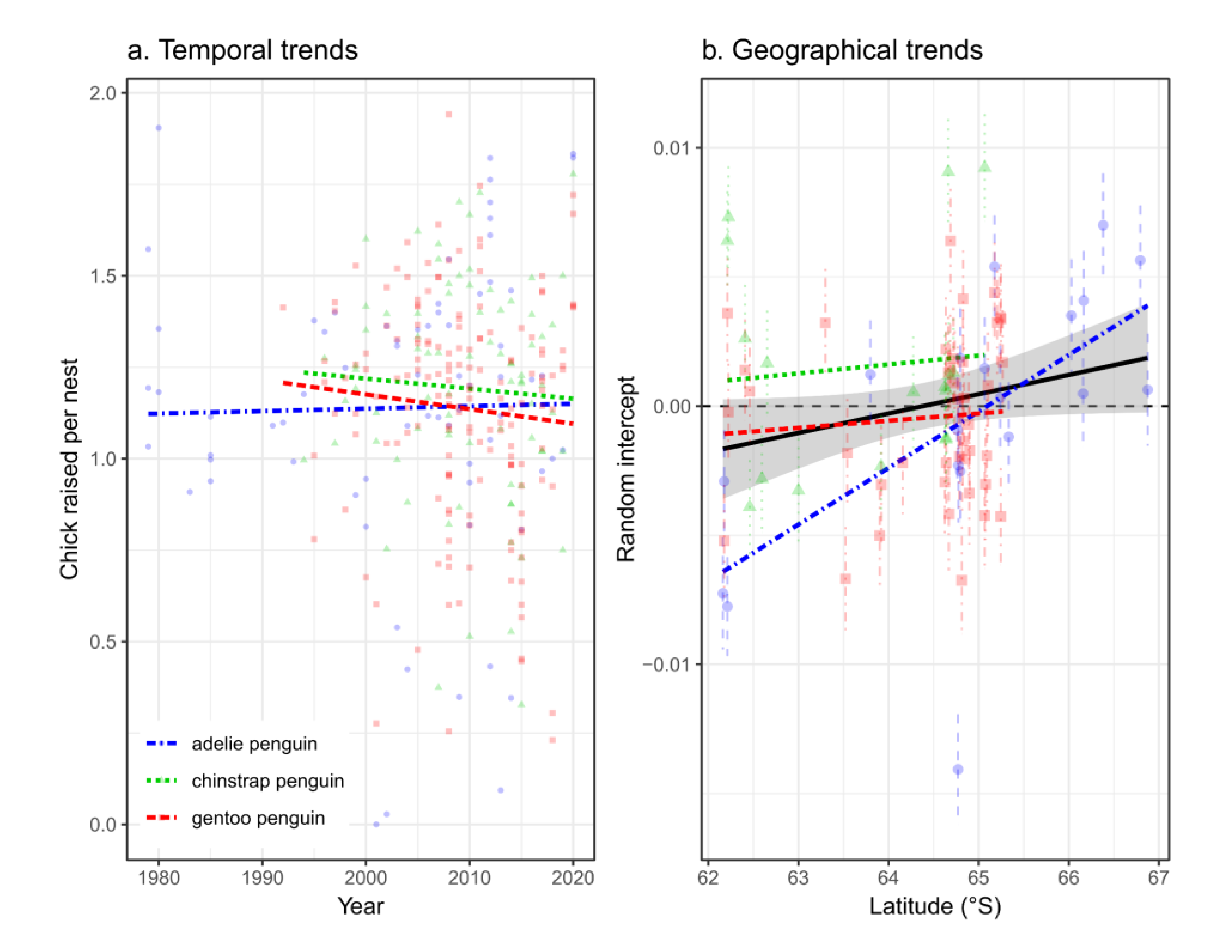
The number of chicks per nest varied from 0.0 to 1.94. While the trend for all species together indicated stability (F
1,311=90.90, simulated P=0.812), the large variability in success (
Figure 3a) is better explained by latitudinal differences (F
1,75=87.99, simulated P=0.041) than species (Chinstrap:Adélie F
2,74=6.35, simulated P=0.312; Gentoo:Adélie F
2,74=5.17, simulated P=0.612). Northerly breeding sites had significantly lower intercept than those southerly, indicating that colonies on northern latitudes had a higher tendency for a decrease of breeding success than those located in southern latitudes (
Figure 3b).
