1. Introduction
Hidradenitis Suppurativa (HS) is a dermatologic condition characterized by inflamed lesions that appear in flexural areas rich in apocrine glands1. The onset of the disease is usually after puberty and is most common during the 3rd decade of life2.
The disease is underdiagnosed in many countries, including Israel. Its prevalence ranges from 0.053% in some countries to 4% in others, which indicates under-diagnosing3. HS is considered more common in women than in males (with a ratio of 3:1)1.
There are several contributors to the pathogenesis of HS, such as genetic predisposition and immune dysregulation. A recent study has shown that up to 34% of patients with HS had a positive family history4. In addition, there is a dysregulation of the inflammatory response, including both innate and acquired immune responses3. Bacterial infection is also considered to play a role in the pathogenesis of HS5.
The severity of HS disease is classified by the Hurley grading system, which classifies HS into 3 groups according to the presence of abscesses and sinuses. Involvement of multiple areas with scarring and infected subdermal fistulas may lead to functional disturbances6. The burden is further intensified by multiple comorbidities, some of which are common such as smoking, metabolic syndrome (including hypertension, hyperlipidaemia and excess weight) in 40% of the patients7, polycystic ovary syndrome in 38%8, malignancy in up to 50%9,10, depression, in up to 42%11, and inflammatory bowel disease in 17-38%12.
Treatment of HS is challenging and includes a wide range of topical and systemic antibiotics13,14, hormone therapy15, oral retinoids16, corticosteroids, and biological treatments, mainly anti-TNF alpha17,18. Additional options are surgical and laser therapies19,20,21.
A previous epidemiological study on HS patients from Israel22, analysed patients treated in the community setting (Clalit Health Services), and was based on digitally annotated data.
The purpose of this study is to characterize the differences in clinical characteristics of Israeli patients with HS of Arab and Jewish origin and to review their clinical characteristics, disease course, risk factors, and different treatment options.
2. Materials and Methods
This study is a retrospective observational study in which we gathered clinical, demographic and laboratory results from the computerized files of 219 patients diagnosed and treated for HS at Rambam Healthcare Campus dermatology clinic – a tertiary hospital located at the north of Israel between 2015 and 2018. Patients with missing data (n = 55) were excluded, resulting in a total of 164 patients included in this study.
The diagnosis was determined by a single dermatologist, based on clinical characteristics, medical history, and physical examination of each patient. Each patient’s age, sex, smoking history, age at diagnosis, duration of the disease, diagnostic delay, location of the lesions, and severity of the disease were recorded according to Hurley stage. We also recorded treatment and family history, body mass index (BMI), associated cutaneous and systemic diseases, and other comorbidities. A positive family history was determined if patients reported first- or second-degree relatives with HS.
The evaluation of the treatment response was classified into 4 groups:
(1) Complete remission - improvement of 100%
(2) Significant response: improvement of 50% or more (but not 100%). ‡
(3) Mild response: improvement of less than 50 % (but not 0%).
(4) No response: - improvement of 0%
The assignment as obesity was classified according to BMI (equal or greater than 30). Smoking history was classified as past or current smoker or non-smoker. Hypertension and type 2 diabetes were determined according to common clinical practice.
Demographic data was compared with the general population according to 22,085 patient files registered with Clalit Health Services22.
2.1. Statistical analysis
Variation of treatment response is shown according to sub-categories (gender, overweight, hypertension, etc.) and calculated using Pearson Chi square.
Treatment response for quantitative measurements (age and duration till diagnosis) was done by ANOVA or Kruskal-Wallis Test. data processing was done by SPSS 25. Significance shown when p<0.05.
Descriptive statistics in terms of mean, standard deviation, percentage, median and percentiles were calculated to the whole parameters in the study. Differences between the data from Rambam medical center vs. data from the literature were tested by Fisher exact test or T-test. Differences between three groups (complete remission + significant, mild and no effect) were tested by Kruskal-Wallis with adjustment for multiple comparisons. P<0.05 was consider as significant. SPSS program was used for all statistical analysis.
3. Results
Characteristics of the patient are presented in
Table 1. Of the 164 patients with HS, 96 (58.5%) were men and 68 (41.5%) were women. The average age at the first visit to the patient was 33.9 years (range 11-75, 33.9±13.8) years. The average age of onset was 27.5 years. Consequently, the interval between the onset and diagnosis of the disease was 4 years. While most of the patients (68.1%) presented between 10 and 39 years, 42% of the male patients had an average age of onset equal to or above 40 years, compared to only 28% of the females (
Table 2).
