1. Introduction
The development of effective methods of processing natural and associated petroleum gases into fuel and valuable chemical products has been an urgent research area for decades [
1,
2,
3,
4,
5,
6]. Huge reserves of natural gas (4036.9 trillion m
3) [
7], as well as recent achievements in the field of shale gas production, force researchers to develop new and improve existing methods of processing methane as the main component of natural gas. Natural gas is a mixture of methane (70-98%), ethane, propane, butane, pentane, and other components. In addition, methane is the main component of associated petroleum and shale gases, biogas and gas hydrates, the so-called unconventional resources. Therefore, the problem of processing natural hydrocarbon gases is primarily the problem of methane processing.
Despite the huge reserves of natural gas, its use as a chemical raw material remains low. There are still no direct ways to convert methane into chemical products and fuel. Currently, natural gas is mainly burned to generate electrical and thermal energy, and only 5% of natural gas is sent for chemical processing, of which 70% is to produce ammonia, 20% for the synthesis of methanol, and the rest to produce various chemicals [
8].
One of the promising directions of natural gas processing is the process of non-oxidative conversion of methane into aromatic hydrocarbons (MDA: 6 CH
4 ⇋ C
6H
6 + 9H
2) on high-silica zeolites (HSZ) modified with various transition metals [
2,
8,
9,
10,
11,
12,
13]. The target product of MDA is benzene – one of the most popular chemical products. Benzene is the most important raw material for the chemical industry, since it is used both as a starting reagent for the synthesis of a wide variety of compounds, and as a solvent for other reactions. The Mo/ZSM-5 catalytic system exhibits the highest activity and stability during the conversion of methane into aromatic hydrocarbons [
2,
14,
15,
16,
17,
18]. However, narrow zeolite channels formed by micropores complicate the mass transfer process and contribute to intensive coke formation and, therefore, a rapid drop-in catalyst activity. Obtaining catalysts based on zeolites with a micro-mesoporous structure is an urgent problem and opens new possibilities for their application [
19,
20]. In recent years, the attention of many research groups has been directed to the creation of zeolite-containing catalysts with an additional mesoporous structure [
19,
21,
22,
23,
24,
25,
26]. The synthesis of zeolite of the ZSM-5 type with an ordered hierarchical pore system is a promising direction for obtaining a catalyst with higher activity and stability in the methane dehydroaromatization reaction compared to microporous zeolite. On the one hand, the modification of the zeolite carrier by forming secondary mesoporosity leads to an improvement in the dispersion of the active component and an increase in the availability of active catalyst centers, which is of great importance for the effective course of the methane dehydroaromatization reaction. On the other hand, the combination of micro- and mesopores in the structure of the zeolite carrier facilitates the diffusion of reagents to the active centers inside the zeolite channels and the products formed from them, which makes it possible to increase the activity of the metal-containing zeolite catalyst and reduce the intensity of coke formation on its surface.
In this regard, the purpose of this work was to develop methods for the synthesis of zeolites of the ZSM-5 type with a micro-mesoporous structure using the second template of carbon black and the preparation of Mo-containing catalysts based on them, as well as to study the physicochemical and catalytic properties of the obtained zeolite-containing systems in the process of methane dehydroaromatization.
2. Materials and Methods
Synthesis of zeolites. High-silica zeolites (HSZ) with a micro-mesoporous structure were obtained by hydrothermal synthesis using hexamethylenediamine and carbon black as structure-forming agents. Carbon black grade P354, obtained at the Institute of Hydrocarbon Processing Problems SB RAS (Omsk), has the following composition: С – 96.4%, О – 2.5%, S – 0.5%, N and Н – 0.3%, and a specific surface area of – 110 m2/g. Carbon particles are homogeneous, have a globular shape with sizes from 30 to 60 nm. A feature of carbon black is the presence of photogenic functional groups on its surface – ion adsorption centers. Oxygen in the composition of carbon black is in hydroxyl, carbonyl, ether, and carboxyl groups. Before use, carbon black was pre-activated for 1 hour while stirring in 1 M NaOH solution at room temperature, after which it was dried at a temperature of 100 ° C.
Carbon template was added to alkaline alumosilicon in an amount of 0.5-5.0 wt%, based on the mass of the resulting zeolite. A solution of sodium silicate (liquid glass composition) was used to prepare alkaline aluminum silica gel: 19.0% SiO2, 7.2% Na2O). The resulting reaction mass was placed in a steel autoclave with a Teflon liner and kept at a temperature of 170 °C for 4 days. The resulting reaction mixture corresponded to the following molar composition: Al2O3 : 40SiO2 : 5.4ГМДА : 15Na2O : 2200H2O : 2С.
After the synthesis was completed, the crystalline solid phase was filtered on a Buchner funnel, washed with distilled water to pH = 7, dried at 100 °C for 6 hours and calcined at 550 °C for 6 hours to remove organic and carbon templates. The sodium form of zeolite obtained after crystallization was converted to ammonium form by treatment with a 25% solution of ammonium chloride at 90 °C for 2 hours with constant stirring. After ion exchange, the zeolite was filtered on a Buchner funnel, washed with distilled water, dried at 100 °C for 6 hours. To obtain the hydrogen form of zeolite, the ammonium form was calcined at 550 °C for 6 hours.
Synthesized zeolites ZSM-5 have the following designations: ZSM-5 – without the use of carbon template; ZSM-5/0.5-5.0 – with the addition of 0.5-5.0 wt% carbon black.
Catalysts preparation. Catalysts of 4%Mo/ZSM-5 were prepared by dry mechanical mixing of zeolites in H-form with nanoscale Mo powder obtained by the method of electric explosion of a conductor in an argon medium. The Mo content in the catalysts is 4.0 wt%. The average particle size of Mo is 70-110 nm. The resulting samples were calcined at a temperature of 550 °C for 4 hours. The prepared catalysts have the following designation: Mo/ZSM-5 – without using a carbon template; Mo/ZSM–5/0.5-5.0 – with the addition of 0.5-5.0 wt% carbon black.
Catalystcharacterization.
The quality of the synthesized zeolites was monitored using IR spectroscopy and X-ray diffraction (XRD) methods. The IR spectra of the samples were taken on a Nicolet 5700 IR Fourier spectrometer (Thermo Electron Corporation, USA) in the region of 4000-400 cm
-1. The degree of crystallinity was determined by the method described in [
27].