3.2. Penguin breeding success and removed krill biomass
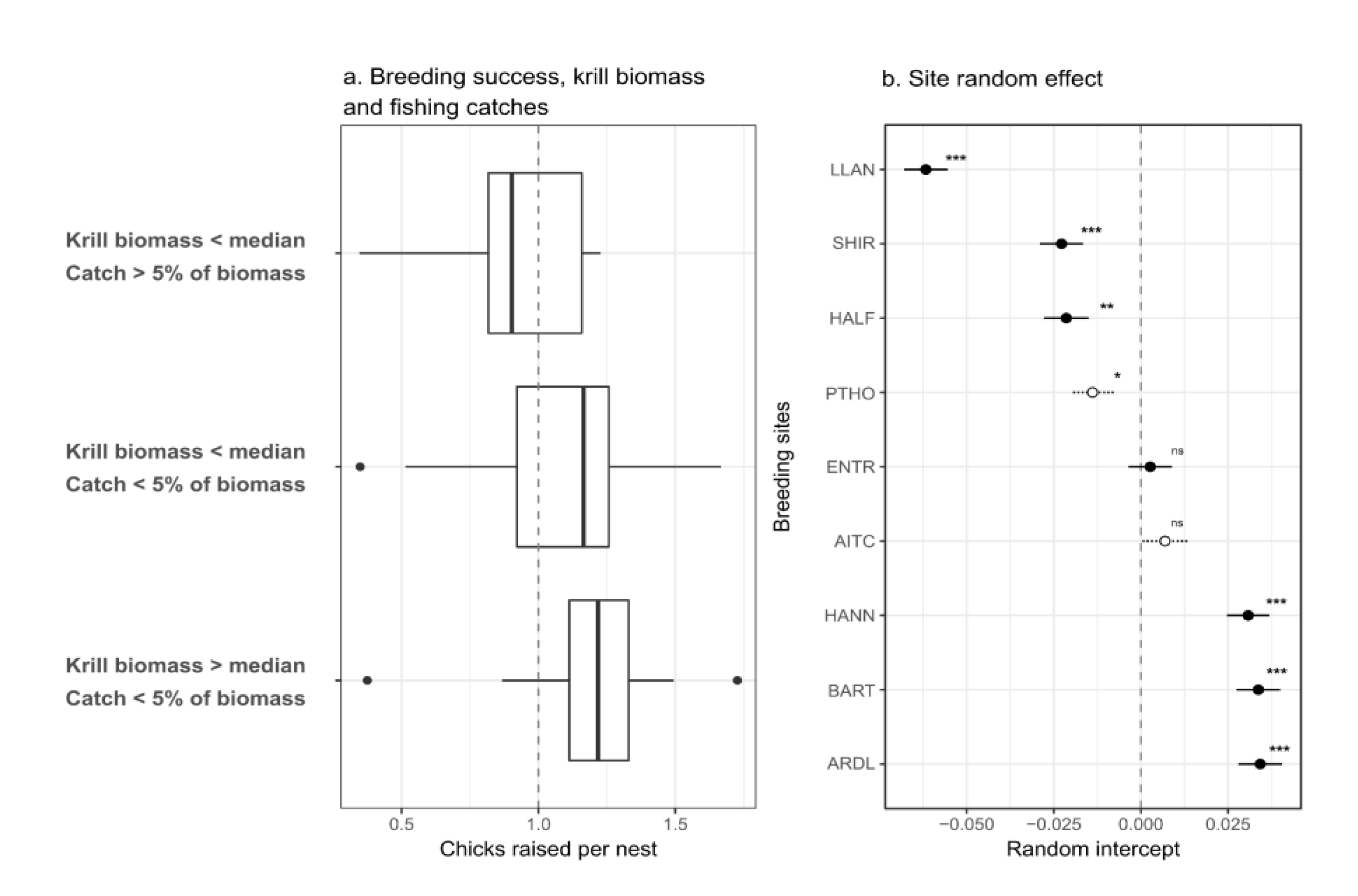
The median number of chicks raised per nest in the colonies within the fishing strata was lower than 1.00 in years when harvest rate was above 5% and krill biomass was below the median. In contrast, the number of chicks raised per nest ranged between 0.9 and 1.25 when catch was <5% and krill biomass was below median, and between 1.10 and 1.30 with krill biomass above median (F
3,62=90.91 simulated P=0.008;
Figure 4a). Site effect was important for the differences, and 4 sites had the random intercept (difference of the site mean in comparison with the mean of all sites) significantly lower than 0 (Fig. 4b), coinciding with sites where in the past fishery has fished close to the colony during summer (
Figure 5).
