1. Introduction:
Intracranial pressure (ICP) monitoring is critical in the clinical management of patients with intracranial insults/lesions.[
1,
2,
3] Monitoring with intracerebral pressure probes has become more prevalent as compared to ventricular drain measurements (i.e., the “gold standard”).[
4,
5,
6] It is however prudent to note that no consensus exists with regard to which ICP monitoring modality is ultimately employed clinically.[
1] There are several types of intracerebral pressure sensors (e.g., fiberoptic or stain-gauge); these sensors require calibration procedures and carry the risk of a silent zero drift.[
7] To overcome such limitations an air-pouch balloon based sensor was introduced ~20 years ago by Spiegelberg (Aesculap, Inc., Center Valley, PA).[
8] One of the benefits of this probe is the automatic zeroing after connection to the monitoring unit; this process is repeated every hour reducing the risk of a silent zero drift.[
8] Previous work has proven that Spiegelberg air-pouch measurements are reliable and correlate with ICPs ascertained via a ventricular drain.[
8,
9]
Despite the promise of Spiegelberg air-pouch balloon based ICP measurements, herein we present a clinical scenario in which we were misled by discordant ICPs values secondary to clot adherence on the ICP probe. Based on this experience, we sought to clarify our initial clinical findings experimentally (i.e., at the bench) and ultimately returned to the bedside in an effort to validate our experimentally findings (i.e., via a clinically pilot).
Case illustration
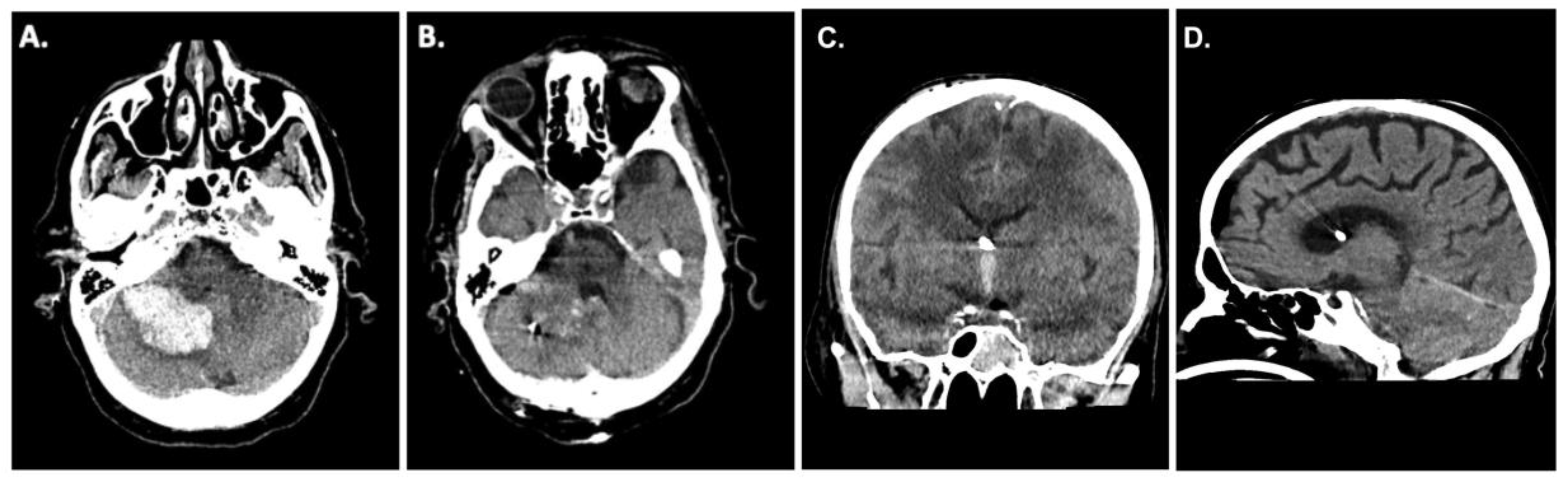
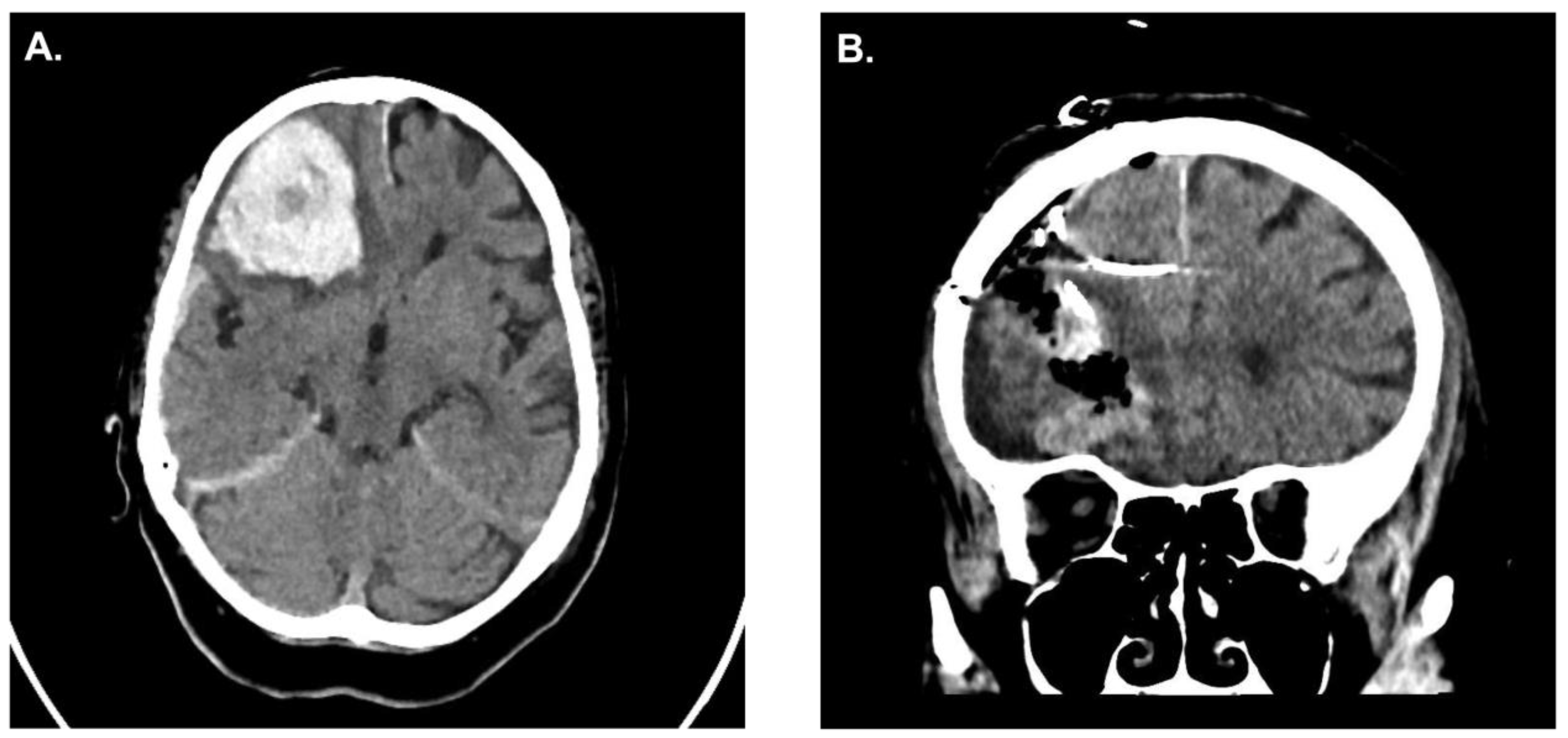
A 62-year-old patient presented to the emergency room at our institution after collapsing. Due to worsening mental status he was electively intubated and a computed tomography (CT)-scan of the brain revealed a cerebellar hemorrhage with compression of the fourth ventricle/resultant hydrocephalus (