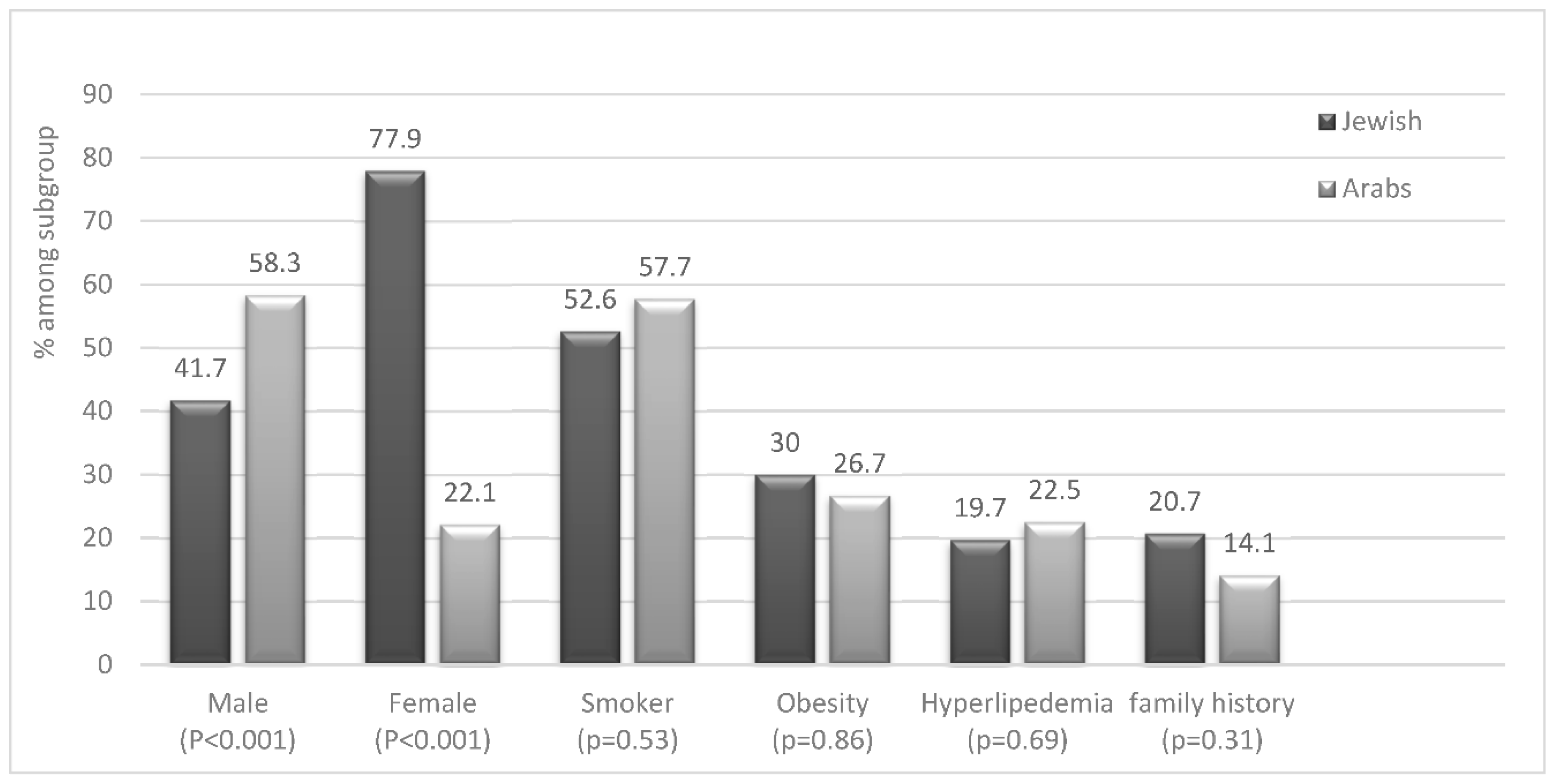
Most of the patients (57%) were Jewish while the remainder (43%) were Arabs. However, when we adjusted the prevalence according to the total visits to the clinic during the same period, we obtained a higher adjusted prevalence of HS in Arab patients rather than in Jewish patients (56% vs 44%, respectively). Additionally, the majority (57%) of Jewish patients with HS were females (P<0.001) while the opposite was observed between Arab patients (Graph 1). This indicates a significant difference in the predominance of male gender in the HS population in comparison to the Jewish one.
Eighteen percent of all patients had a family history of HS and was more common among Jewish patients 20.7% compared to Arab patients (14%) (Graph 1).
Graph 1.
– disease characteristics of HS patients according to ethnicity.
Graph 1.
– disease characteristics of HS patients according to ethnicity.
When we compared clinical data of patients in this study (n = 164) with data derived from HS cohort in Israel (
Table 3), registered at Clalit Health Services
22, (n=22,085), we found that the percentage of Arabs was 4 times higher in our cohort than in the general population.
Graph 2.
- disease characteristics of HS patients according to gender.
Graph 2.
- disease characteristics of HS patients according to gender.
Data of general population in Israel according to study done from patient’s data registered with Clalit Health Services 22.