4. Discussion
Summer krill biomass on the northwestern Antarctic Peninsula has been on a slight decreasing trend between 1995 and 2020. Recent estimates of Antarctic krill biomass ([
12,
36], this study) indicated a smooth tendency of decrease, therefore matching trends verified by other sources of information, such as research net sampling [
12,
13] or fishery-dependant variables [
31].
Conversely, the fishing catches in subarea 48.1 have shown an increasing trend reaching the catch limit of 155,000 tonnes constantly in the last 4 years. Based on the high levels of inter-annual krill variability, it seems that the level of catches (associated to the trigger level) may represent a substantial amount of the biomass on a given strata in some years. During recent years, around 70% of the allocated catch limit for subarea 48.1 has been caught on the Bransfield strata alone [
39]. Although, in recent fishing seasons the fleet has moved to a more autumn-winter operation in the area, if similar levels of catch occur in the future, in conditions like those in years 2001, 2002, 2004 and 2010 (see results) the catch could represent a substantial amount of the local standing krill stock (meaning eventually that a substantial amount of spawning population could be caught [
40]), periods when competition with krill-predators can occur [
15,
16,
19]. In addition, the rapid recovery of baleen whales after reaching critical levels due to whaling [
41,
42], could potentially increase competition for krill [
43], as baleen whales are important krill consumers in the area [
44,
45].
Our results indicated that decreases of breeding success of the three species of
Pygoscelis penguins were observable throughout the WAP. A geographical coherence was noted where populations north of 65°S tended to have steeper decreases, which are sectors experiencing faster warming [
46,
47] with consequent lower sea ice coverage [
48,
49], increased krill fishing catches [
16,
50] and spatiotemporal fishery concentration [
31]. Localized reductions of penguin populations have been observed by other studies [
51,
52,
53], which might be linked to a reduction in breeding success and recruitment [
5] as a response to lower availability of krill under climate change [
21]. For instance, in years when winter sea ice was low, summer krill biomass also was low, which reflected in increased foraging effort by penguins and reduced breeding success [
21]. Therefore, sustained decreases in breeding success found in this study are consistent with a decrease in krill biomass.
The hypothesis that increased fishing catches in periods of low krill availability implied in lower penguin breeding success was supported by the results, therefore indicating that the current strategy management based on a fixed catch limit (trigger level) may not prevent potential effects of the fishery on predators when local krill biomass is low. This mechanism was previously proposed to reductions in the number of breeding pairs and on breeding performance [
15,
16].
