1. Introduction
Cisplatin (CDDP) has been widely used as a first-line therapy for malignant tumors owing to its potent antitumor effect since its discovery by Rosenberg et al. in 1965 [
1]. CDDP incorporated into cancer cells preferentially binds to the nitrogen atom at the 7-position of purine bases, especially guanine bases in the nucleus due to the substitution of chloride ligands by a water molecule, and bridges two adjacent purine bases [
2]. The various DNA-platinum covalent adducts that are formed inhibit transcription factors and polymerases and cause chromatin disruption, ultimately inducing apoptosis in cancer cells [
3]. CDDP-based chemotherapy is a central component of several curative approaches for patients with malignant diseases, including gastric, esophageal, lung, ovarian, testicular, and head and neck cancers [
4,
5,
6,
7,
8,
9]. CDDP has a high tumor regression effect; however, it causes serious side effects and severely impairs the patient's quality of life (QOL) [
10]. Gastrointestinal symptoms such as nausea, vomiting, and loss of appetite, which occur in many patients, are particularly severe in patients receiving various anticancer agents and are frequently treated using antiemetic agents [
11]. The most serious side effects are kidney failure and other kidney dysfunctions [
11,
12], for which measures, such as infusing patients with large volumes of fluids and using diuretics to increase urine output and reduce nephrotoxicity, are mandatory. However, these measures can severely interfere with a patient's QOL. In addition, there is concern that CDDP may cause neurotoxicity and a decrease in leucocytes, leading to a decrease in immune function due to myelosuppression [
12]. Therefore, it is essential to fully monitor the patient's condition while conducting tests of each function to ensure safety and QOL when treating with CDDP. Furthermore, several researchers have reported that CDDP causes adverse side effects due to its small molecular weight and indiscriminate distribution in normal tissues [
13,
14,
15,