3.1. Associated Diseases
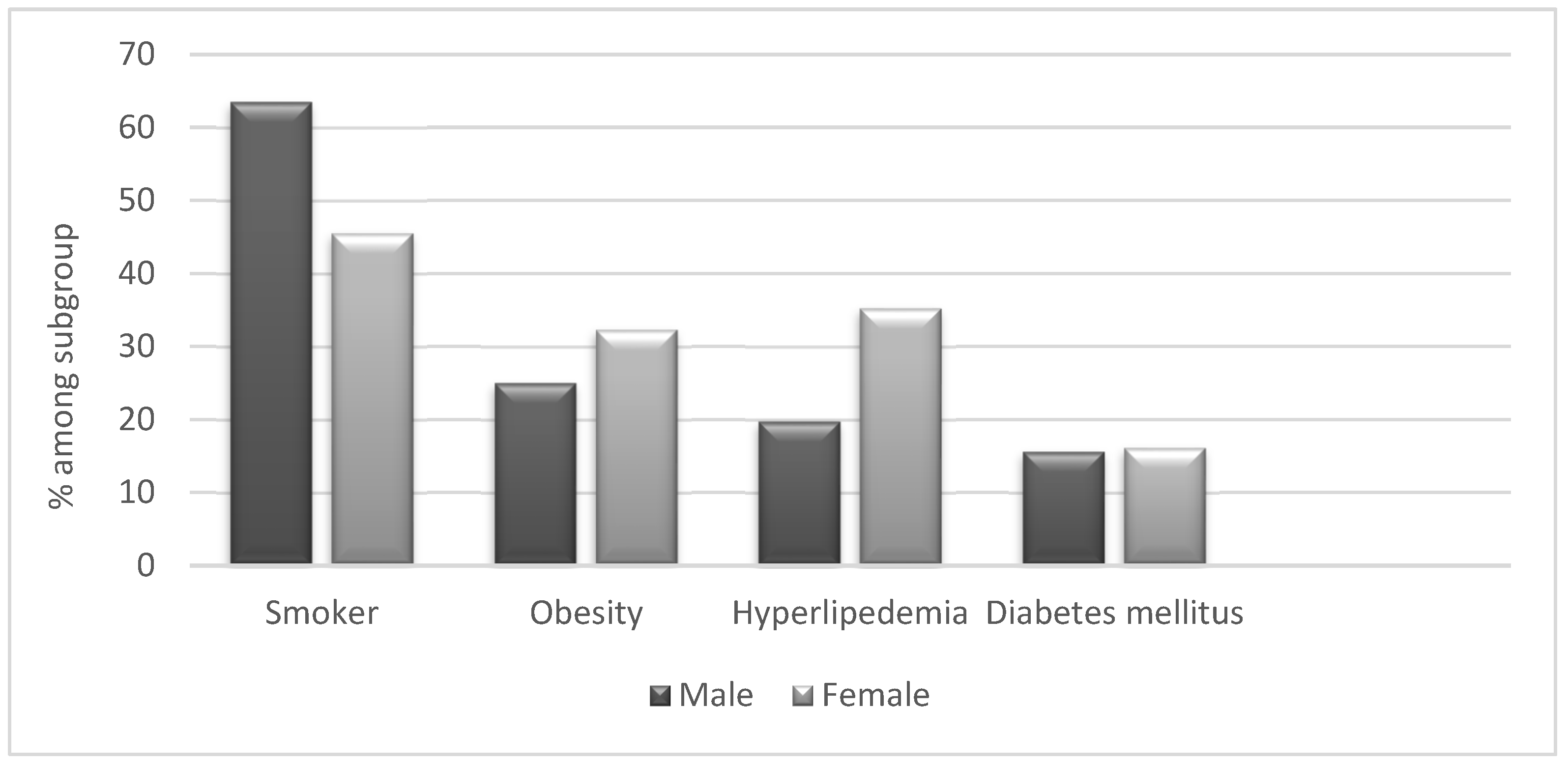
When analysing co-morbidities, we did not find significant differences between Arab and Jewish patients with HS (Graph 1 & 2) We found a high prevalence of obesity (28%) in HS patients compared to the general population (7%). However, the prevalence of hypertension and diabetes was similar among HS patients and controls. Furthermore, 55% of the patients were active or past smokers, among them 63.5% men, compared to 12.9% of the general population (
Table 3). 57.7% of Arab patients with HS and 52.6% of Jewish patients are smokers (Graph 1).
The two most common skin comorbidities were eczema 12% and acne 11%. Less common were psoriasis and other diseases such as SCC (squamous cell carcinoma), drug allergies, burns, and skin infections.