Figure 1A). CT angiography failed to demonstrate any underlying vascular pathology. Due to the size of the hemorrhage and its mass effect, the patient was taken for an emergency clot evacuation and surgical decompression. Just prior to the decompression a combined external ventricular drain-ICP probe from Spiegelberg was placed in the right frontal ventricle; the sub-occipital craniotomy with hematoma evacuation was uncomplicated. During the case an additional infratentorial ICP probe was inserted in the hematoma cavity as per our institutional standard. After replacing the bone, the infratentorial probe exhibited an ICP value of 27mmHg whilst the supratentorial probe reported 13mmHg, respectively. Despite several attempts to re-zero, the infratentorial ICP value did not change. As such, we felt it was prudent to explore the surgical site yet upon reopening the surgical field showed no sign of rebleeding and/or massive swelling of the cerebellum. Even the cerebellum was under the level of the dura, which was discordant to the elevated ICP value of 27mmHg. We removed the ICP sonde and a new ICP probe was inserted into the cerebellum (
Figure 1B). Observing the previous ICP probe, we noticed a thin clot adhering to the air-pouch of the ICP probe. After revising the probes position, the infratentorial ICP was noted to be 15mmHg. The postoperative CT scan showed sufficient hematoma evacuation with proper location of the combined EVD-ICP probe in the right ventricle (
Figure 1B/1C). In addition, there was no sign of transtentorial herniation in the postoperative CT-scan (
Figure 1D). The remainder of the patients post-operative course was uneventful and they were ultimately discharged to a rehabilitation facility given residual imbalance and dysmetria.