The management of the krill fishery is based on a precautionary fixed catch limit applied for the entire subarea 48.1. While such approach balances and redistributes catch, the krill biomass is averaged over 20 years periods to calculate the impact fishing will have [
54]. But the krill biomass is not homogeneously distributed in space and is not constant in time [
40,
55]; the catch levels being calculated using fixed biomass estimations (i.e. long term biomass averages and constant recruitment rates) to estimate impact of fishing does not reflect the reality of krill dynamics [
40,
56]. Such a management approach, might not be as precautionary as previously thought [
15]. Proposed solutions would be to use the lower 95% value of the biomass to estimate allowed catch, allocate catch limits according risk assessment and to re-evaluate the krill parameters in shorter intervals within the management strata [
32,
35], which in light of our results, would be the better approach to avoiding unexpected impacts. As indicated by our results, the effects of fishing up to the catch limit was detrimental to penguins breeding success in years when krill biomass was low. Taking into account not only the period of fishing but also the availability of krill is necessary to prevent impacts, as it is possible that under low krill availability, the effect of fishing could be carried on to the following years [
16].
5. Conclusions
Our analysis indicates that krill removals have had detectable impact over breeding success of
Pygoscelis penguins in the West and Bransfield strata. In periods of low krill biomass, the catch limit can represent a substantial amount of the local krill biomass, likely resulting in interference competition. Our results identified localized effects of the krill fishery in the WAP, reinforcing the need to allocate catch limits over smaller spatial scales, to ensure the application of CCAMLR's precautionary management approach. On the other hand, the revised management framework must consider the natural variability of krill populations and even more so in the context of climate change in the WAP. Such modifications would avoid krill fishing becoming an additional pressure reducing penguins’ capacity to adapt to the fast changes occurring in the Antarctic Peninsula. Finally, finding indicators that are able to predict when those periods of low krill biomass will occur - winter sea ice for instance [
21]- could be of help in planning in advance the allowed catch levels in a given year.
Author Contributions
Conceptualization, L.K., F.S. and C.C.; methodology, L.K., F.S., M.M.; formal analysis, L.K.; resources, C.C.; writing—original draft preparation, L.K.; writing—review and editing, C.C., F.S., M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Instituto Antártico Chileno through Programa Áreas Marinas Protegidas (AMP 24 03 052), and by ANID – Millennium Science Initiative Program – ICN2021_002.
Acknowledgments
The authors thanks to the CCAMLR Secretariat for providing data on Krill fishery submitted by members and access to krill acoustic data.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Full reproducible R code and detailed analytic procedures used in this study.
Appendix B
Available data for reproducing analysis used in this study, which is composed of comma-separated files (.csv) of penguin counts, summarized yearly fishing catches, krill acoustic estimations and shapefiles (.shp) of the Strata for estimating krill acoustic abundance.