16]. These reports are based on the following theory: polymerization of CDDP into polymeric polymers can accumulate CDDP in tumor tissues through the enhanced permeability and retention (EPR) effect and mitigate adverse drug reactions by reducing its distribution in normal tissues.
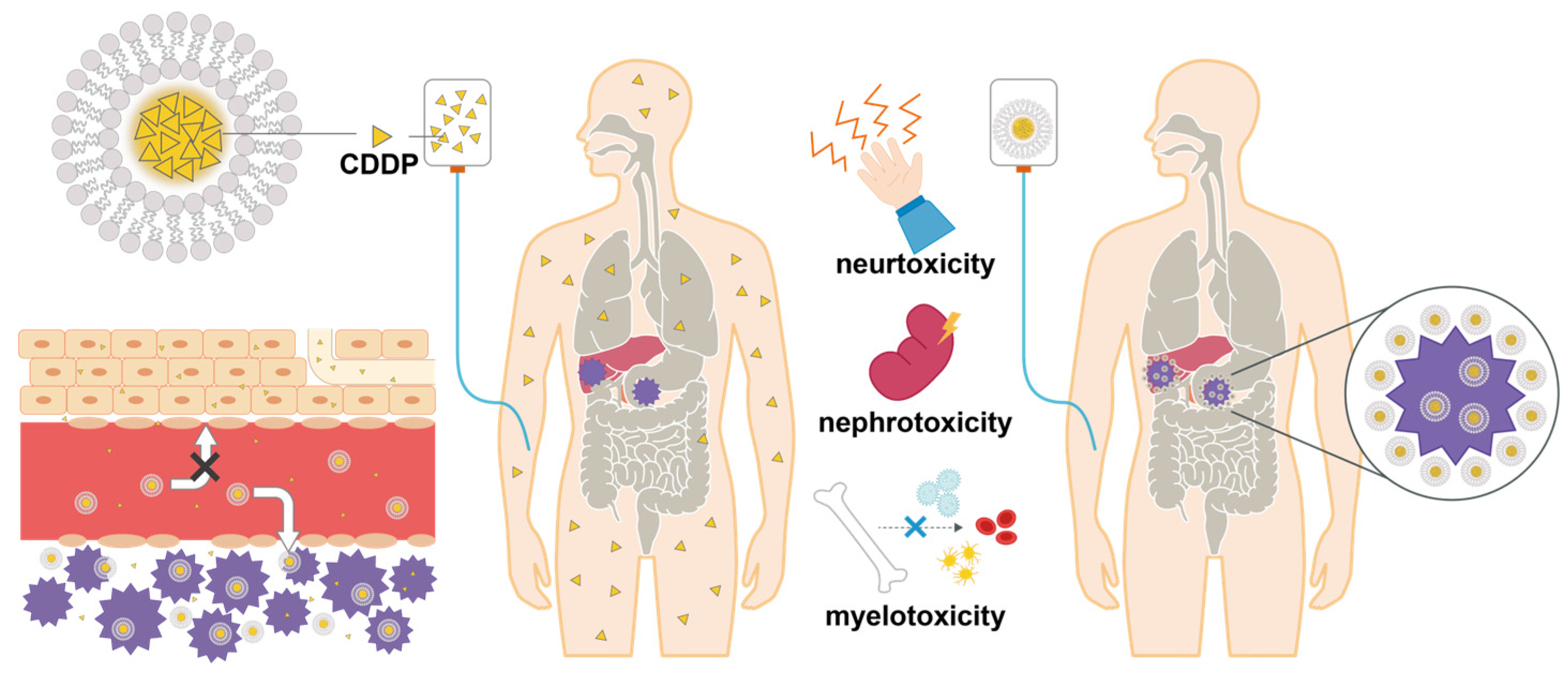
We have previously reported that a complex of styrene-co-maleic acid (SMA) encapsulated CDDP (SMA-CDDP) accumulates in tumors through the EPR effect and suppress cancer without harmful side effects in mice transplanted with cancer cells [
16]. However, SMA-CDDP has the disadvantage of undergoing chemical synthesis for several days [
15], making it less convenient for clinical use. Our research team previously reported a clinical case in which indocyanine green (ICG) encapsulated liposomes (ICG liposomes) were accumulated in a tumor and demonstrated therapeutic efficacy with photodynamic therapy [
17]. Therefore, we used liposomes in the present study because of guaranteed tumor accumulation and safety owing to the EPR effect in clinical practice and because they are easy to prepare and suitable for encapsulation of low molecular weight compounds, such as CDDP (
Figure 1).
In the present study, we reported a clinical case in which CDDP liposomes were administered to two patients, one with multiple recurrent liver metastases from metastatic nasal cancer and the other who underwent partial pancreatectomy and jejunal biliary anastomosis for biliary tract cancer, to evaluate their safety and efficacy.
2. Materials and Methods
2.1. Ethical Review and Informed Consent
This study was approved by the Ethics Review Committee at the IGT Clinic on December 21, 2022, under approval number 19. Participants provided written informed consent before the clinical trial. This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical Research Involving Human Subjects established by the Japanese Ministry of Health, Labor, and Welfare.
General disability and administration site conditions were diagnosed by the physician according to CTCAE v5.0-JCOG before and after administering the CDDP liposome treatment.