2. Methods:
The study was approved by the ethics committee, [BLINDED FOR REVIEW]. Formal consent of the patients described/presented within the text were obtained. The majority of the reported work involved an experimental study/apparatus (i.e., no animal or human involvement).
2.1. Experiment setting
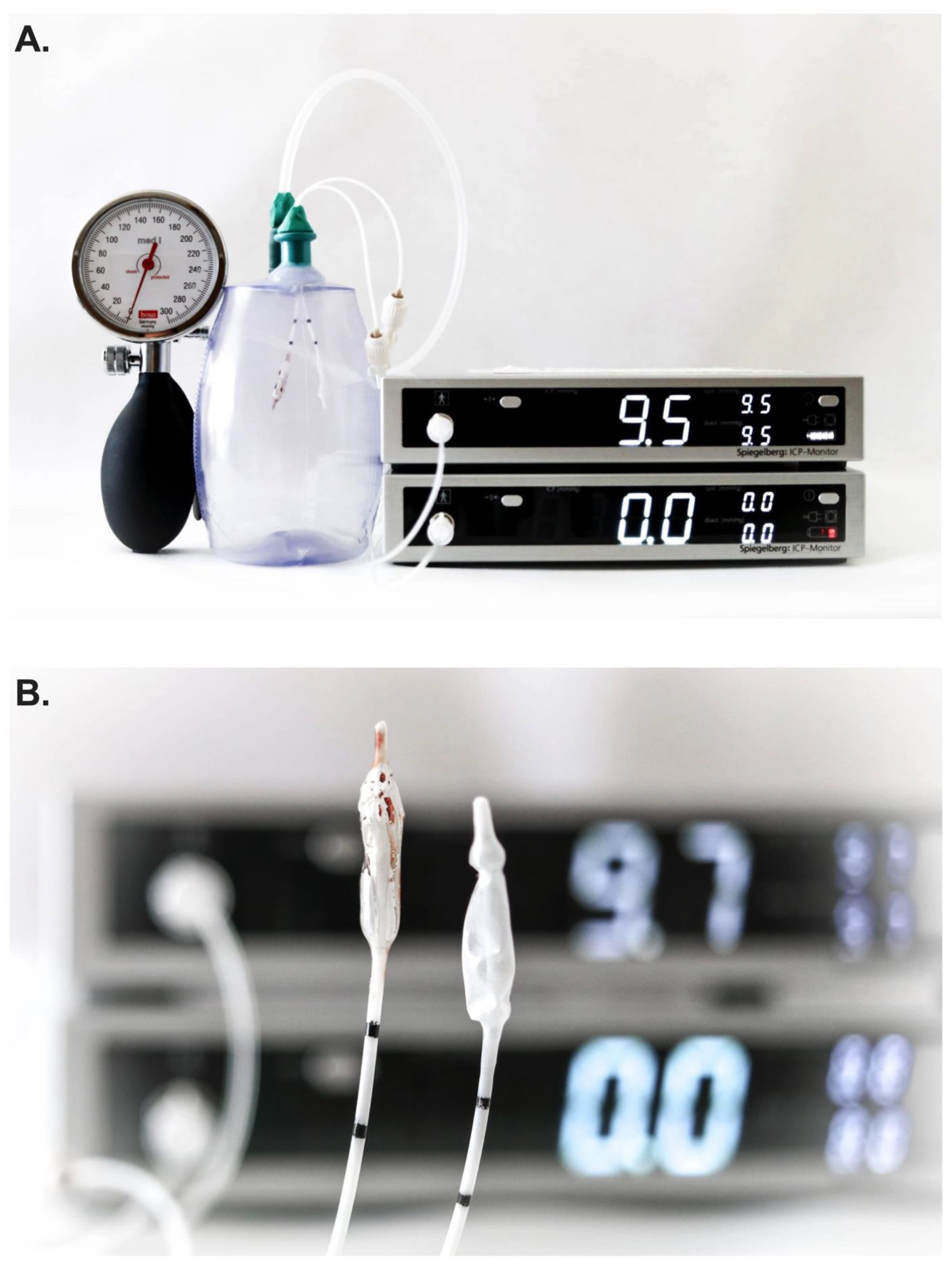
Two Spiegelberg 3PN-sensors were simultaneously inserted into a closed drain system and were connected to two separate ICP monitors thereby allowing for simultaneous ICP measurements (PRIVAC@, Primed Halberstadt, Medizintechnik GmbH, Halberstadt, Germany). Through a separate port, a 12F tube was connected to the drain system and a blood pressure manometer (BOSO, BOSCH + SOHN GmbH u. Co.KG, Jungingen). In so doing, the pressure within the closed drain system could be altered in a controlled manner (
Figure 2A).