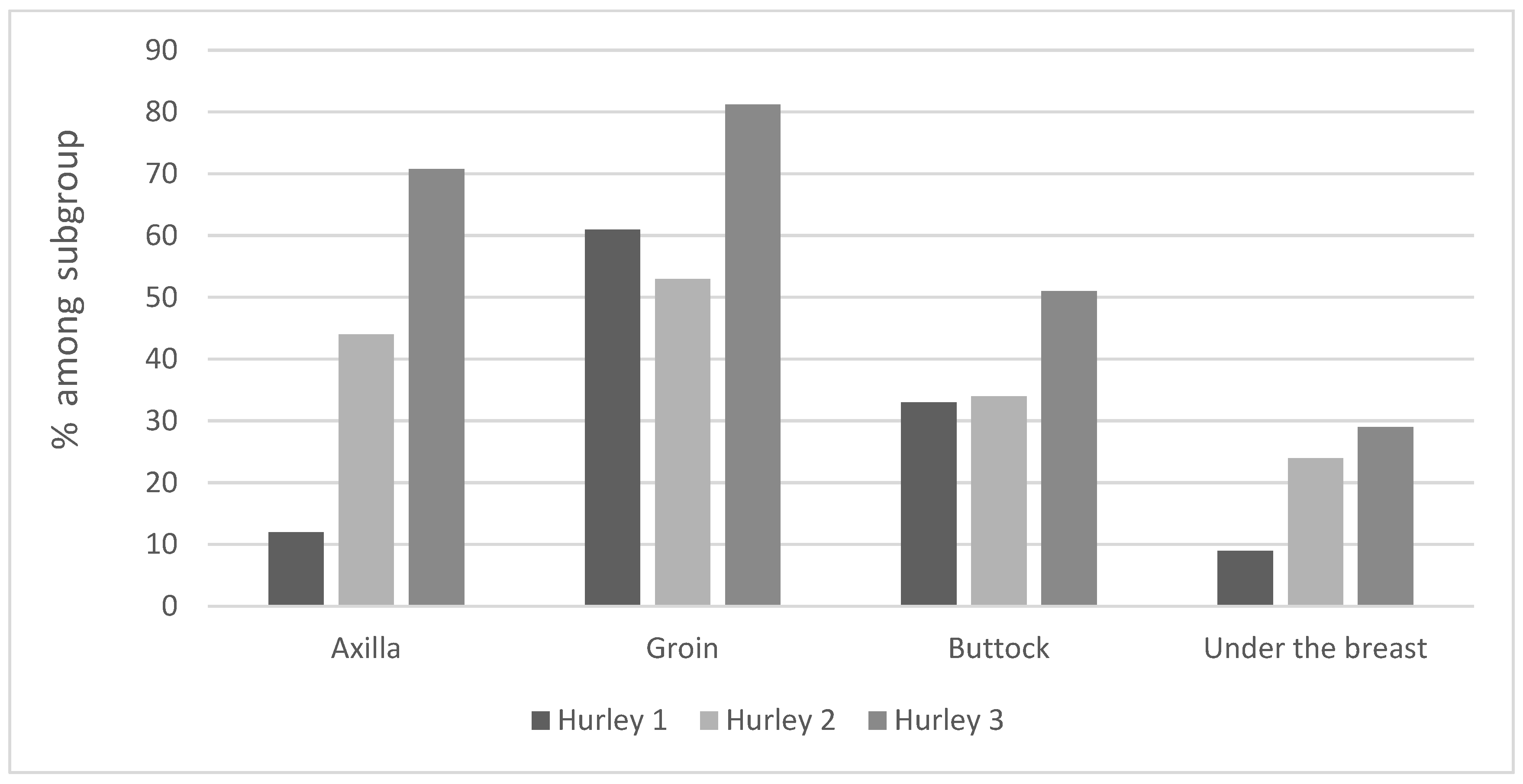
Affected areas – The areas most frequently affected were the groin (74%), followed by the axilla (63%), buttocks (43%), and sub mammary folds (27%). When analyzed by gender, genital lesions were more common in males; 56% of the patients with groin lesions and 66% of the patients with buttock lesions were men. 50% of the patients with axillary lesions and 60% of the patients with sub mammary lesions were females. There were no differences in affected areas between Arab and Jews.