2.2. Preparation of CDDP liposomes
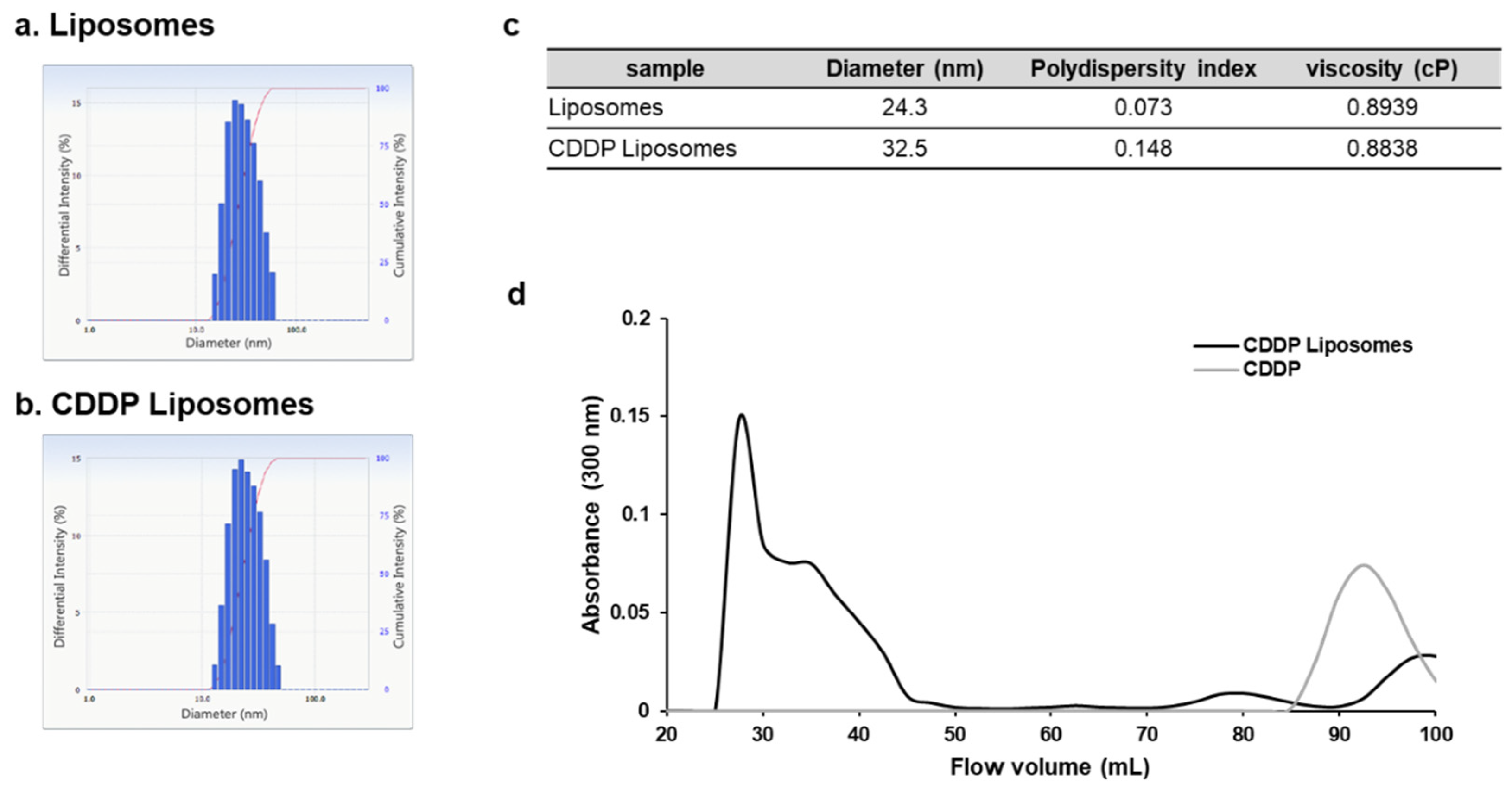
1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) was purchased from Yuka-Sangyo Co., Ltd. (Tokyo, Japan), and CDDP was purchased from Nichi-Iko (Sogawa, Japan). Liposomes were prepared as previously reported (17). Briefly, DMPC was dissolved in a 5% glucose solution at a concentration of 8.85 mM using a Bransonic® CPX8800H-J Ultrasonic Cleaner (Branson Ultrasonic Co., Ltd., Danbury, CT, USA) and then sonicated at 40 kHz for 60 min under 45 °C. Subsequently, the liposomes were purified using sterile filtration through a 0.20 μm pore size filter. The particle size of liposomes was determined using an ELSZ-2000 (Otsuka Electronics Co., Ltd., Osaka, Japan). CDDP liposomes were prepared by mixing 10 mg/20 ml CDDP and 8.85 mM/10 ml liposomes and sterile-filtered through a 0.20 mm filter. Liposomalization of CDDP was performed with gel filtration chromatography using a Sephadex® G-25 column (Merck, German).
2.3. CDDP liposome therapeutic intervention
CDDP (10 mg) was mixed with 300 mg of DMPC-derived liposomes, purified using a 0.2 μm filter, diluted to 100 ml with saline (Hikari Pharmaceutical Co., Ltd., Tokyo, Japan), and then injected intravenously at 2 mL/kg. Immediately after CDDP administration, 2 g/20 ml sodium thiosulfate (Nichi-Iko Pharmaceutical Co., Ltd., Tokyo, Japan) was administered intravenously.