2.2. Study design
Two Spiegelberg 3PN-sensors were inserted in the closed drain system and a simultaneous measurement of pressure was performed during steady increases in pressure within the chamber (i.e., from 0 to 30mmHg in 5mmHg increments and from 30 to 60mmHg in 10mmHg increments). To minimize potential sources bias, this experiment was repeated > 5 times on different days by two independent investigators (S.W. and S.H.).
Once the system was validated and concordance of measurements between probes confirmed in the experiment setting, one Spiegelberg 3PN-sensor was placed into a petridish filled with 5ml of fresh whole human blood obtained from the hospital’s laboratory core just prior to the experiment. After waiting for the coagulation (~30 minutes), a thin-layer of clot was subsequently visible on the air-pouch balloon sensor (
Figure 2B). Thereafter, simultaneous measurements of pressure were again performed as per the above using both ICP probes in the experimental setting (i.e., clot-layered and control).
2.3. Translation of experimental data (pilot clinical case)
Given our index case and the results obtained during our experimental study we felt it was critical to attempt to validate our findings in a relevant clinical setting.
A 50-year-old patient was admitted to our hospital secondary to hypertensive intracerebral hemorrhage within the basal ganglia. After consent, the patient was taken to the operating room for surgical placement of two ICP monitoring devices. One probe air-pouch probe was placed within the hematoma cavity and the other one in the adjacent cerebral parenchyma. After surgery, the patient was transferred to the neuro-intensive care unit (ICU) for monitoring/care.
2.4. Statistical analyses