The chemical composition of the samples was analyzed using an «EDX-800HS» (Shimadzu, Japan) energy dispersive X-ray fluorescence spectrometer with a Rh anode X-ray tube (voltage 15-50 kV, current 20-1000 μA, vacuum, collimator 3-5 mm) and a PFA-378 flame photometer.
The phase composition and degree of crystallinity of the samples were assessed by X-ray diffraction (XRD). Before analysis, the samples were heat treated at 600 °C for 3 h to remove the template. Diffraction patterns were recorded on an «DISCOVER D8» (Bruker, Germany) diffractometer equipped with a Lynx-Eye detector using monochromatic CuKα radiation. Scanning was carried out in the range of angles 2θ = 6-90°, scan step was 0.02 degrees and accumulation time was 3 s. The relative degree of crystallinity was calculated from the ratio of the total integrated intensity from the crystalline phase to the sum of the total integrated intensities from the crystalline and amorphous phases. X-ray phase analysis was performed using the PDXL software package by comparing the obtained diffraction patterns with the PDF2 database.
The morphology and crystal size of the samples were investigated by scanning electron microscopy (SEM) on a «JEOL JSM-6490LV» (JEOL, Japan) electron microscope. The SEM images were recorded in the secondary electron mode at an accelerating voltage of 20 kV and a working distance of 10 mm. Before recording, the samples were placed on the surface of a 25 mm in diameter aluminum table and fixed with a conductive adhesive tape.
The study of the structure and microstructure of the samples was carried out by high–resolution transmission electron microscopy (HR-TEM) – on an electron microscope «ThemisZ» (Thermo Fisher Scientific, USA) with an accelerating voltage of 200 kV and a maximum resolution of 0.07 nm and in the scanning mode of electrons scattered at large angles (HAADF STEM) [
28]. The images were recorded using a Ceta 16 CCD matrix (Thermo Fisher Scientific, USA). The device is equipped with an energy dispersive spectrometer of X-ray characteristic radiation (EDS) Super (Thermo Fisher Scientific, USA) with a semiconductor Si detector with an energy resolution of 128 eV.
The acidic properties of the obtained catalysts were studied by the method of thermoprogrammed desorption of ammonia (TPD-NH
3), which made it possible to determine the number and the strength distribution of acid sites [
29]. The adsorption of ammonia was carried out at 100 °C until the sample was completely saturated, then the physically adsorbed ammonia was removed by helium purging at the same temperature for 2 h, after which desorption was carried out in the temperature range 100-550 °C at a temperature rise rate of 10 K/min.
The characteristics of the porous structure were determined by the method of low-temperature adsorption-desorption of nitrogen (77 K) on an «ASAP-2020» sorbtometer (Micromeritics, USA). Before analysis, the samples were evacuated at 350 °C for 6 h. The specific surface area was calculated by the BET method at a relative partial pressure P/P0 = 0.2. The pore size distribution was calculated from the desorption curve by the BJH method (Barrett-Joyner-Halenda). The total pore volume was determined by the BJH method at a relative partial pressure P/P0 = 0.95, while the volume of micropores in the presence of mesopores was determined by the t method of Lippens and de Boer.
Catalytic evaluation. The process of non-oxidative conversion of methane (99.99% purity) was investigated in a flow-through reactor at 750 °С, a volumetric feed rate of 1000 h
–1, and atmospheric pressure. A quartz reactor was charged with 1 cm
3 of catalyst (fractional composition: from 0.5 to 1.0 mm). The reaction products were analyzed by gas-liquid chromatography using a «Chromatek-Kristall 5000.2» (Chromatek, Russia) chromatographer every 40 min of a catalytic experiment. To assess the catalytic properties of the samples, the degree of methane conversion, the yield, and selectivity of the formation of reaction products were determined [
30].
3. Results
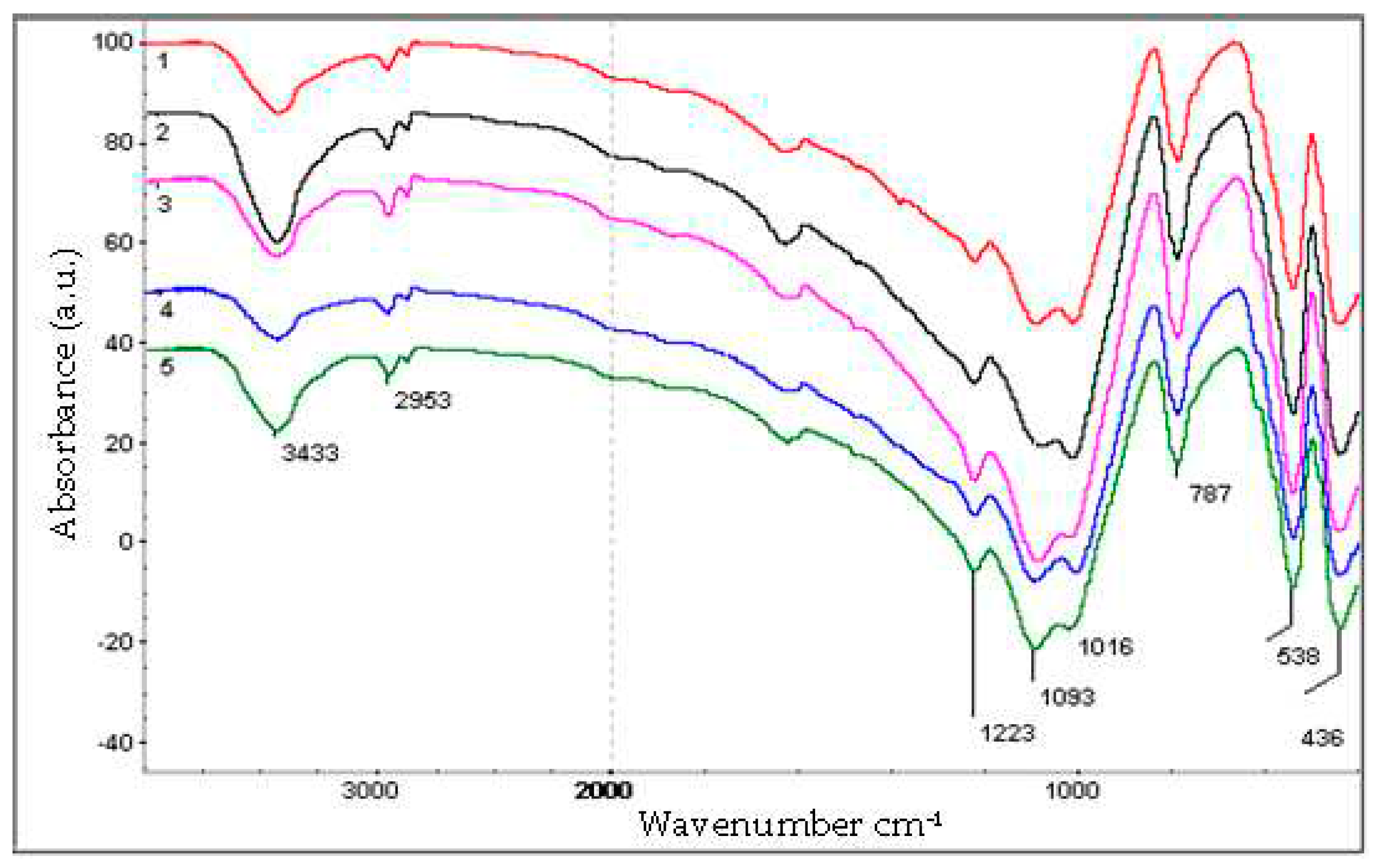
IR-spectroscopy. Figure 1 shows the IR spectra of zeolites of a high degree of crystallinity obtained without and with the addition of carbon black in the synthesis of the reaction mixture.