3.2. Diagnostic factors for predicting disease severity according to Hurley stages:
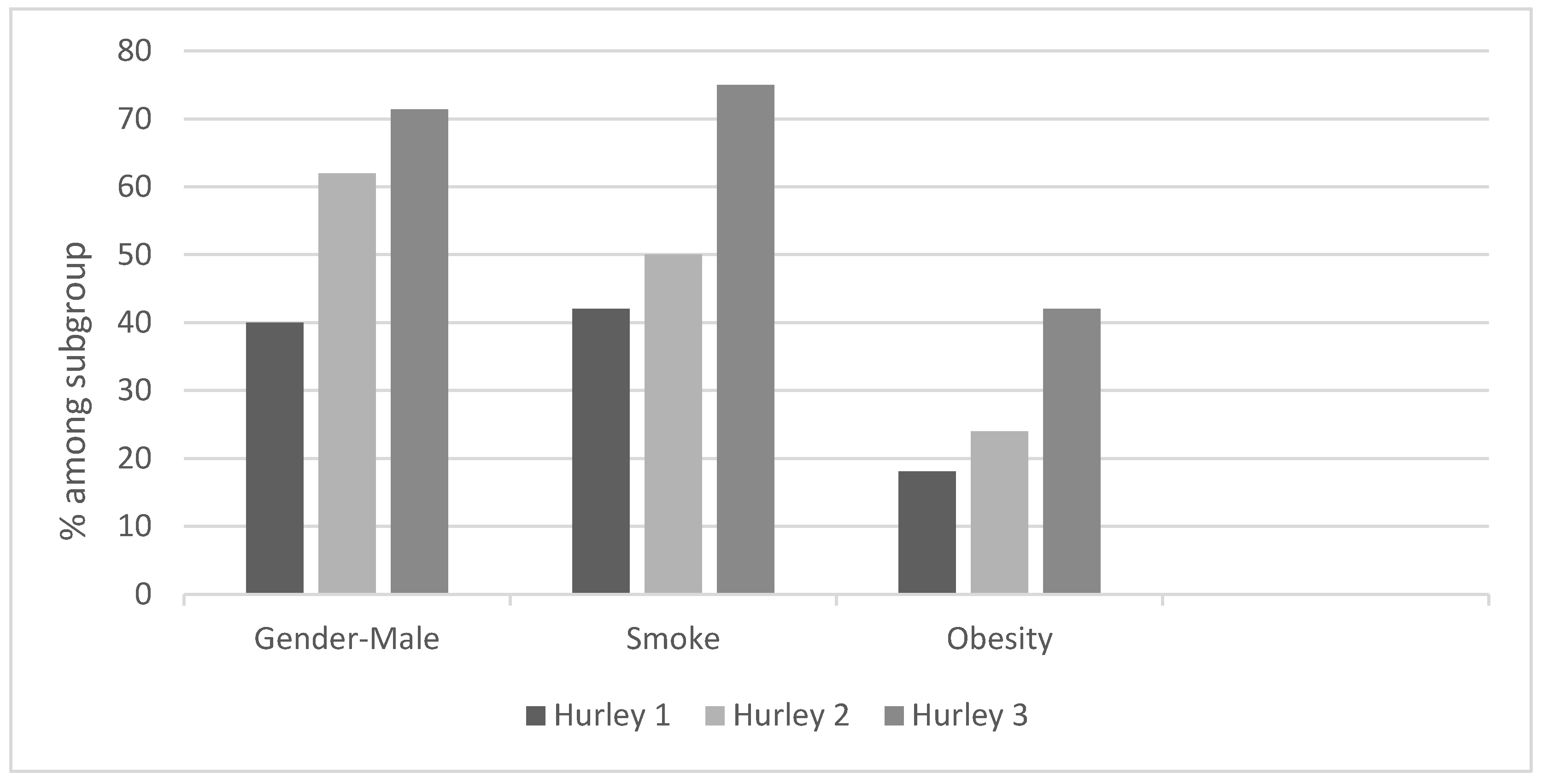
Hurley stage I was seen in 20%, stage II in 51%, and stage III in 29% of the patients. Males had a higher risk factor for more severe disease. 62% of the patients classified as Hurley II and 71.4% of the patients classified with Hurley III were men (Graph 3). Smoking and obesity were also found to be risk factors for a more serious disease: 50% of Hurley II and 75% of Hurley III patients were smokers, 24% of Hurley II and 42% of Hurley III patients were overweight.
Regarding affected areas (Graph 4) - Axillary and buttock lesions were found to predispose to a more severe disease: 34% of Hurley II and 51% of Hurley III patients presented buttock lesions. 44% of Hurley II and 70.8% of Hurley III patients had axillary lesions.
No correlation was found between family history, ethnicity, and disease severity.
Graph 3.
– disease characteristics and their relationship with disease severity according to Hurley classification.
Graph 3.
– disease characteristics and their relationship with disease severity according to Hurley classification.
Graph 4.
– lesion location and its relationship with disease severity according to Hurley classification.
Graph 4.
– lesion location and its relationship with disease severity according to Hurley classification.
3.3. Treatment response
Our cohort was treated in a tertiary medical centre; therefore, most patients had already failed several treatment modalities upon referral. These mainly included short courses in oral antibiotics and topical treatment.
There was no difference in response to treatment between Arabs and Jews.
Topical antibiotic- Almost all patients received topical treatment in combination with oral antibiotic, a fact that made this modality of treatment difficult to assess.
Oral antibiotic treatment – the most common antibiotic used was Clindamycin (128 patients). 21% of the patients showed a significant improvement in response to this treatment.
The second most common antibiotic was rifampin (62 patients), which was administered mainly in combination with clindamycin.
Prednisone treatment – several patients were treated with oral prednisone (n=24), most of them had a short-term partial response.