2.4. Examination of circulating tumor cells
Patients had their blood drawn on their first visit for circulating tumor cells (CTC) testing. Obtained blood samples were immediately transported to Research Genetics Cancer Center International (RGCC; GmBH, Switzerland) for analysis. We received the test report.
3. Results
3.1. A case in which CDDP liposomes were administered to a patient with recurrence of multiple liver metastases from metastatic nasal cancer
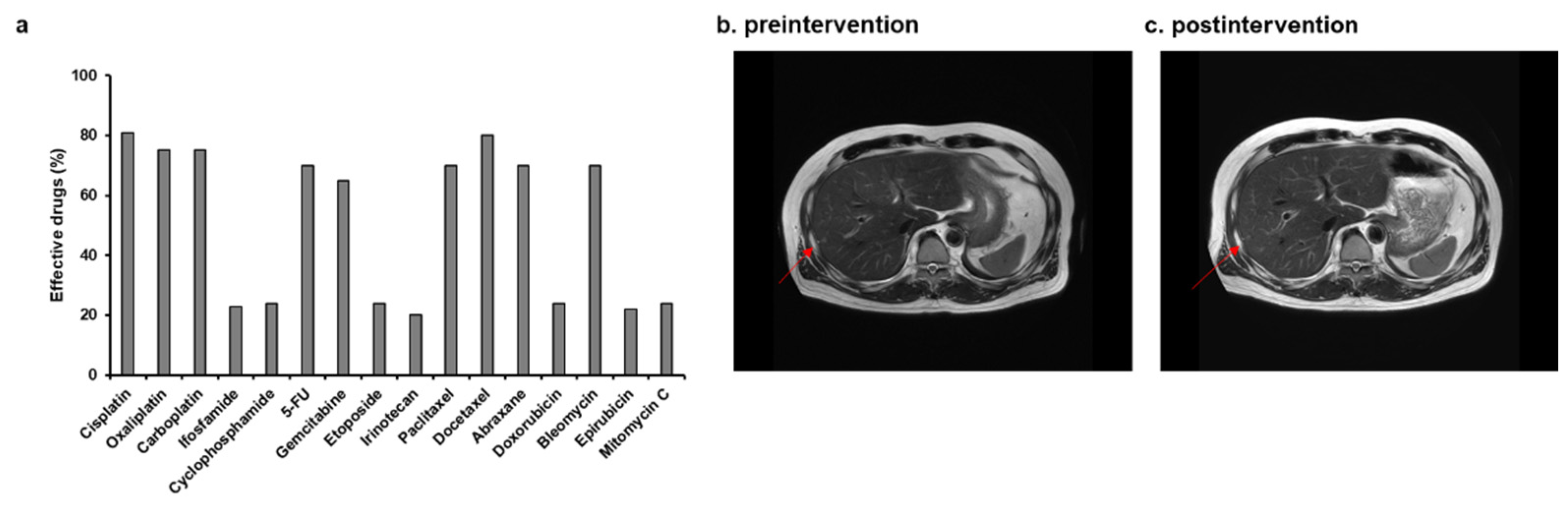
A 58-year-old man with a height of 165 cm, weight of 60 kg, and PS of 0 gave informed consent to participate in our clinical trial. His medical history is as follows. He had a recurrence of multiple liver metastases in 2019, despite a complete response to selective administration of CDDP and radiotherapy for stage-1 nasal cancer in 2012. He demonstrated a partial response after 12 courses of systemic chemotherapy with CDDP and irinotecan for the recurrence of multiple liver metastases. However, during this therapy, he experienced nausea, malaise, and lingering side effects, such as numbness in his fingers, and his QOL was greatly reduced. He was seen at our hospital in October 2020 while receiving standard treatment at another hospital.