Absorption bands at 436, 538, 787 and 1093 cm-1 characteristic of the ZSM-5 are observed on the IR spectra. The bands at 436 and 1093 cm–1 refer to vibrations inside the SiO4 and AlO4 tetrahedra and are not responsible for the zeolite structure. The wide intense absorption band in the region of 950-1200 cm–1 refers to antisymmetric valence vibrations of Si, AlO, and the absorption band at 436 cm–1 refers to deformation vibrations inside the SiO4 and AlO4 tetrahedra. The absorption band at 787 cm–1 is associated with valence vibrations of SiO4 and AlO4 tetrahedra. The absorption band at 538 cm–1, which relates to fluctuations in the external bonds of the Al, SiO4 tetrahedra of the frame, is due to the presence of double four- and six-membered rings in the frame and determines the structure of the zeolite. The presence of this band in the infrared spectrum of the zeolite indicates that it belongs to the ZSM-5 type. The ratio of the intensities of the absorption bands at 538 and 436 cm–1 allows us to judge the purity of the synthesized samples and the degree of their crystallinity. The crystallinity of all synthesized zeolites was 100%.
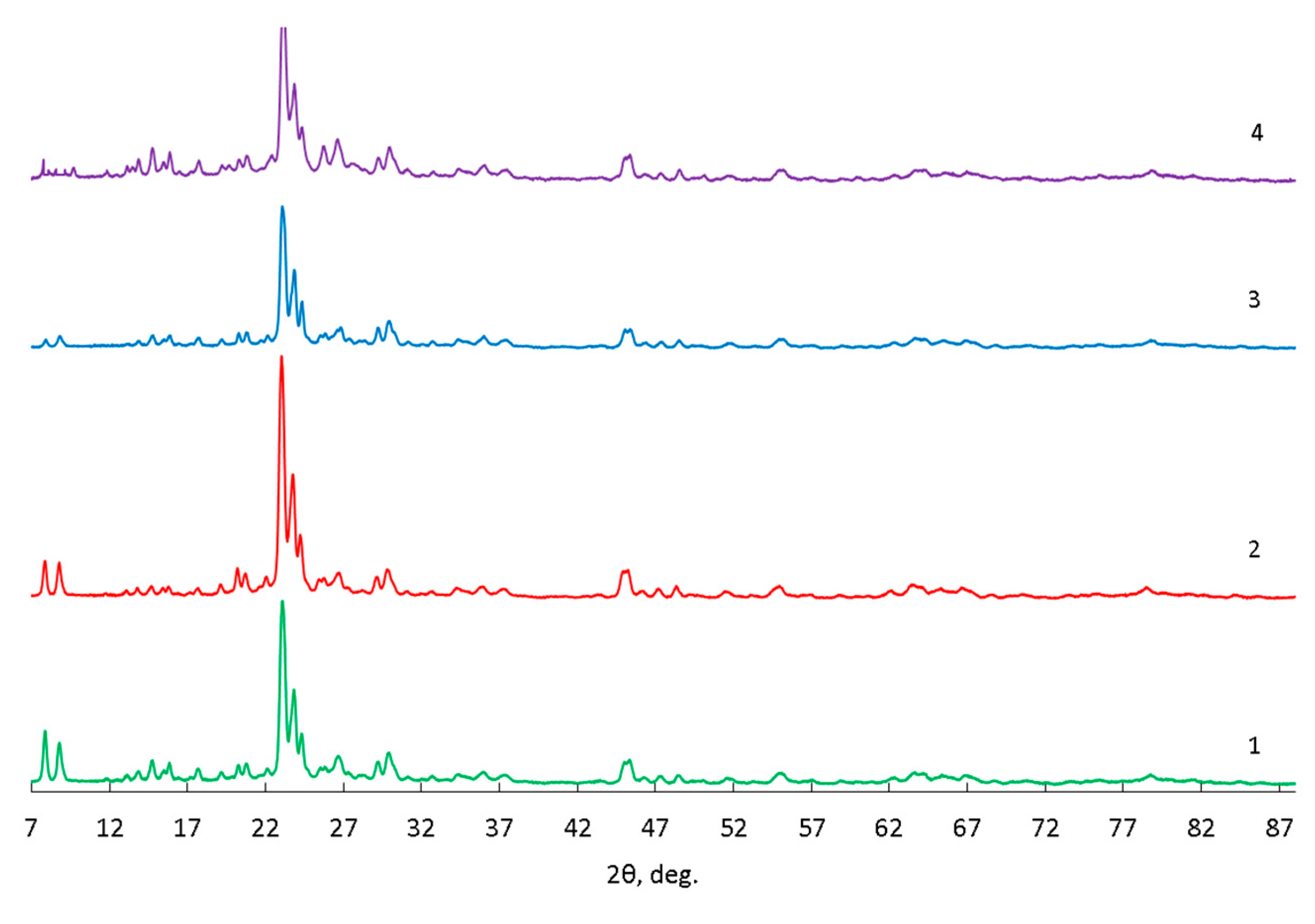
XRD. The obtained zeolites were studied by the X-ray phase method.