References
- Krüger, L. Decreasing Trends of Chinstrap Penguin Breeding Colonies in a Region of Major and Ongoing Rapid Environmental Changes Suggest Population Level Vulnerability. Diversity 2023, 15, 327. [Google Scholar] [CrossRef]
- Strycker, N.; Wethington, M.; Borowicz, A.; Forrest, S.; Witharana, C.; Hart, T.; Lynch, H.J. A Global Population Assessment of the Chinstrap Penguin (Pygoscelis Antarctica). Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.J.; Naveen, R.; Trathan, P.N.; Fagan, W.F. Spatially Integrated Assessment Reveals Widespread Changes in Penguin Populations on the Antarctic Peninsula. Ecology 2012, 93, 1367–1377. [Google Scholar] [CrossRef]
- Hinke, J.T.; Salwicka, K.; Trivelpiece, S.G.; Watters, G.M.; Trivelpiece, W.Z. Divergent Responses of Pygoscelis Penguins Reveal a Common Environmental Driver. Oecologia 2007, 153, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Trivelpiece, W.Z.; Hinke, J.T.; Miller, A.K.; Reiss, C.S.; Trivelpiece, S.G.; Watters, G.M. Variability in Krill Biomass Links Harvesting and Climate Warming to Penguin Population Changes in Antarctica. Proc. Natl. Acad. Sci. 2011, 108, 7625–7628. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, P.; Naveen, R.; Forrest, S.; Poncet, J.; Lynch, H.J. A Comprehensive Coastal Seabird Survey Maps out the Front Lines of Ecological Change on the Western Antarctic Peninsula. Polar Biol. 2015, 927–940. [Google Scholar] [CrossRef]
- Herman, R.; Borowicz, A.; Lynch, M.; Trathan, P.; Hart, T.; Lynch, H. Update on the Global Abundance and Distribution of Breeding Gentoo Penguins (Pygoscelis Papua). Polar Biol. 2020, 43, 1947–1956. [Google Scholar] [CrossRef]
- Polito, M.J.; Trivelpiece, W.Z.; Karnovsky, N.J.; Ng, E.; Patterson, W.P.; Emslie, S.D. Integrating Stomach Content and Stable Isotope Analyses to Quantify the Diets of Pygoscelid Penguins. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Dimitrijević, D.; Paiva, V.H.; Ramos, J.A.; Seco, J.; Ceia, F.R.; Chipev, N. Isotopic Niches of Sympatric Gentoo and Chinstrap Penguins: Evidence of Competition for Antarctic Krill ? Polar Biol. 2018, 41, 1655–1669. [Google Scholar] [CrossRef]
- McMahon, K.W.; Michelson, C.I.; Hart, T.; McCarthy, M.D.; Patterson, W.P.; Polito, M.J. Divergent Trophic Responses of Sympatric Penguin Species to Historic Anthropogenic Exploitation and Recent Climate Change. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 25721–25727. [Google Scholar] [CrossRef]
- Panasiuk, A.; Wawrzynek-Borejko, J.; Musiał, A.; Korczak-Abshire, M. Pygoscelis Penguin Diets on King George Island, South Shetland Islands, with a Special Focus on the Krill Euphausia Superba. Antarct. Sci. 2020, 32, 21–28. [Google Scholar] [CrossRef]
- Atkinson, A.; Hill, S.L.; Pakhomov, E.A.; Siegel, V.; Reiss, C.S.; Loeb, V.J.; Steinberg, D.K.; Schmidt, K.; Tarling, G.A.; Gerrish, L.; et al. Krill (Euphausia Superba) Distribution Contracts Southward during Rapid Regional Warming. Nat. Clim. Chang. 2019, 9, 142–147. [Google Scholar] [CrossRef]
- Atkinson, A.; Hill, S.L.; Reiss, C.S.; Pakhomov, E.A.; Beaugrand, G.; Tarling, G.A.; Yang, G.; Steinberg, D.K.; Schmidt, K.; Edwards, M.; et al. Stepping Stones towards Antarctica: Switch to Southern Spawning Grounds Explains an Abrupt Range Shift in Krill. Glob. Chang. Biol. 2022, 28, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- WG-ASAM Results from the WG-ASAM Intersessional e-Group on Krill Biomass Estimates from Acoustic Surveys; 2021.