Gene therapy using p53 and TRAIL lentivectors combined with intravenous infusion of high-dose ascorbic acid resulted in almost complete remission. For approximately 1 year, we tried to prevent cancer recurrence in the patient by administering high-dose ascorbic acid; however, two recurrent lesions were observed in the patient’s liver in October 2022, and he was recommended systemic chemotherapy with CDDP and irinotecan as standard treatment at another hospital. The patient refused because he was concerned that his QOL would deteriorate tremendously like before. Based on previously successful results of combined therapy with CDDP and irinotecan and the favorable response to CDDP in the CTC test, we administered CDDP liposomes to the patient with his consent (
Figure 2a). We added 100 ml of saline to 30 ml of CDDP liposomes and administered this treatment to the patients once weekly from October 22 to December 20, 2022. After the intervention, the patient only complained of mild fatigue (
Table 1), and no other adverse side effects were observed (
Table 1 and
Table 2). The intervention was completed without any evidence of exacerbation of renal function in blood test data. Subsequently, magnetic resonance imaging (MRI) examination showed stable disease (SD) (
Figure 2b,c).
TP, total protein; ALB, albumin; T-Bil, total bilirubin; ALP, alanine phosphotransferase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LD, lactate dehydrogenase; γ-GT, gamma-glutamyl transferase; Ch-E, cholinesterase; GLU, glucose; HbA1c, haemoglobin A1C; TC, total cholesterol; TG, triglyceride; UN, urea nitrogen; CRE, creatinine; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; WBC, white blood cell; RBC, red blood cell; Hgb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCHC, mean corpuscular haemoglobin concentration; PLT, platelet; MYELO, myelocyte; MET. M, Metamyelocyte; NEUT, neutrophil; EOS, eosinophil; BASO, basophil; LYMP, lymphocyte. AT. LY, atypical lymphocyte; Mono, monocyte.
3.2. A case in which CDDP liposomes were administered to a patient undergoing partial pancreatic resection and jejunociliary anastomosis for biliary tract cancer
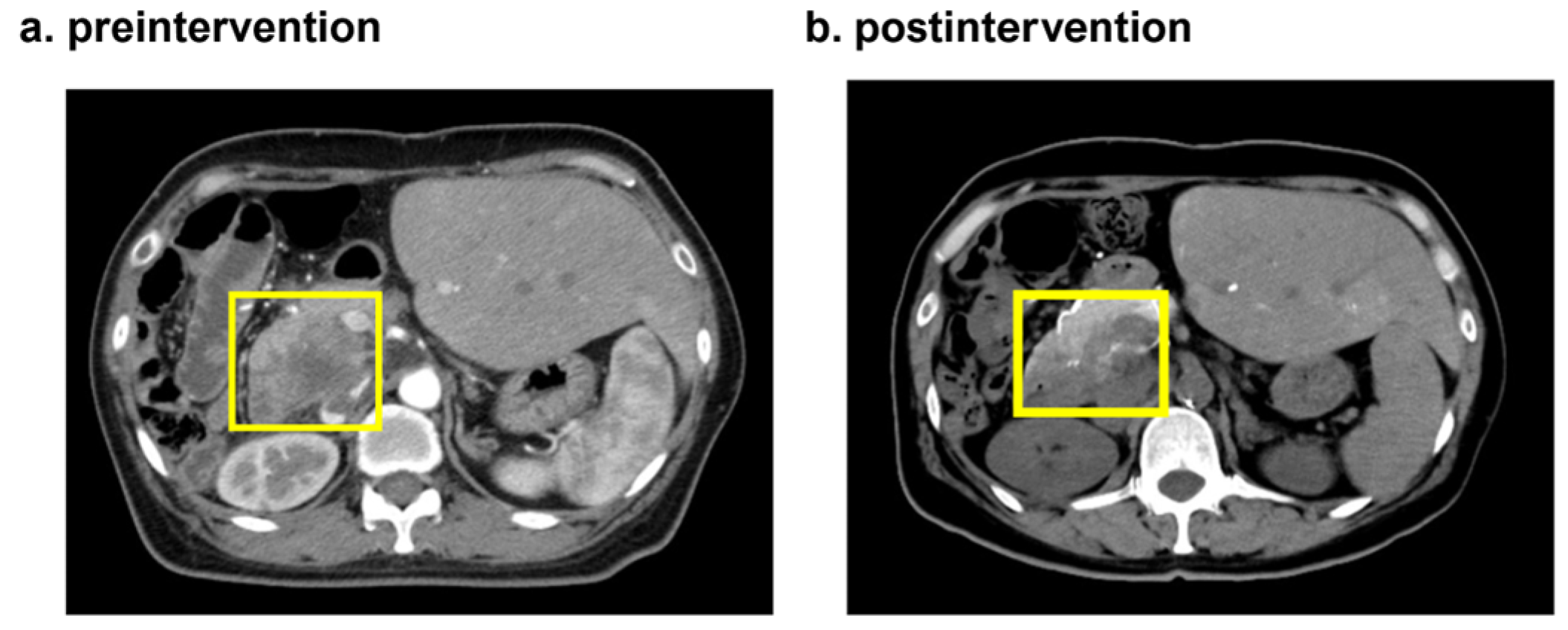
A 52-year-old woman with a height of 160 cm, weight of 57 kg and PS = 0 provided informed consent to participate in our clinical trial. Her past medical history is as follows. In October 2021, she was diagnosed with well-differentiated adenocarcinoma by biopsy after a computed tomography (CT) and autopsy ultrasonography (AUS) scan revealed masses in her pancreas and left lung. Although she underwent micro-RNA capsule gene therapy at another hospital in May and October 2022, she visited our hospital in January 2023 due to progressive disease (PD). She agreed to our proposal of an integrated approach using CDDP liposomes, and commenced treatment. On January 16, 100 ml of saline was added to a standard formulation of CDDP liposomes (CDDP 10 mg + liposome 300 mg) and administered to her, as a result, she experienced mild fatigue on the day (