Figure 2 shows diffractograms of zeolites synthesized both without the addition of carbon black and using a carbon template. All samples are characterized by the presence of well-resolved peaks in the range of angles 2θ = 6-34°, which indicate their belonging to the zeolite type ZSM-5 [
31]. The absence of traces of impurity phases and broadening of peak lines indicates a high degree of crystallinity of synthesized zeolites equal to 100%.
Thus, the studies of zeolites by IR spectroscopy and X-ray phase analysis have shown that the addition of a carbon template during the synthesis of zeolites does not affect their structural properties, all synthesized zeolites belong to the ZSM-5 type and have a high degree of crystallinity.
Chemical composition. Studies of the chemical composition of the obtained zeolites by X-ray fluorescence analysis showed that their silicate modulus (SiO
2/Al
2O
3) is 40 (
Table 1). Thus, the addition of a carbon template during the synthesis of zeolite does not affect its silicate module.
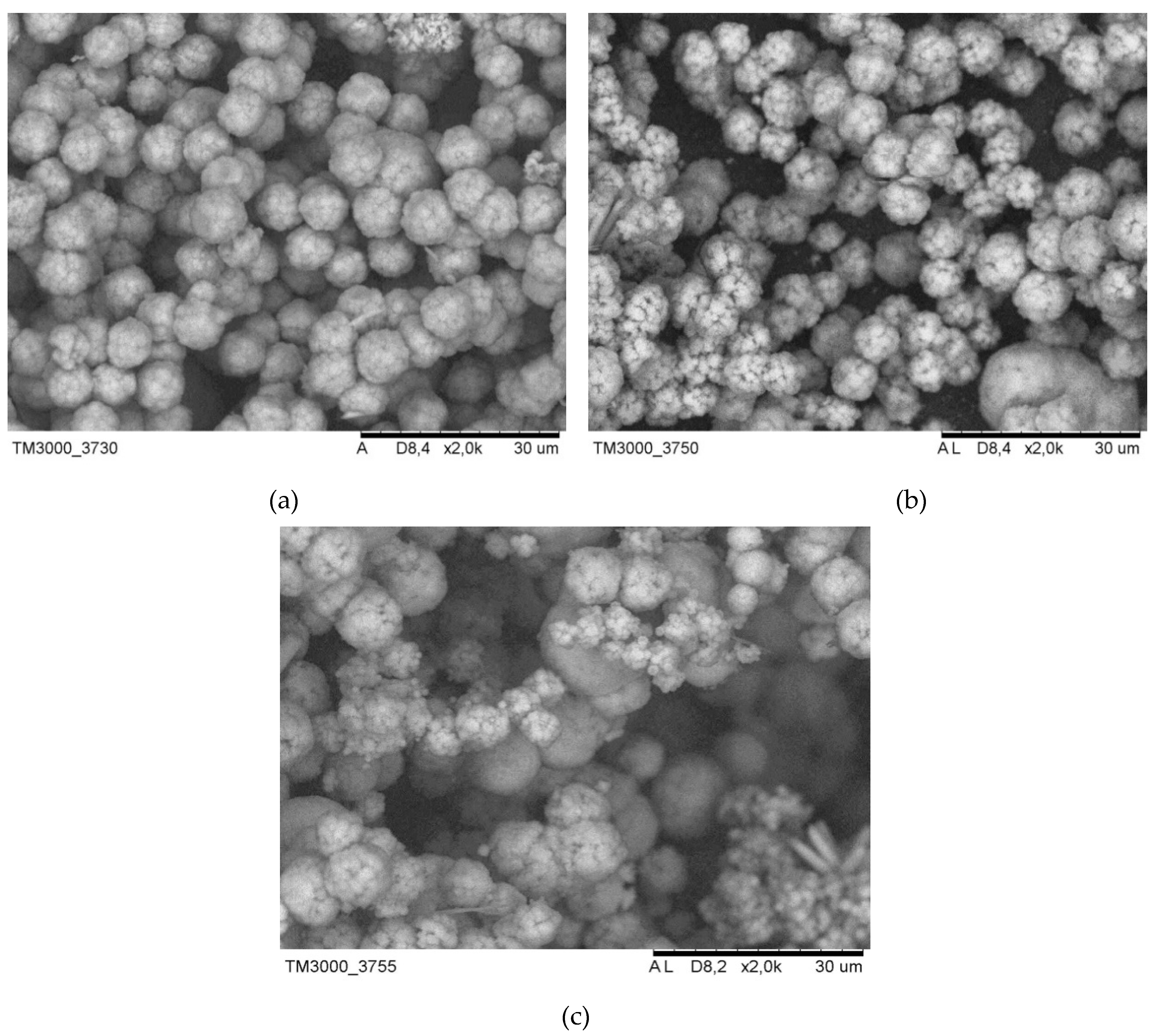
SEM. Figure 3 shows electron microscopic images of synthesized zeolites. Zeolite particles synthesized without the addition of carbon black are homogeneous in their composition and have the form of polycrystalline spheroids with sizes from 6 to 8 microns (
Figure 3a). In the electronic image of zeolite synthesized with the addition of carbon black in an amount of 1.0%, a looser connection (articulation) of crystals in polycrystalline spheroids is observed, while they are less homogeneous, the size of the crystals varies from 4 to 8 microns (
Figure 3b). When the carbon black content in the reaction mixture increases during the synthesis of zeolite to 3.5% and its subsequent calcination, inhomogeneous particles are formed (
Figure 3b). The size of polycrystalline spheroids varies from 4 to 9 microns, in addition, the formation of larger particles with sizes up to 12 microns is observed.
Thus, with an increase in the concentration of carbon black added during the synthesis of zeolite from 1.0 to 5.0%, the number of inhomogeneous zeolite crystals formed increases and their size increases.
TPD-NH3. Since metal-containing zeolite catalysts of MDA have a bifunctional nature associated with the presence of both metallic active centers and acidic zeolite centers, it was of interest to investigate the acidic characteristics of micro-mesoporous zeolites and catalysts derived from them [
32].