Retinoids treatment – In response to Acitretin treatment (n = 27), 22% of the patients showed a significant improvement, although most patients have shown only a partial response with recurrence of the disease. Isotretinoin (n=40) was indicated primarily in patients with a co-occurrence of acne and showed partial improvement in 25% of treated patients. 65% of treated patients were classified as nonresponsive to treatment.
Surgical treatments – data are presented in
Table 4. The most common surgical procedure was abscess drainage. The response to treatment was temporary and most of the patients had recurrence. A minority of patients underwent a broad excision procedure and showed signs of recurrence at the same site. Seven patients were treated with an Er:YAG laser using a deroofing method to treat inflamed sinuses, all of whom showed healing of the treated areas.
Adalimumab - Patients who did not receive several treatment options were selected for biological treatment with adalimumab (n=36). 8% fully responded, 33% of patients markedly improved, 42% had partial improvement, and 17% did not respond. Patients who did not respond to adalimumab were treated with Infliximab (n=5). Of these, one patient had a complete response, one had marked improvement, two had partial improvement, and one patient did not have a therapeutic response.
Prognostic factors of the disease and response to adalimumab treatment – we searched for parameters that predict treatment response in patients treated with adalimumab (
Table 5). Most parameters including ethnicity, gender, Hurley stage, age, hypertension, excess weight, hyperlipidaemia, smoking, leukocyte count, and liver function test were not found to have an influence on treatment response. Two parameters: time to diagnosis and location of the involvement (groin, axilla, buttocks, and sub mammary) had a significant effect in predicting response to adalimumab treatment. Although a better response was observed in patients with a shorter interval until diagnosis (p = 0.049), a worse response was observed when lesions involved the buttocks (P=0.037). All patients who did not respond to adalimumab had buttock lesions, compared to 40% of patients who had partial response and 47% of patients who had marked improvement.
4. Discussion
In this single-centre study, we included 164 patients, Arabs and Jews, with HS who were diagnosed and treated by the same dermatologist. We investigate the severity, risk factors, and demographic, clinical, and treatment characteristics of HS. Although there are several studies in western countries, epidemiological data for patients from the Middle East and Asia is rare 23. Recently, a study held in Israel described the demographic and clinical characteristics of HS patients, extracted from the Clalit Health Services database 22. This study was carried out in an ambulatory setting (community) and was based on digitally annotated data. On the other hand, our study was conducted in a single centre, in which we examined all patient files, the treatment regimen was consistent between patients, and examined all patient files and validated diagnosis and comorbidities.
We documented a high proportion of Arabs among HS patients: 56% of our cohort. This is compatible with the findings by Shalom et al. 22. In their study, 25% of the patients with HS were Arabs compared to only 11% of Arabs in the control population. The over -representation of the Arab population among HS patients may suggest the effect of environmental factors (nutrition, smoking) which could be affected by the lower socio-economic situation among Arabs; genetic predisposition could be another explanation. In fact, a slightly higher prevalence of smoking was found among our Arab patients with HS.
HS disease in our study has been found to be more prevalent in men (58%). This difference was prominent among the Arab population, where the incidence in males was 77.4%. On the contrary, in the Jewish population, the majority of patients were women (52.7%). The findings in the Arab population contradict previously reported data suggesting a higher prevalence of HS in women. Shalom et al.
22 also showed female predominance (60%). This difference is probably due to a selection bias of more severe patients referred to our tertiary centre (suggested by the high percentage of Hurley II and III patients (80%) (
Table 1). In particular, a male predominance was reported in more severe stages of the disease in other cohorts
8.
The average age of onset was higher in men than in women (Table2), which corresponds to previous reports 28. This is most probably due to hormonal changes in females. Additionally, the mean age of diagnosis was 39.9 years, which corresponds to the mean age found in the study conducted by Guy Shalom et al. (38.5 years) 22. The mean age of diagnosis found in Israel is higher than the age of diagnosis found in other cohorts. In Italy, the mean age of diagnosis is 23.9 24, more than 10 years younger than the age of diagnosis in Israel. This is despite the fact that the younger population is higher in Israel than in Europe. These findings may indicate a low awareness of HS disease among patients and physicians, leading to late diagnoses.
The co-morbidities of HS in our study, corresponds to previous reports 25. We found a high percentage of smokers, most of them males (66%) among patients. Additionally, we found a higher prevalence of obesity in HS patients compared to the general population.
Regarding family history, only 18% of the patients had a positive family history of HS (20.4% among Jewish patients compared to 14% among Arab patients). This differs from the data found in the literature, suggesting that 30-40% of patients had a positive family history 26. This finding could be unique to patients with more severe HS referred to our tertiary centre and / or genetic characteristics of Arab and Jewish patients in Israel. Genetic profiling of HS patients should be investigated in more depth.