- Watters, G.M.; Hinke, J.T.; Reiss, C.S. Long-Term Observations from Antarctica Demonstrate That Mismatched Scales of Fisheries Management and Predator-Prey Interaction Lead to Erroneous Conclusions about Precaution. Sci. Rep. 2020, 10, 2314. [Google Scholar] [CrossRef] [PubMed]
- Krüger, L.; Huerta, M.F.; Santa Cruz, F.; Cárdenas, C.A. Antarctic Krill Fishery Effects over Penguin Populations under Adverse Climate Conditions: Implications for the Management of Fishing Practices. Ambio 2021, 50, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Hinke, J.T.; Cossio, A.M.; Goebel, M.E.; Reiss, C.S.; Trivelpiece, W.Z.; Watters, G.M. Identifying Risk: Concurrent Overlap of the Antarctic Krill Fishery with Krill-Dependent Predators in the Scotia Sea. PLoS One 2017, 12, e0170132. [Google Scholar] [CrossRef] [PubMed]
- Warwick-Evans, V.; Ratcliffe, N.; Lowther, A.D.; Manco, F.; Ireland, L.; Clewlow, H.L.; Trathan, P.N. Using Habitat Models for Chinstrap Penguins Pygoscelis Antarctica to Advise Krill Fisheries Management during the Penguin Breeding Season. Divers. Distrib. 2018. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Deagle, B.; Love, K.; Polanowski, A.; Fielding, S.; Wood, A.G.; Hill, S.; Grant, S.; Belchier, M.; Fleming, A.; et al. Changes in Prey Fields Increase the Potential for Spatial Overlap between Gentoo Penguins and a Krill Fishery within a Marine Protected Area. Divers. Distrib. 2021, 27, 552–563. [Google Scholar] [CrossRef]
- Phillips, J.A.; Fayet, A.L.; Guilford, T.; Manco, F.; Warwick-Evans, V.; Trathan, P. Foraging Conditions for Breeding Penguins Improve with Distance from Colony and Progression of the Breeding Season at the South Orkney Islands. Mov. Ecol. 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Salmerón, N.; Belle, S.; Cruz, F.S.; Alegria, N.; Finger, J.V.G.; Corá, D.H.; Petry, M.V.; Hernández, C.; Cárdenas, C.A.; Krüger, L. Contrasting Environmental Conditions Precluded Lower Availability of Antarctic Krill Affecting Breeding Chinstrap Penguins in the Antarctic Peninsula. Sci. Rep. 2023, 13, 5265. [Google Scholar] [CrossRef]
- Paiva, V.; Geraldes, P.; Ramirez, I.; Werner, A.; Garthe, S.; Ramos, J. Overcoming Difficult Times: The Behavioural Resilience of a Marine Predator When Facing Environmental Stochasticity. Mar. Ecol. Prog. Ser. 2013, 486, 277–288. [Google Scholar] [CrossRef]
- Paiva, V.H.; Pereira, J.; Ceia, F.R.; Ramos, J.A. Environmentally Driven Sexual Segregation in a Marine Top Predator. Sci. Rep. 2017, 7, 2590. [Google Scholar] [CrossRef]
- Boyd, C.; Grünbaum, D.; Hunt, G.L.; Punt, A.E.; Weimerskirch, H.; Bertrand, S. Effects of Variation in the Abundance and Distribution of Prey on the Foraging Success of Central Place Foragers. J. Appl. Ecol. 2017, 54, 1362–1372. [Google Scholar] [CrossRef]
- Osborne, O.E.; Hara, P.D.O.; Whelan, S.; Zandbergen, P.; Hatch, S.A.; Elliott, K.H. Breeding Seabirds Increase Foraging Range in Response to an Extreme Marine Heatwave. Mar. Ecol. Prog. Ser. 2020, 646, 161–173. [Google Scholar] [CrossRef]
- Lowther, A.D.; Trathan, P.; Tarroux, A.; Lydersen, C.; Kovacs, K.M. The Relationship between Coastal Weather and Foraging Behaviour of Chinstrap Penguins, Pygoscelis Antarctica. ICES J. Mar. Sci. 2018, 75, 1940–1948. [Google Scholar] [CrossRef]
- Krafft, B.A.; Lowther, A.; Macaulay, G.; Chierici, M.; Biuw, M.; Renner, A.; Klevjer, T.A.; Arata, J.; Makhado, A.; Reiss, C.; et al. Development of Methods Relevant to Feedback Management (FBM) for the Krill Fishery. CCAMLR Work. Gr. Ecosyst. Monit. Manag. WG-EMM-18/08.
- Lowther, A.D.; Krafft, B.; Cardenas, C.; Zhao, X.; Bergstad, O.A. Empirically-Driven Feedback Management Incorporating Multi-Scale Risk Assessment and an Experimental Framework to Facilitate Adaptive Improvement. CCAMLR Work. Gr. Ecosyst. Monit. Manag. WG-EMM-19/06.