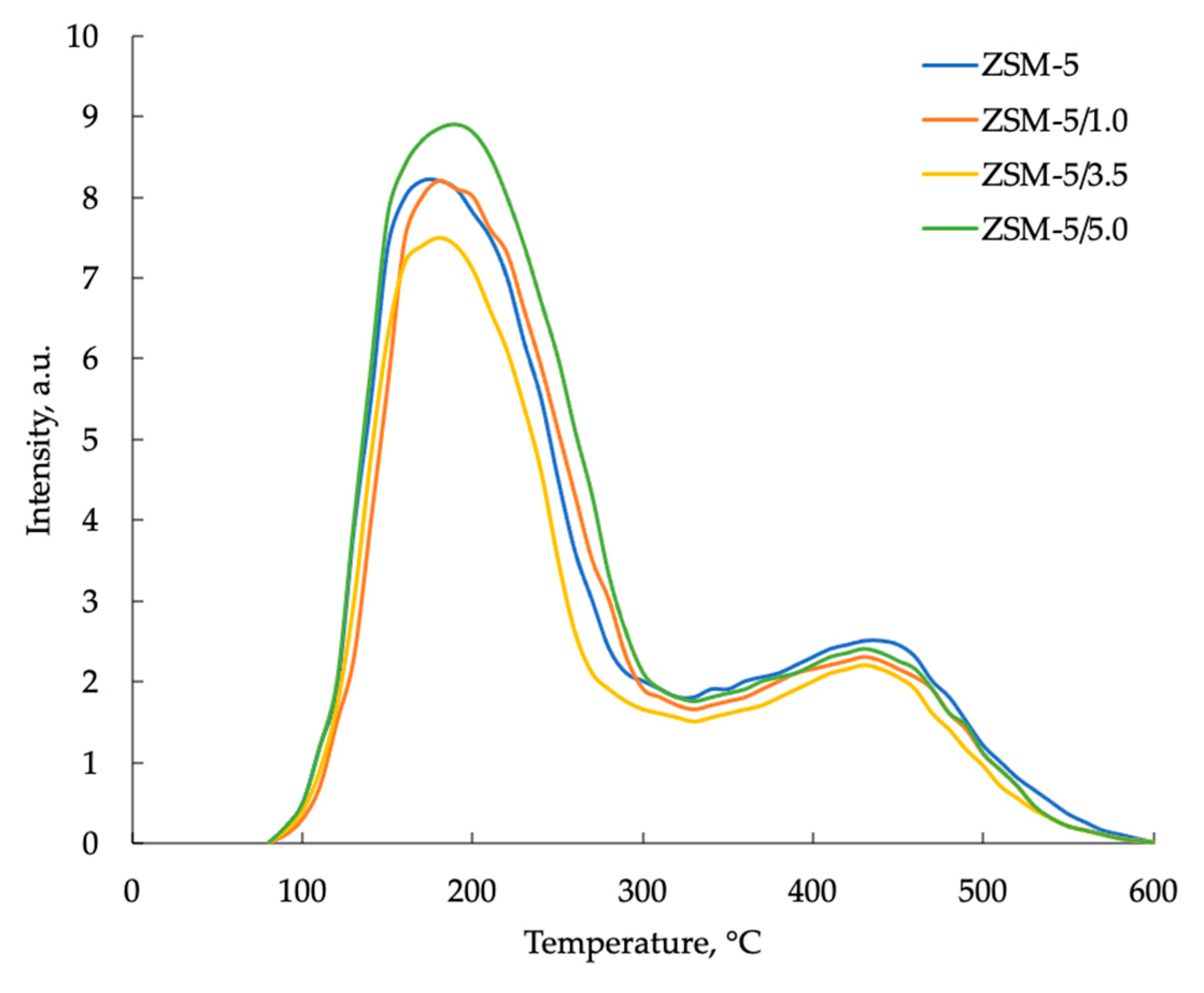
Figure 4 shows the thermal desorption spectra of ZSM-5 zeolites synthesized without and using a carbon template. On all curves there are two peaks with clearly defined temperature maxima in the region of 180-200 °C and 430-440 °C, indicating the presence of two types of acid centers – weak and strong. Strong acid centers belong mainly to the Brensted acid centers (BAC), which are responsible for the formation of aromatic hydrocarbons [
33,
34].
The intensity of the high-temperature peak in the region of 430-460 °C for zeolites synthesized with the addition of carbon black is slightly weaker than for zeolite ZSM-5, which is associated with a decrease in the number of Brensted acid centers.
The addition of 1.0% carbon black at the stage of zeolite synthesis practically does not affect its acidity (
Table 2). The concentration of strong acidic centers of zeolites obtained without and with the addition of 1.0% carbon black is 388 and 389 mmol/g, respectively. An increase in the concentration of carbon black in the composition of the reaction gel to 5.0% leads to a decrease in the content of strong acid centers in zeolite to 322 mmol/g and to an increase in the concentration of its weak acid centers to 824 mmol/g. This change is probably due to the fact that the addition of carbon at the stage of zeolite synthesis leads to differences in the distribution of Al atoms in the zeolite structure, i.e. there is a change in the ratio of Lewis and Brensted acid centers. At the same time, the total concentration of acidic zeolite centers with the addition of carbon black practically does not change and amounts to 1146 mmol/g.
The addition of Mo to the microporous zeolite ZSM-5 leads to a decrease in the strength and concentration of strong acid centers from 388 to 355 mmol/g. The decrease in acidity is associated with the interaction of Mo with the Brensted acid centers of zeolite. Modification of Mo of zeolite synthesized with the addition of 1.0% carbon black leads to a more significant decrease in the concentration of strong acid centers, which is due to a more uniform distribution of Mo in the zeolite channels due to the creation of a micro-mesoporous structure [
14,
32,
35].
Table 3 presents the results of studies of the characteristics of the porous structure of zeolites synthesized without and with the addition of carbon black and a 4% Mo/ZSM-5/1.0 catalyst.
From the data given in
Table 3, the ZSM-5 sample is characterized by a micropore volume of 0.13 cm
3/g and has a specific surface area of 285 m
2/g. The addition of 1.0% carbon black at the stage of zeolite synthesis leads to an increase in its specific surface area and mesopore volume [
36]. With an increase in the concentration of carbon black added during the synthesis of zeolite, the total volume of its pores increases because of the formation of mesopores, while the volume of micropores decreases slightly. The addition of Mo to zeolite ZSM-5/1.0 leads to a decrease in its specific surface area and total pore volume, and the volume of micropores decreases more significantly than the volume of mesopores, which indicates that molybdenum blocks micropores in zeolite [
37].