All statistical analyses were conducted using IBM SPSS© (version 27, IBM Corp., Armonk, NY, USA) or GraphPad Prism (version 9.2.0, San Diego, CA, USA). Mean values +/- standard deviation (SD) were calculated, and a student’s t-test was performed for ICP values; Bonferroni corrections were applied as appropriate. A p-value of 0.05 was defined to be statistically significant.
3. Results:
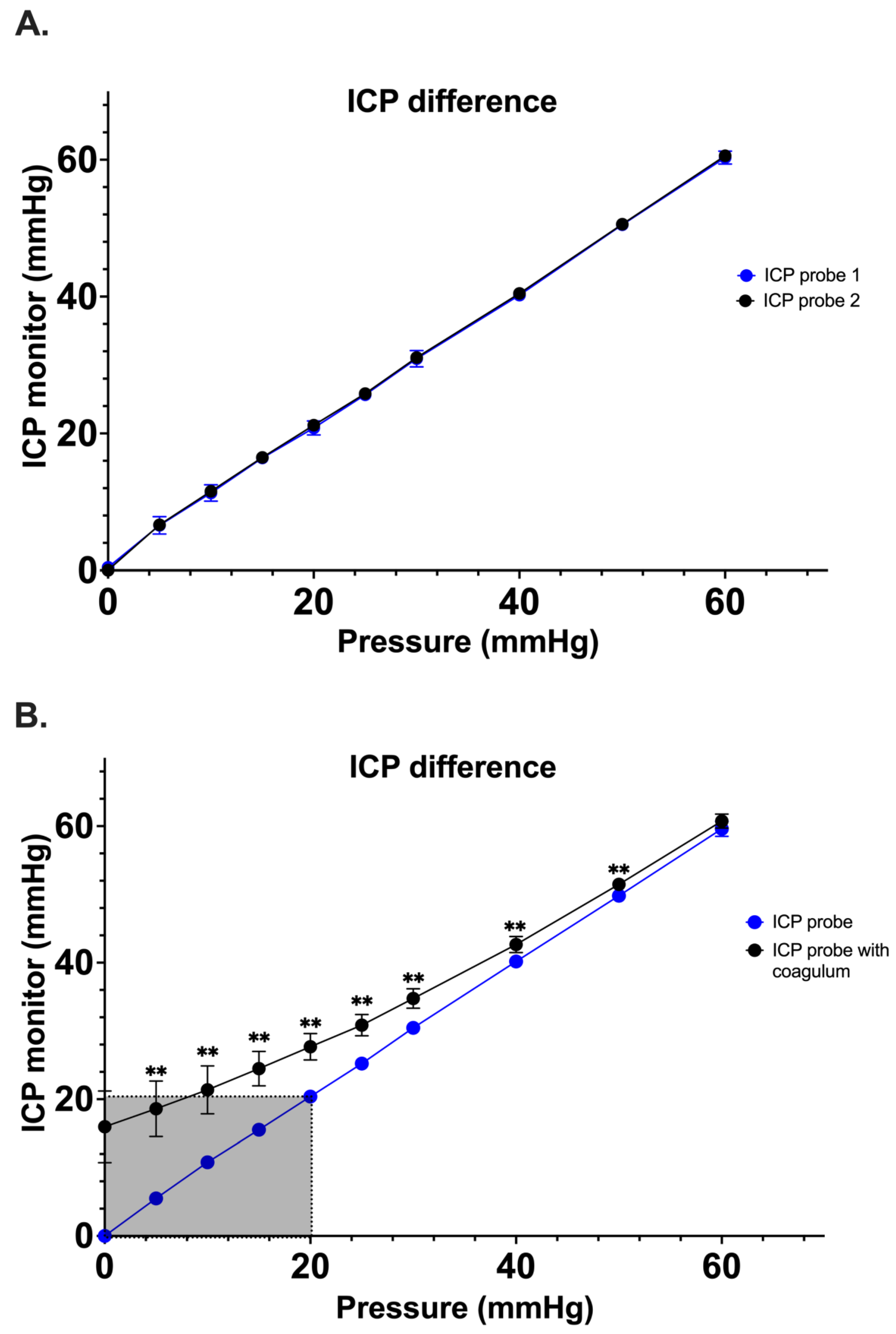
Simultaneous pressure measurements by two control Spiegelberg-3PN air-pouch ICP sensors demonstrated good correlation with no significant difference having been noted between the pressure values ascertained between 0 and 60 mmHg (n=5; range 0-1.6 mmHg) (
Figure 3A). Once the experimental system was optimized, a secondary study comparing ICP probes coated with clotted blood vs control ICP probes was performed (n=10). At 0 mm Hg, the measured mean pressure of the ICP+clot probe was 16.0 5.3 mmHg as compared to a mean pressure of 00 mmHg of the control ICP probe (p<0.001). Interestingly, a significant difference between both probes was observed up to 50 mmHg (51.5 1.0 mmHg vs 49.8 0.4 mmHg, p<0.001) (
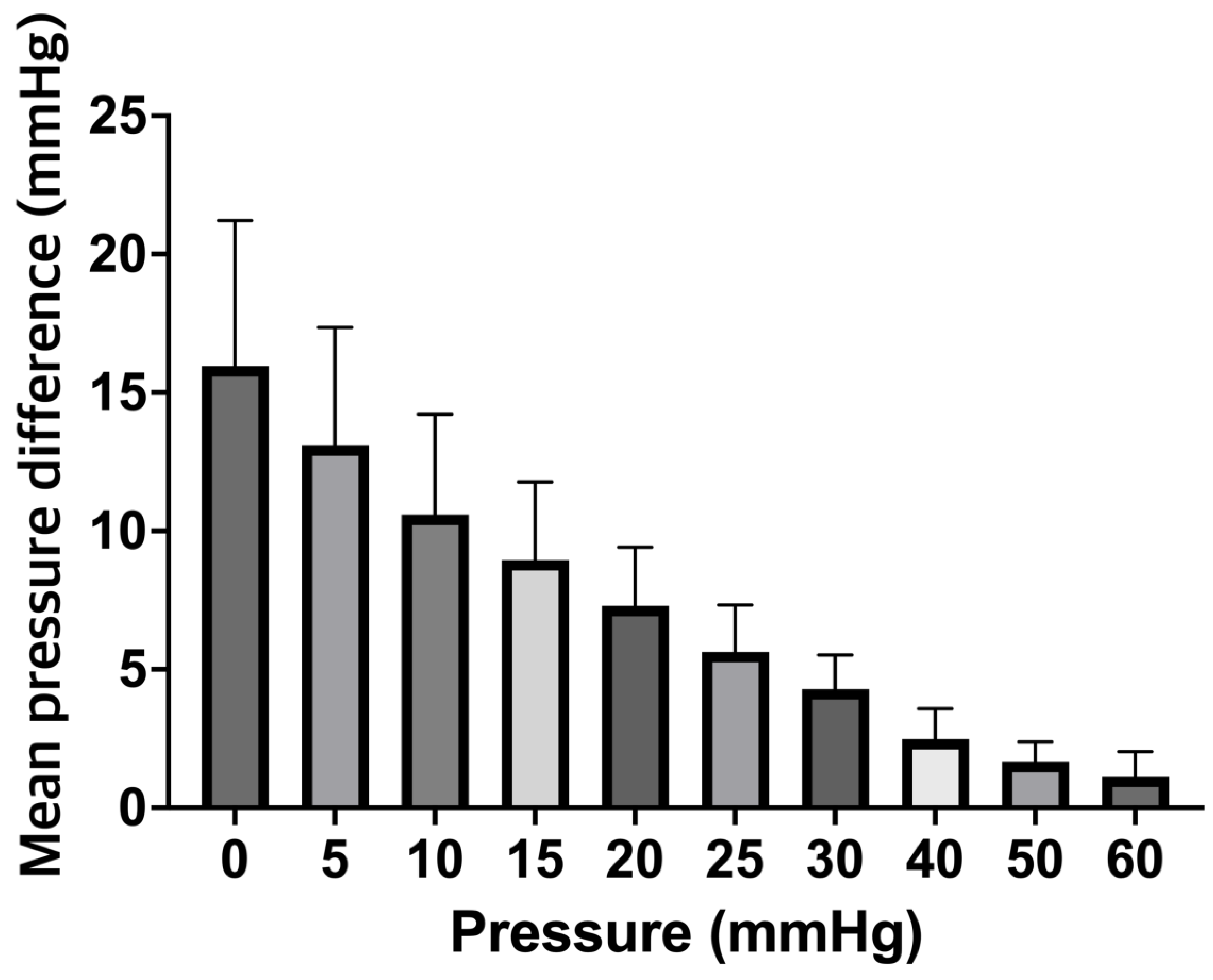
Figure 3B). It is also important to note that we observed falsely high mean ICP values over a range of clinically relevant ICPs for the ICP+clot probe. For example, the control probe correctly displayed a value of 10 mmHg when subjected to a pressure of 10 mmHg, while the ICP+clot probe displayed values of 21.43.5mmHg; this differences are illustrated in
Figure 4.
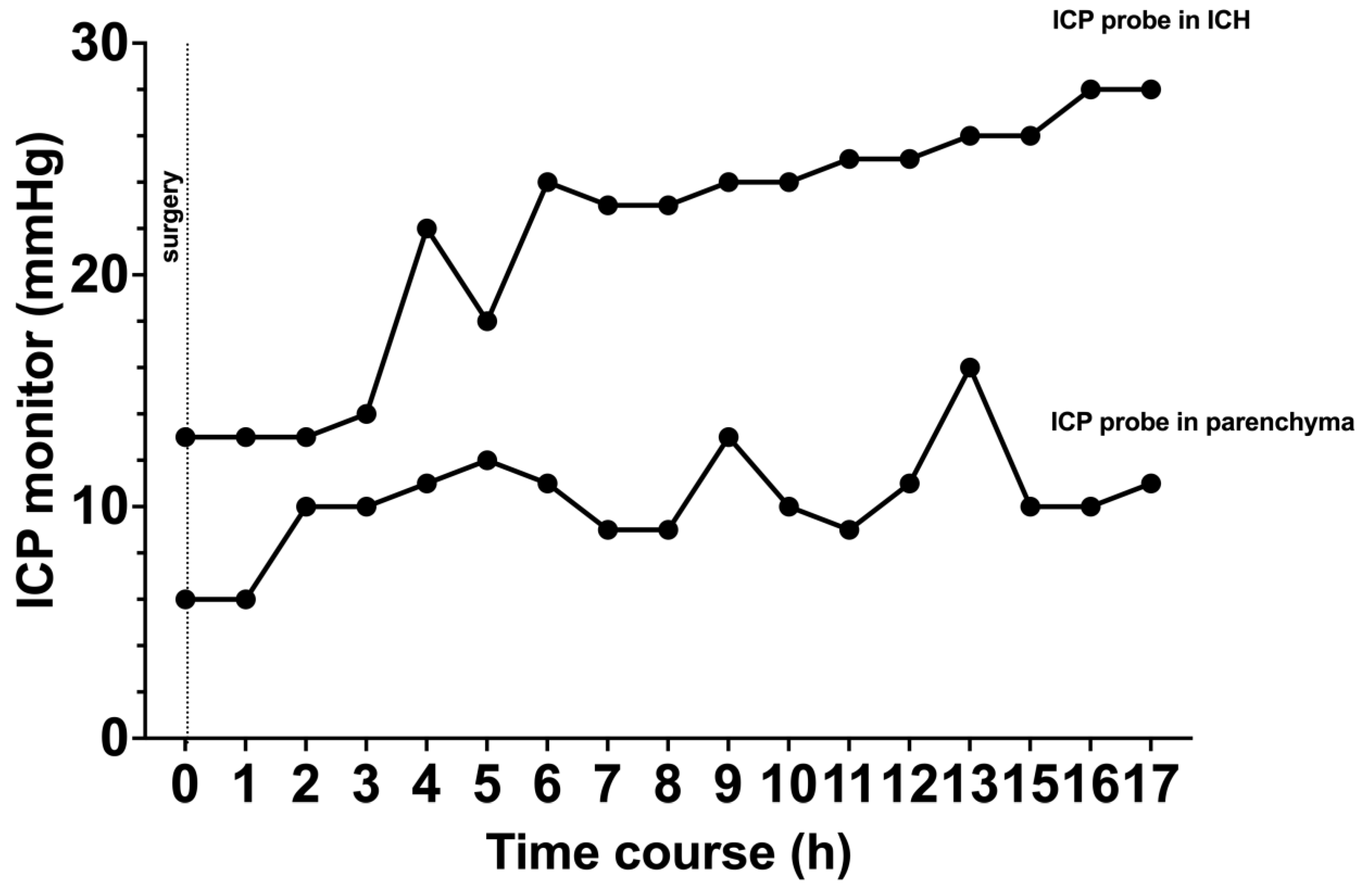
In the clinical pilot, similar results were observed in our patient that underwent simultaneous ICP measurement; the patient presented with a right frontal ICH and the indication for surgical evacuation was made (
Figure 5A). After hematoma evacuation, ICP probes were inserted (
Figure 5B). ICP probe located within the hematoma cavity consistently display higher ICPs values when compared to the ICP probe located in the adjacent parenchyma (