- Humphries, G.R.W.; Naveen, R.; Schwaller, M.; Che-Castaldo, C.; McDowall, P.; Schrimpf, M.; Lynch, H.J. Mapping Application for Penguin Populations and Projected Dynamics (MAPPPD): Data and Tools for Dynamic Management and Decision Support. Polar Rec. (Gr. Brit). 2017, 53, 160–166. [Google Scholar] [CrossRef]
- Lynch, H.; Che-Castaldo, C.; Humphries, G.; Naveen, R. MAPPPD (Mapping Application for Penguin Populations and Projected Dynamics) Available online:. Available online: https://www.penguinmap.com/ (accessed on 23 August 2022).
- Santa Cruz, F.; Krüger, L.; Cárdenas, C.A. Spatial and Temporal Catch Concentrations for Antarctic Krill: Implications for Fishing Performance and Precautionary Management in the Southern Ocean. Ocean Coast. Manag. 2022, 223, 106146. [Google Scholar] [CrossRef]
- CCAMLR Report of the Forty-First Meeting of the Scientific Committee; Hobart, Australia, 2022.
- Hadfield, J.D. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–21. [Google Scholar] [CrossRef]
- Hadfield, J. MCMC Generalised Linear Mixed Models 2022.
- CCAMLR Report of the Working Group on Ecosystem Monitoring and Management; Hobart, Australia, 2022.
- Constable, A. Managing Fisheries to Conserve the Antarctic Marine Ecosystem: Practical Implementation of the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR). ICES J. Mar. Sci. 2000, 57, 778–791. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. 2022.
- Krafft, B.A.; Macaulay, G.J.; Skaret, G.; Knutsen, T.; Bergstad, O.A.; Lowther, A.; Huse, G.; Fielding, S.; Trathan, P.; Murphy, E.; et al. Standing Stock of Antarctic Krill (Euphausia superba Dana, 1850) (Euphausiacea) in the Southwest Atlantic Sector of the Southern Ocean, 2018–19. J. Crustac. Biol. 2021, 41, 1–17. [Google Scholar] [CrossRef]
- CCAMLR Secretariat Fishery Report 2021: Euphausia superba Reported Catch. 2022.
- Meyer, B.; Atkinson, A.; Bernard, K.S.; Brierley, A.S.; Driscoll, R.; Hill, S.L.; Marschoff, E.; Maschette, D.; Perry, F.A.; Reiss, C.S.; et al. Successful Ecosystem-Based Management of Antarctic Krill Should Address Uncertainties in Krill Recruitment, Behaviour and Ecological Adaptation. Commun. Earth Environ. 2020, 1, 28. [Google Scholar] [CrossRef]
- Johannessen, J.E.D.; Biuw, M.; Lindstrøm, U.; Ollus, V.M.S.; Martín López, L.M.; Gkikopoulou, K.C.; Oosthuizen, W.C.; Lowther, A. Intra-Season Variations in Distribution and Abundance of Humpback Whales in the West Antarctic Peninsula Using Cruise Vessels as Opportunistic Platforms. Ecol. Evol. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Pallin, L.J.; Kellar, N.M.; Steel, D.; Botero-Acosta, N.; Baker, C.S.; Conroy, J.A.; Costa, D.P.; Johnson, C.M.; Johnston, D.W.; Nichols, R.C.; et al. A Surplus No More? Variation in Krill Availability Impacts Reproductive Rates of Antarctic Baleen Whales. Glob. Chang. Biol. 2023, 1–14. [Google Scholar] [CrossRef]
- Trebilco, R.; Melbourne-Thomas, J.; Constable, A.J. The Policy Relevance of Southern Ocean Food Web Structure: Implications of Food Web Change for Fisheries, Conservation and Carbon Sequestration. Mar. Policy 2020, 115, 103832. [Google Scholar] [CrossRef]