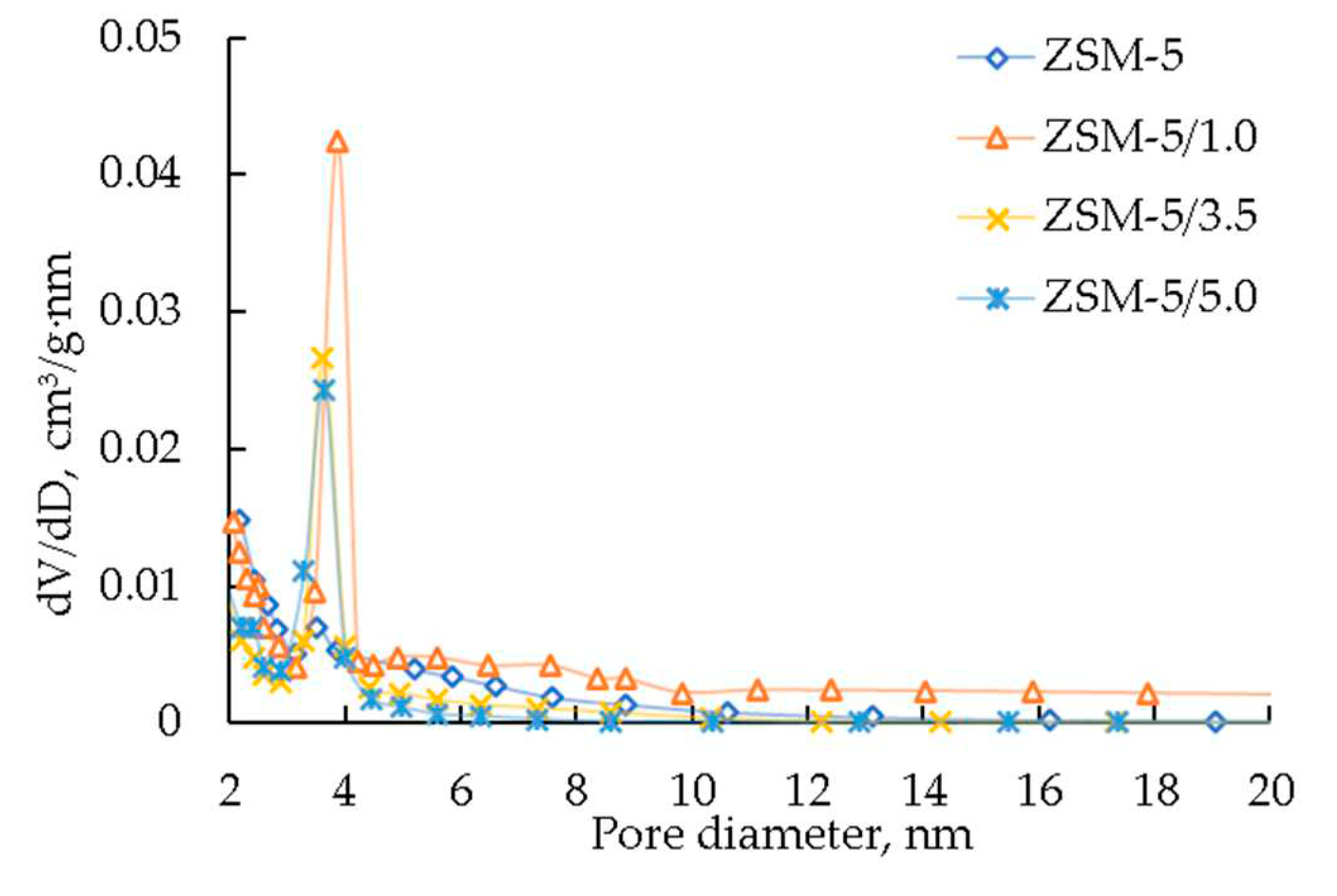
Figure 5 shows data on the pore size distribution in synthesized zeolites.
For zeolite ZSM-5 synthesized without the addition of a carbon template, the peak in the range of 3-4 nm corresponding to mesopores is practically absent, only a small shoulder is present. When a carbon template is added at the stage of zeolite synthesis, micropores are formed, the size of which is 3.5-20.0 nm.
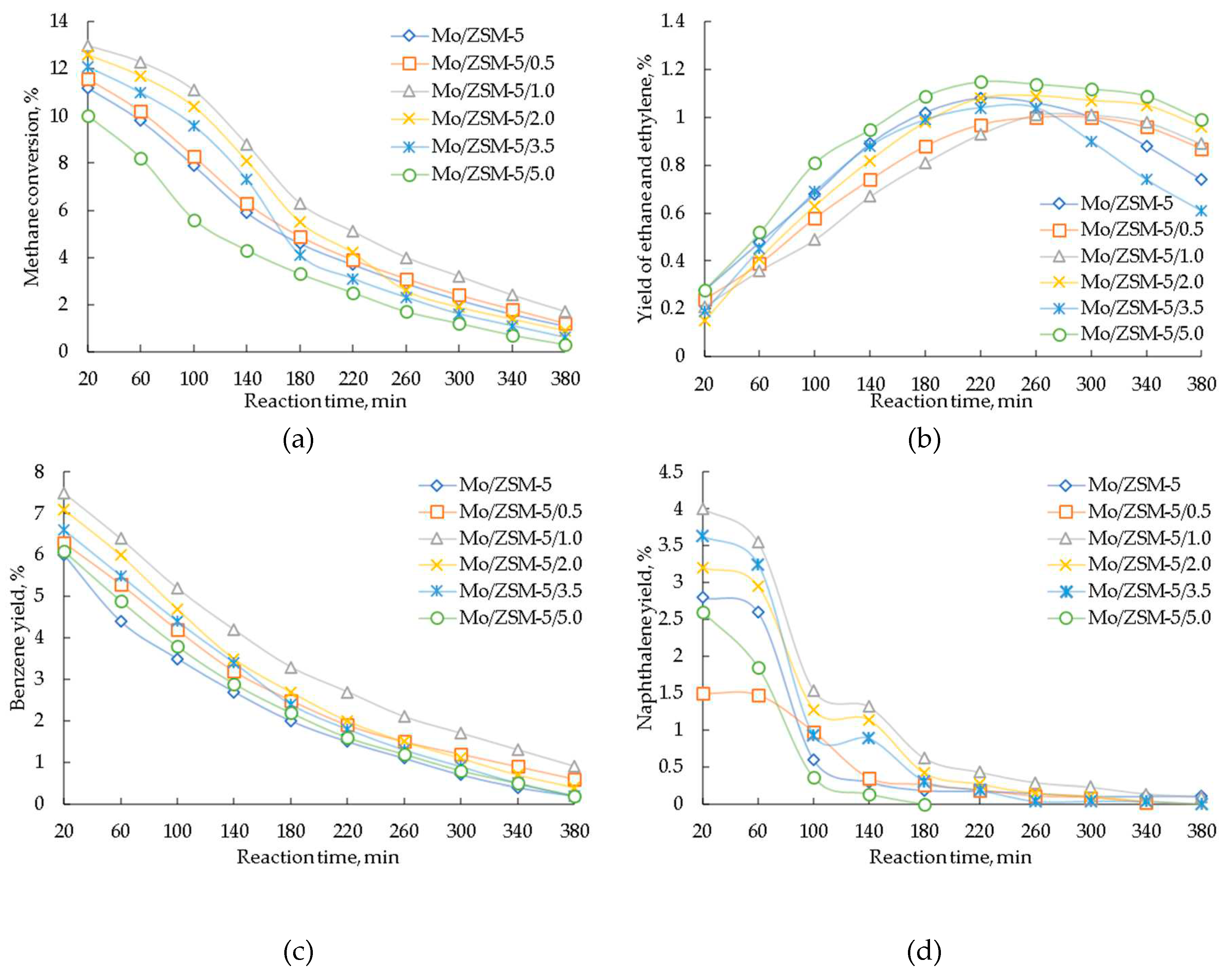
Catalytic results. The results of testing 4%Mo/ZSM-5 catalysts obtained based on zeolites synthesized without and with the addition of various amounts of carbon black in the process of methane dehydroaromatization are shown in
Figure 6. In all cases, except for the Mo/ZSM-5/5.0 sample, the use of carbon in the synthesis of zeolite leads to obtain a catalyst with a higher activity compared to the Mo/ZSM-5 sample obtained on the basis of zeolite synthesized without the addition of carbon black.