Figure 6). No clinical or radiological signs of elevated ICP were observed during the course of observation in the neuro-ICU.
4. Discussion:
The clinical relevance of ICP monitoring has been demonstrated in a myriad of studies and treatment parameters guided by ICP have been associated with improved outcome(s) and mortality.[
3,
10,
11] A threshold of 22 mmHg has been defined as pathological by the Brain Trauma Foundation guidelines; such ICPs warrant an escalation of medical and/or surgical care.[
1] Therefore, a simple and reliable measure of ICP is crucial as physicians/surgeons manage critical ill patients.
As noted above certain classes of ICP probes require zeroing procedures prior to implantation. Further such probes may drift (e.g., from -13 to 22mmHg with the Camino device (Camino Laboratories, San Diego, CA)) or an average difference of 10 mmHg of all recordings in Codman microsensors (Codman/Johnson & Johnson, Raynham, MA).[
8,
12,
13,
14] Given these concerns, the Spiegelberg air-pouch balloon based ICP system has a number of pertinent advantages (i.e., an automated zeroing process after connection to the ICP monitor and the repeated hourly zeroing process correcting potential zero drift). In line with such advantages, the reliability of this class of ICP probe has been reported in several experimental and clinical studies. [
8,
9,
15] Despite the noted benefits of the Spiegelberg ICP system and its correlation with gold standard ventricle measurements, herein we identified/highlighted a potential pitfall in the correct application/measurement related to probe placement. The air-pouch balloon-assisted sensor used in this study has a defined volume in the pouch and 0.1ml of air is pumped into the pouch during the zeroing process. Our clinical experiences and experimental studies suggest that clot adherence to the air pouch may lead to aberrant measurements. While the exact mechanism for such findings remains to be fully elucidated we would posit that they related directly to perturbations in total pouch volume.