- Trathan, P.N.; Warwick-Evans, V.; Young, E.F.; Friedlaender, A.; Kim, J.H.; Kokubun, N. The Ecosystem Approach to Management of the Antarctic Krill Fishery - the ‘Devils Are in the Detail’ at Small Spatial and Temporal Scales. J. Mar. Syst. 2022, 225, 103598. [Google Scholar] [CrossRef]
- Warwick-Evans, V.; Kelly, N.; Dalla Rosa, L.; Friedlaender, A.; Hinke, J.T.; Kim, J.H.; Kokubun, N.; Santora, J.A.; Secchi, E.R.; Seyboth, E.; et al. Using Seabird and Whale Distribution Models to Estimate Spatial Consumption of Krill to Inform Fishery Management. Ecosphere 2022, 13, 1–24. [Google Scholar] [CrossRef]
- Bozkurt, D.; Bromwich, D.H.; Carrasco, J.; Hines, K.M.; Maureira, J.C.; Rondanelli, R. Recent Near-Surface Temperature Trends in the Antarctic Peninsula from Observed, Reanalysis and Regional Climate Model Data. Adv. Atmos. Sci. 2020, 37, 477–493. [Google Scholar] [CrossRef]
- Carrasco, J.F.; Bozkurt, D.; Cordero, R.R. A Review of the Observed Air Temperature in the Antarctic Peninsula. Did the Warming Trend Come Back after the Early 21st Hiatus? Polar Sci. 2021, 28, 100653. [Google Scholar] [CrossRef]
- Eayrs, C.; Li, X.; Raphael, M.N.; Holland, D.M. Rapid Decline in Antarctic Sea Ice in Recent Years Hints at Future Change. Nat. Geosci. 2021, 14, 460–464. [Google Scholar] [CrossRef]
- Parkinson, C.L. A 40-y Record Reveals Gradual Antarctic Sea Ice Increases Followed by Decreases at Rates Far Exceeding the Rates Seen in the Arctic. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 14414–14423. [Google Scholar] [CrossRef]
- Santa Cruz, F.; Ernst, B.; Arata, J.A.; Parada, C. Spatial and Temporal Dynamics of the Antarctic Krill Fishery in Fishing Hotspots in the Bransfield Strait and South Shetland Islands. Fish. Res. 2018, 208, 157–166. [Google Scholar] [CrossRef]
- Petry, M.V.; Valls, F.C.L.; Petersen, E.D.S.; Krüger, L.; Piuco, R.D.C.; dos Santos, C.R. Breeding Sites and Population of Seabirds on Admiralty Bay, King George Island, Antarctica. Polar Biol. 2016, 39, 1343–1349. [Google Scholar] [CrossRef]
- Petry, M. V.; Valls, F.C.L.; Petersen, E.S.; Finger, J.V.G.; Krüger, L. Population Trends of Seabirds at Stinker Point, Elephant Island, Maritime Antarctica. Antarct. Sci. 2018, 30, 220–226. [Google Scholar] [CrossRef]
- Barbosa, A.; Benzal, J.; De León, A.; Moreno, J. Population Decline of Chinstrap Penguins (Pygoscelis antarctica) on Deception Island, South Shetlands, Antarctica. Polar Biol. 2012, 35, 1453–1457. [Google Scholar] [CrossRef]
- Constable, A.J.; De La Mare, W.K. A Generalised Model for Evaluating Yield and the Long-Term Status of Fish Stocks under Conditions of Uncertainty. CCAMLR Sci. 1996, 3, 31–54. [Google Scholar]
- Hofman, R.J. Sealing, Whaling and Krill Fishing in the Southern Ocean: Past and Possible Future Effects on Catch Regulations. Polar Rec. 2017, 53, 88–99. [Google Scholar] [CrossRef]
- Kinzey, D.; Watters, G.M.; Reiss, C.S. Estimating Recruitment Variability and Productivity in Antarctic Krill. Fish. Res. 2019, 217, 98–107. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).