The micro-mesoporous catalyst Mo/ZSM-5/1.0 shows the greatest activity and stability of operation in the process of MDA (
Figure 6a). The conversion of methane on this catalyst is 13% in 20 minutes of reaction. An increase in the concentration of carbon black to 3.5% leads to a decrease in the activity of the Mo-containing zeolite catalyst (conversion of 12.1% in 20 minutes of reaction), at the same time its activity during 180 minutes of reaction is higher than that of the Mo/ZSM-5 sample, after which the conversion of methane is approaching the conversion methane for the Mo/ZSM-5 catalyst. An increase in the concentration of carbon black to 5.0% leads to the production of a Mo-containing catalyst, which is significantly inferior in activity to the Mo/ZSM-5 catalyst (the conversion of methane in 20 minutes is 10%).
Analysis of the composition of gaseous products formed during the conversion of methane shows that they consist mainly of ethane and ethylene, the yield of which increases as the process proceeds, reaching maximum values in the reaction time interval of 220-300 minutes, and does not exceed 1.2% (
Figure 6b). Then the concentration of ethane and ethylene decreases because of carburization of the active centers of Mo-containing zeolite catalysts. Micro-mesoporous zeolite catalysts are characterized by a higher concentration of gaseous products, the largest amount of which is formed on the Mo/ZSM-5/5.0 catalyst.
The composition of liquid methane conversion products contains aromatic hydrocarbons, mainly benzene and naphthalene (
Figure 6b,d). The largest amount of benzene is formed in the first 20 minutes of the reaction on all studied catalysts, after which its yield gradually decreases. The addition of carbon black to the reaction mixture during the synthesis of zeolites leads to the production of Mo-containing catalysts, on which a larger amount of benzene is formed. The highest benzene yield is achieved on the Mo/ZSM-5/1.0 sample and is 7.0% in 20 minutes of reaction. The largest amount of naphthalene is formed in 20-60 minutes of reaction, then its yield decreases. The sharpest decrease in naphthalene concentration is observed on the Mo/ZSM-5/5.0 catalyst.
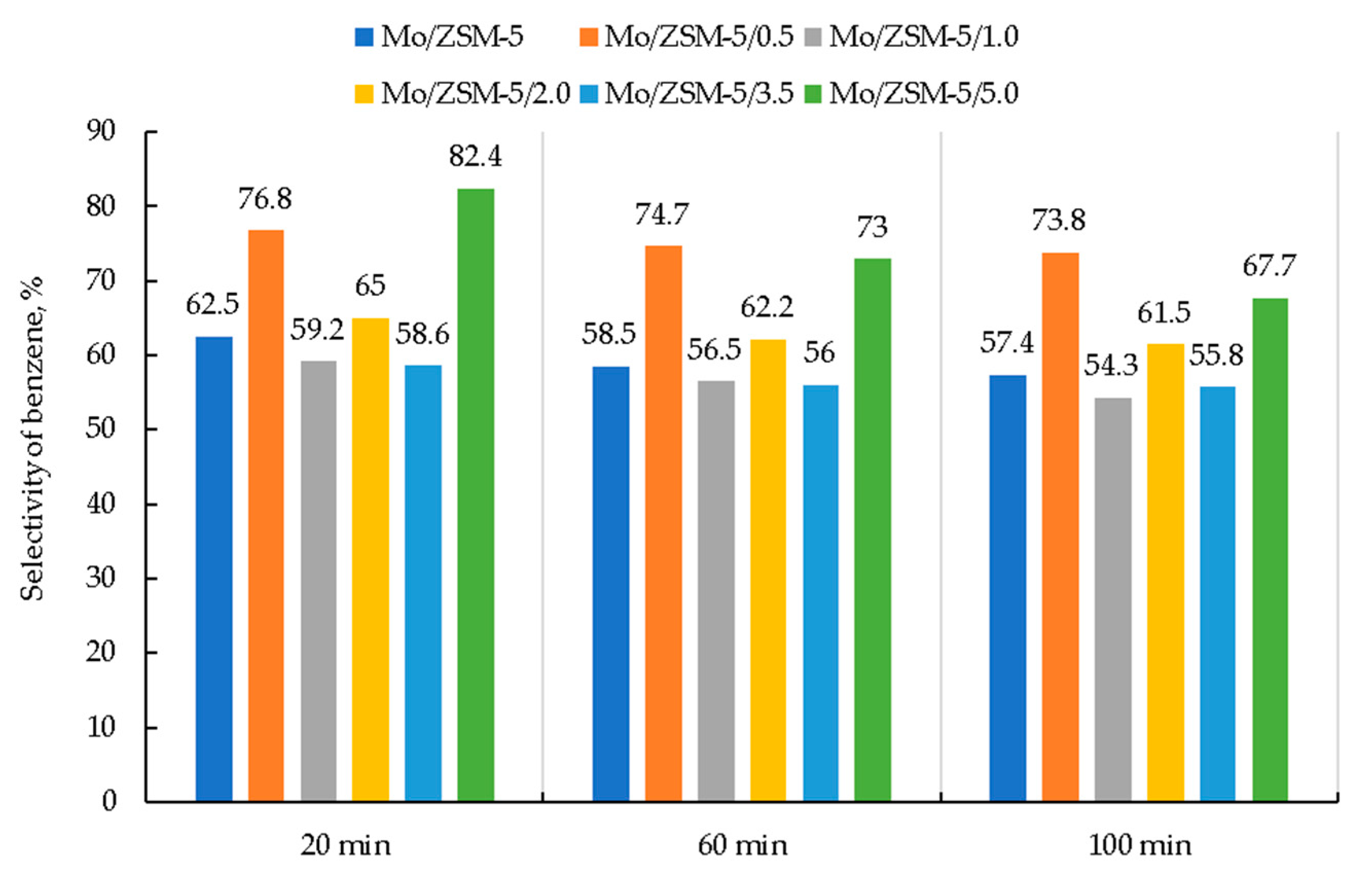
Figure 7 shows the dependence of the selectivity of benzene formation on the operating time of 4%Mo/ZSM-5 catalysts obtained based on zeolites with a micro-mesoporous structure.