Table 1). Therefore, two days later, on January 18, we reduced CDDP formula to 3.3 mg while keeping liposome content at 300 mg and added 100 ml of normal saline and administered to her via an intravenous drip. Our attempts were successful, and she did not experience any adverse side effects from the CDDP liposomes. Subsequently, we applied hyperthermia to her on January 20, and then, an angiographic CT scan diagnosed her with a partial response (PR) on January 23 (
Figure 3).
4. Discussion
Despite the increase in new anticancer agents, such as molecularly targeted drugs and immune checkpoint inhibitors [
18,
19], CDDP is still considered the first treatment choice for many cancer patients in clinical practice. This is because it has a high antitumor effect against various cancers, including gastric, esophageal, lung, ovarian, testicular, and head and neck cancers [
4,
5,
6,
7,
8,
9], and potential synergistic effects when combined with other types of anticancer agents and radiation therapy [
20,
21]. Our research group previously reported that CDDP combined with a Lentiviral vector with tumor suppressor genes, including p53 and p16, and phosphatase and tensin homolog (PTEN)-mediated gene therapy inhibited the growth of non-muscle invasive bladder cancer (NMIBC) [
22]. Despite CDDP’s usefulness and versatility, its use is often discouraged based on physician judgment or rejected by patients because of the occurrence of gastrointestinal symptoms such as chemotherapy-induced nausea and vomiting (CINV) and, in severe cases, neurological disorders, immunosuppression, and renal toxicity [
10,
11,
12]. Aprepitant, 5-HT
3 receptor antagonists, and dexamethasone are used to counter the side effects of CDDP, primarily to prevent CINV. Aprepitants and 5-HT3 receptor antagonists are metabolized by hepatic cytochrome P-450s, such as CYP3A4, and therefore demonstrate a dose-dependent inhibitory effect on CYP3A4 [
23] and may interact with concomitant drugs, including antineoplastic agents, which may cause excessive increases in blood levels because they are metabolized primarily in the liver, thus limiting their use in patients with severe liver impairment. Steroidal agents, such as dexamethasone, have been shown to prevent allergies and CINV; however, there is a concern regarding their immunosuppressive effects [
24]. Because higher-grade tumors require higher doses of CDDP, such patients require higher doses of CINV prophylaxis drugs. Therefore, physicians and patients must be more concerned about the side effects of CINV prophylaxis drugs and CDDP. Furthermore, the development of anticancer drugs that accumulate only in tumors without affecting other organs has been eagerly awaited.