According to our study, the body area that was the most involved was the groin, followed by the axilla and buttocks. This corresponds to the results of Revuz’s study showing that the groin, axilla and perianal region are the area’s most frequently affected areas 2. We found that lesions in the axilla and buttocks are predisposing factors to severe HS. Schrader et al. 27 also demonstrated that the involvement of the axilla, buttocks, and breast regions increases the risk of severe disease, but statistical significance was shown only with univariate analysis (analysis of a single area in the body) and not with multivariate analysis of different regions. In addition, we found that men who smoke and are overweight are predisposed to a more severe form of the disease. This corresponds to the results of Shalom et al. 22 as well as those of Canoui-Poinrine 28 and Schrader et al. 27. On its own, ethnicity did not correlate with severe disease.
The treatment of HS disease is considered a great challenge for dermatologists. topical and systemic antibiotics, oral retinoids, hormone therapy, corticosteroids, and biological treatments, mainly anti-TNF alpha and surgical or laser modalities are the main treatment options and are used according to disease severity 29 ‘30,31. Adalimumab, is the only treatment approved for the treatment of HS in moderate and severe cases and was found effective and well tolerated by patients with HS with moderate to severe disease 32,33. In our study, the response rates to Adalimumab treatment were consistent with previous studies and showed good results. 41% of the patients had marked improvement (8% of them had complete remission), 42% had partial improvement, and only 17% did not show response. Both the latency of diagnosis and the location of the lesions affected the response to Adalimumab: long latency and buttock lesions were the worse prognostic factors. However, it was found that ethnicity and sex did not affect the response.
In summary, we found differences in the Arab and Jewish HS population especially with respect to the high prevalence of the disease in the Arab population, mainly in Arab males. This could be attributed by their low socio-economic situation. In the other hands This should be further explored using genetic studies to identify differences in predisposing mutations 34.
In addition, we show significant effectiveness of Adalimumab treatment in most patients with moderate and severe HS in our cohort composed of Jews and Arabs. Response was better in patients with early diagnosis, emphasizing the importance of an early diagnosis. This is especially important for our patients, where we recognized the high mean age of diagnosis and the significant delay in diagnosis.
Increased awareness of HS diagnosis among patients and physicians may result in earlier diagnoses, which, together with lifestyle adjustment, may improve the prognosis of HS patients. Future studies should address and answer the unmet need for better treatment options.
5. Limitation
the main limitation of the study is its retrospective nature. Statistical analysis of demographic and clinical influences on treatment response has some limitations: first, part of the collected data is based on the personal statements of patients. Additionally, the combination of different treatments does not allow an accurate evaluation of each therapy as a single therapy. A third limitation is the differences in duration of treatment among patients that may affect treatment response.
Author Contributions
Assist. professor Ziad Khamaysi, the corresponding author, devised the project, the main conceptual ideas and proof outline. Dr Anan Hammud , processed the experimental data, performed the analysis, drafted the manuscript and designed the figures assisted assist. professor Ziad Khamaysi, Dr. Emily Avitan-Hersh supervised the study and helped in the data processing.
Statements
Statement of Ethics: We confirm that this work is original and has not been published elsewhere nor is it currently under consideration for publication elsewhere. All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content. Study approval statement: The protocol was approved by the Institutional Helsinki Committee (IRB), approval number (033818). Consent to participate statement: Because it was a retrospective study (we just examined the files), there was no need for inform consent.
Data Availability Statement
All data generated or analysed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Conflicts of Interest Statement
There was no conflict of interest.
References
- Jemec, G. B. E. Clinical practice. Hidradenitis suppurativa. N. Engl. J. Med. 366, 158–164 (2012). [CrossRef]
- Revuz, J. Hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. JEADV 23, 985–998 (2009).
- Gill, L., Williams, M. & Hamzavi, I. Update on hidradenitis suppurativa: connecting the tracts. F1000Prime Rep. 6, (2014). [CrossRef]
- Fitzsimmons, J. S. & Guilbert, P. R. A family study of hidradenitis suppurativa. J. Med. Genet. 22, 367–373 (1985). [CrossRef]
- Lapins, Jarstrand & Emtestam. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br. J. Dermatol. 140, 90–95 (1999). [CrossRef]
- Roenigk RK, H. H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. N. Y. 729–739 (1989).
- Rabasseda, X. A report from the 22nd Congress of the European Academy of Dermatology and Venereology (October 2-6, 2013 - Istanbul, Turkey). Drugs Today Barc. Spain 1998 49, 667–677 (2013). [CrossRef]
- Kraft, J. N. & Searles, G. E. Hidradenitis Suppurativa in 64 Female Patients: Retrospective Study Comparing Oral Antibiotics and Antiandrogen Therapy. J. Cutan. Med. Surg. 11, 125–131 (2007). [CrossRef]
- Lapins, J., Ye, W., Nyrén, O. & Emtestam, L. Incidence of Cancer Among Patients With Hidradenitis Suppurativa. Arch. Dermatol. 137, 730–734 (2001).