The greatest selectivity of benzene formation on all studied catalysts is observed in the first 20 minutes of the reaction. With an increase in the duration of the process, the selectivity of benzene formation on Mo/ZSM-5 catalysts decrease. The highest selectivity of benzene formation is achieved on Mo/ZSM-5 catalysts obtained based on zeolites synthesized with the addition of carbon black in the amount of 0.5 and 5.0%, and amounts to 76.8 and 82.4%, respectively. The selectivity of benzene formation on a microporous Mo/ZSM-5 catalyst for 20 minutes of reaction is 62.5%.
The difference in the activity of the microporous sample and micro-mesoporous catalysts is probably due to the distribution of the Mo active phase in the zeolite structure.
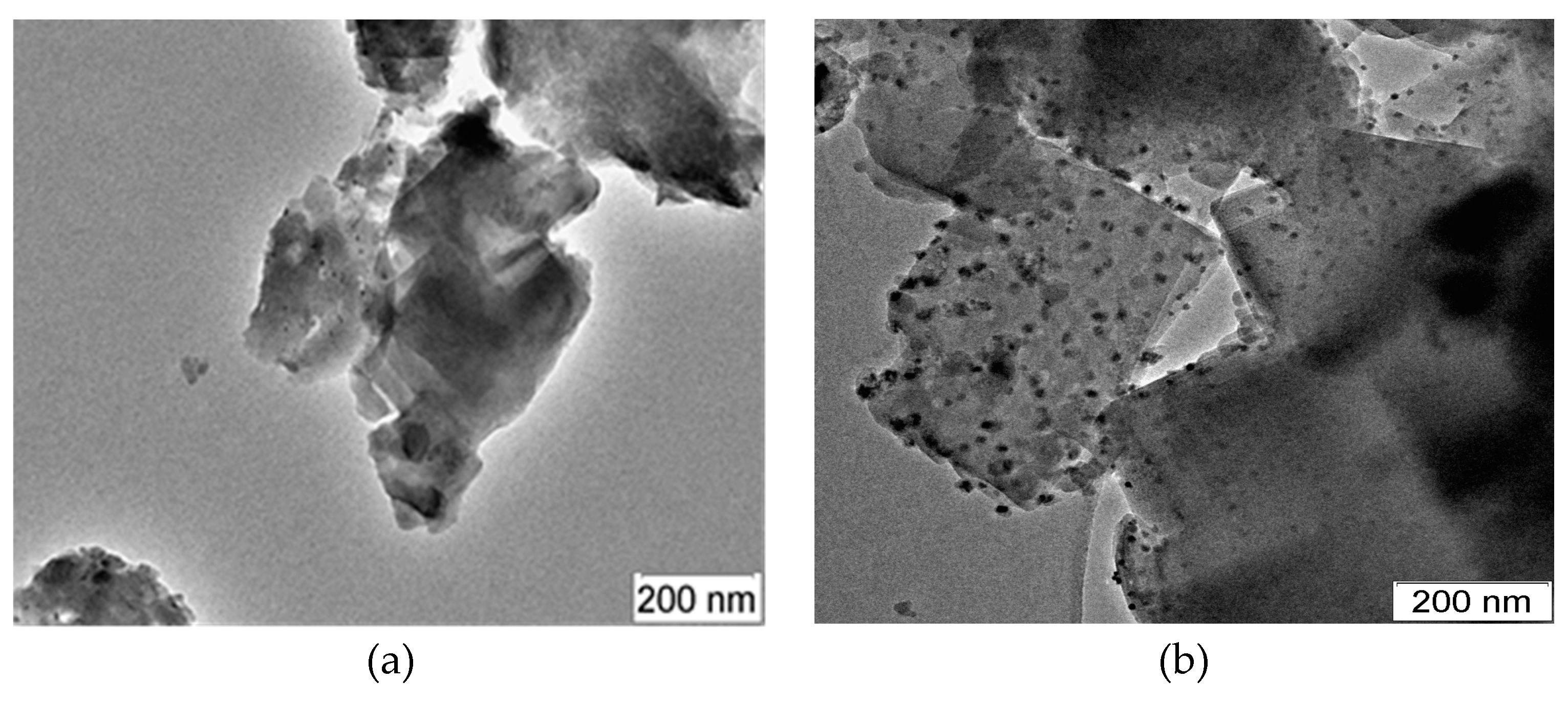
Figure 8 shows micrographs of Mo/ZSM-5 catalysts based on zeolites with a microporous and micro-mesoporous structure, which worked during the dehydroaromatization of methane for 380 min.
For a catalyst obtained based on microporous zeolite, small clusters of Mo with a size of less than 1 nm are observed in the zeolite channels and particles with a size of up to 10 nm on its surface (
Figure 8a). In the 4%Mo/ZSM-5 catalyst prepared using zeolite with a micro-mesoporous structure, there are clusters of Mo about 1 nm in size with a very dense arrangement in the zeolite channels and particles up to 10 nm in size on the surface, which indicates a more uniform distribution of molybdenum in the zeolite volume (
Figure 8b).