Given our initial clinical case and experimental findings we felt it was extremely important to obtain prospective clinical data. The differences between ICP values for the probe located the hematoma vs adjacent parenchyma was even more extreme than expected. Given these data and the risks associated with inaccurate ICP measurement in neurocritical care we feel it imperative to make the following recommendations:
First, this class of ICP probe should not be placed in the regions prone to blood accumulation (e.g., a hematoma cavity or within epidural/subdural spaces). Second, the location of an air-pouch ICP probe should not be placed in the immediate proximity of other tissue that may affect the extension of the air-pouch (i.e., bone, dura mater and/or the falx).
5. Limitations:
We acknowledge that the experimental set-up employed is truly reductionist in nature and does not replicate the true complexity of an in vivo environment. In reality, the ICP probe would be surrounded by tissue and would experience continuous fluid exchange at physiologic temperature. Although, our clinical pilot (N= 1 patient) yielded results that supported our experimental model it is clear that future prospective studies will be required to validate and expand on this body of work.
6. Conclusions:
The air-pouch balloon assisted ICP probe is both a reliable and simple tool for ICP measurement, however, special attention should be applied when placing the ICP probe.
Author Contributions
SW and SH conceived and designed the study, contributed and analyzed the data, wrote the paper. DD, BB, ST, JB and TM collected the data and performed critical revision. FG designed and supervised the study.
Data Availability
The datasets used and/or analyzed in the current study are available upon request.