- Maclean, G. M. & Coleman, D. J. Three Fatal Cases of Squamous Cell Carcinoma Arising in Chronic Perineal Hidradenitis Suppurativa. Ann. R. Coll. Surg. Engl. 89, 709–712 (2007). [CrossRef]
- Shavit, E. et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 29, 371–376 (2014). [CrossRef]
- Fimmel, S. & Zouboulis, C. C. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol. 2, 9–16 (2010). [CrossRef]
- Jemec, G. B. E. & Wendelboe, P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 39, 971–974 (1998). [CrossRef]
- Gener, G. et al. Combination Therapy with Clindamycin and Rifampicin for Hidradenitis Suppurativa: A Series of 116 Consecutive Patients. Dermatology 219, 148–154 (2009). [CrossRef]
- Joseph, M. A., Jayaseelan, E., Ganapathi, B. & Stephen, J. Hidradenitis suppurativa treated with finasteride. J. Dermatol. Treat. 16, 75–78 (2005). [CrossRef]
- Boer, J. & Nazary, M. Long-term results of acitretin therapy for hidradenitis suppurativa. Is acne inversa also a misnomer? Br. J. Dermatol. 164, 170–175 (2011). [CrossRef]
- Grant, A., Gonzalez, T., Montgomery, M. O., Cardenas, V. & Kerdel, F. A. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: A randomized, double-blind, placebo-controlled crossover trial. J. Am. Acad. Dermatol. 62, 205–217 (2010). [CrossRef]
- Gupta, A. K. & Studholme, C. Adalimumab (Humira) for the Treatment of Hidradenitis Suppurativa. Skin Ther. Lett. 21, 1–4 (2016).
- Mahmoud B.H., Tierney E., Hexsel C.L., Pui j., Ozog D.M., Hamzavi I.H. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser .J Am Acad Dermatol. 62, 637-645(4) (2010). [CrossRef]
- Mikkelsen P.R., Dufour D.N., Zarchi K., Jemec G.B. Recurrence rate and patient satisfaction of CO2 laser evaporation of lesions in patients with hidradenitis suppurativa: a retrospective study. Dermatol Surg. 41,255-260 (2015). [CrossRef]
- Lapins J., Marcusson J.A., Emtestam L. Surgical treatment of chronic hidradenitis suppurativa: CO2 laser stripping-secondary intention technique. Br J Dermatol. 131,551-556 (1994). [CrossRef]
- Shalom G., Babaev M., Freud T., et al. Demographic and health care service utilization by 4417 patients with hidradenitis suppurativa. J Am Acad Dermatol. 77, 1047-1052. (2017). [CrossRef]
- Kurokawa I., Hayashi N. Japan Acne Research Society. Questionnaire surveillance of hidradenitis suppurativa in Japan. J Dermatol. 42,747–749 (2015). [CrossRef]
- Bettoli V., Pasquinucci S., Caracciolo S., et al. The Hidradenitis supurativa patient journey in Italy: current status, unmet needs and opportunities. J Eur Acad Dermatol Venereol. 30, 1965–1970 (2016). [CrossRef]
- Kim W.B., Sibbald R.G., Hu H., et al. Clinical features and patient out-comes of hidradenitis suppurativa: a cross-sectional retrospective study. J Cutan Med Surg. 20, 52–57 (2016). [CrossRef]
- Von Der Werth J.M., Williams H.C., Raeburn J.A. The clinical genetics of hidradenitis suppurativa revisited. Br J Dermatol .142, 947–953 (2000). [CrossRef]
- Schrader A.M., Deckers I.E., Van Der Zee H.H., Boer J., Prens E.P. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J AmAcad Dermatol. 71, 460–467 (2014). [CrossRef]
- Canoui-Poitrine F., Revuz J.E., Wolkenstein P., et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity.J Am Acad Dermatol.61, 51–57 (2009). [CrossRef]
- Gulliver W., Zouboulis C.C., Prens E., et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord.17, 343-351 (2016). [CrossRef]
- Sbidian E., Hotz C., Seneschal J., et al. Antitumour necrosis factor-alpha therapy for hidradenitis suppurativa: results from a national cohort study between 2000 and 2013. Br J Dermatol .174, 667-700 (2016). [CrossRef]
- Martin-Ezquerra G., Masferrer E., Masferrer-Niubo M., et al. Use of biological treatments in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 29, 56-60 (2015). [CrossRef]
- Kimball A.B., Okun M.M., Williams D.A., et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med .375, 422-334 (2016). [CrossRef]
- Kim E.S., Garnock-Jones K.P., Keam S.J. Adalimumab: A Review in Hidradenitis Suppurativa. Am J Clin Dermatol. 17, 545-552 (2016). [CrossRef]
- Pink A.E., Simpson M.A., Brice G.W., et al. PSENEN and NCSTN mutations in familial hidradenitis suppurativa (Acne Inversa). J Invest Dermatol. 131, 70-1568 (2011). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).