Acknowledgment
We thank Spiegelberg GmbH & Co for their technical support.
Conflicts of Interest
JDB has an equity position in Treovir Inc. and is a member of the POCKiT Diagnostics and NeuroX1 boards of scientific advisors.
Declarations
The manuscript complies with all instructions to authors and the final manuscript was approved by all authors. Further, this manuscript has not been published elsewhere and is not under consideration by another journal.
Ethics approval
The study was approved by the ethics committee, University Hospital Rostock, Germany (Registration A2020-0276).
References
- Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery [Internet]. 2017;80:6–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27654000. 2765.
- Hawryluk GWJ, Citerio G, Hutchinson P, Kolias A, Meyfroidt G, Robba C, et al. Intracranial pressure: current perspectives on physiology and monitoring. Intensive Care Med [Internet]. 2022;48:1471–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35816237. 3581.
- Castaño-Leon AM, Gomez PA, Jimenez-Roldan L, Paredes I, Munarriz PM, Perez IP, et al. Intracranial Pressure Monitoring in Patients With Severe Traumatic Brain Injury: Extension of the Recommendations and the Effect on Outcome by Propensity Score Matching. Neurosurgery [Internet]. 2022;91:437–49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35876668. 3587.
- Bales JW, Bonow RH, Buckley RT, Barber J, Temkin N, Chesnut RM. Primary External Ventricular Drainage Catheter Versus Intraparenchymal ICP Monitoring: Outcome Analysis. Neurocrit Care [Internet]. 2019;31:11–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31037639. 3103.
- Liu H, Wang W, Cheng F, Yuan Q, Yang J, Hu J, et al. External Ventricular Drains versus Intraparenchymal Intracranial Pressure Monitors in Traumatic Brain Injury: A Prospective Observational Study. World Neurosurg [Internet]. 2015;83:794–800. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25541084.
- Volovici V, Huijben JA, Ercole A, Stocchetti N, Dirven CMF, van der Jagt M, et al. Ventricular Drainage Catheters versus Intracranial Parenchymal Catheters for Intracranial Pressure Monitoring-Based Management of Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J Neurotrauma [Internet]. 2019;36:988–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30251919. 3025.
- Zacchetti L, Magnoni S, Di Corte F, Zanier ER, Stocchetti N. Accuracy of intracranial pressure monitoring: systematic review and meta-analysis. Crit Care [Internet]. 2015;19:420. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26627204. 2662.
- Lang J-M, Beck J, Zimmermann M, Seifert V, Raabe A. Clinical Evaluation of Intraparenchymal Spiegelberg Pressure Sensor. Neurosurgery [Internet]. 2003;52:1455–9. Available from: https://journals.lww.com/00006123-200306000-00022. 0000.
- Yau YH, Piper IR, Clutton RE, Whittle IR. Experimental evaluation of the Spiegelberg intracranial pressure and intracranial compliance monitor. Technical note. J Neurosurg [Internet]. 2000;93:1072–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11117854. 1111.
- Lindblad C, Raj R, Zeiler FA, Thelin EP. Current state of high-fidelity multimodal monitoring in traumatic brain injury. Acta Neurochir (Wien) [Internet]. 2022;164:3091–100. Available from: http://www.ncbi.nlm.nih.gov/pubmed/36260235. 3626.
- Talving P, Karamanos E, Teixeira PG, Skiada D, Lam L, Belzberg H, et al. Intracranial pressure monitoring in severe head injury: compliance with Brain Trauma Foundation guidelines and effect on outcomes: a prospective study. J Neurosurg [Internet]. 2013;119:1248–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23971954. 2397.
- Piper I, Barnes A, Smith D, Dunn L. The Camino intracranial pressure sensor: is it optimal technology? An internal audit with a review of current intracranial pressure monitoring technologies. Neurosurgery [Internet]. 2001;49:1158–64; discussion 1164-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11846910. 1184.
- Fernandes HM, Bingham K, Chambers IR, Mendelow AD. Clinical evaluation of the Codman microsensor intracranial pressure monitoring system. Acta Neurochir Suppl [Internet]. 1998;71:44–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9779140. 9779.
- Morgalla MH, Mettenleiter H, Bitzer M, Fretschner R, Grote EH. ICP measurement control: laboratory test of 7 types of intracranial pressure transducers. J Med Eng Technol [Internet]. 1999;23:144–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10561825. 1056.
- Chambers IR, Siddique MS, Banister K, Mendelow AD. Clinical comparison of the Spiegelberg parenchymal transducer and ventricular fluid pressure. J Neurol Neurosurg Psychiatry [Internet]. 2001;71:383–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11511715. 1151.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).