With the development of drug delivery in recent years, polymerized anticancer drugs based on micelles and liposomes have been attracting attention as a countermeasure for serious side effects such as CDDP-mediated nephrotoxicity [
13,
14,
15,
16]. Clinical trials have also been conducted on CDDP modified with polyethylene glycol (PEG) and liposomes; however, these have not yet been used in actual clinical practice in Japan. The present study is the first in Japan to reveal that liposomalized CDDP may alleviate CDDP-mediated adverse drug reactions.
In the present study, we conducted a clinical trial on CDDP liposomes and combined sodium thiosulfate with CDDP liposomes to minimize CDDP-mediated adverse drug reactions. Even if the liposome formulation collapses before incorporation into cancer cells and CDDP leaks into the bloodstream, a low incidence of adverse effects due to toxicity in normal cells is expected because sodium thiosulfate chelates CDDP.
Neither of the participants, a 58-year-old man and a 52-year-old woman, in the present study showed abnormal increases in blood tests for clinical markers of nephrotoxicity, such as urea nitrogen (UN) or creatinine, after the CDDP liposome treatment intervention. In addition, these participants showed no abnormalities in their blood leukocytes, suggesting that liposomalization of CDDP may also reduce myelosuppression. The 58-year-old male patient was diagnosed with a metastatic liver tumor that continued to grow; however, some therapeutic benefits were observed because his tumor remained SD during the administration period of the CDDP liposome therapeutic intervention. The 52-year-old female patient showed mild fatigue during treatment with 10 mg encapsulated CDDP liposomes. The patient underwent contrast CT shortly after she arrived in Japan, revealing that the mild fatigue may be due to dehydration. This is because, during Transcatheter Arterial Chemo Embolization (TACE,) approximately 1,500 mg of infusion fluid and 0.75 mg of Aloxi were used as anti-nausea drugs and 10 mg of undiluted CDDP was used without the occurrence of nausea. Therefore, we thought reducing the CDDP dose from 10 to 3.3 mg and liposomalizing it would be sufficient to treat patients without the occurrence of nausea. We observed that for the 58-year-old participant, CDDP liposomes were safe and achieved tumor regression.
To our knowledge, the present study is the first in Japan to reveal that liposomalized CDDP may alleviate CINV and CDDP-mediated adverse drug reactions, such as renal damage and immunosuppression. In addition, CDDP liposome intervention demonstrated some antitumor efficacy in both participants in the present study. The effect of sodium thiosulfate cannot be ruled out in terms of the side effects caused by CDDP in the present study [
25]; however, we believe that the present results are due to the liposomalization of CDDP, as shown in
Figure 4, and previous studies that used similar liposomes have also reported liposome-related EPR effects [
17]. Furthermore, previous reports of CDDP liposomes reducing cytotoxicity in humans in clinical trials and in vitro studies support the present study’s results [
26,
27]. In the future, we will increase the number of participants who consent to participate in a study to test the safety and efficacy of CDDP liposomes in treating various symptoms of cancer. We believe CDDP liposomes will help patients with cancer achieve complete remission while improving their QOL.
Author Contributions
Conceptualization, Y.K. and S.K.; methodology, Y.K.; software, S.K.; validation, Y.K., S.K. and H.M.; formal analysis, Y.K. and S.K.; investigation, Y.K. and S.K..; resources, T.K., Y.H., M.H. and T.O.; data curation, S.K.; writing—original draft preparation, S.K.; writing—review and editing, K.H.; visualization, H.M.; supervision, K.H.; project administration, Y.K.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical Research Involving Human Subjects established by the Japanese Ministry of Health, Labor and Welfare, and approved by the Ethics Review Committee at the IGT Clinic (approval number: 19 and date of approval: December 21, 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data necessary to evaluate the paper’s conclusions are present in the paper and/or in the Supplementary Information. Additional data are available upon request from the corresponding author [K.H.].
Acknowledgments
We would like to express our deepest gratitude to the entire staff of Rinku Medical Clinic for their cooperation in the present study.
Conflicts of Interest
S.K., T.K., Y.H., M.H., and T.O. are employed as researchers at StateArt Inc. Y.K., M.H., K. H. has no conflicts of interest.
References
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698-699. [CrossRef]
- Lippard, S. J. New chemistry of an old molecule: cis-[Pt (NH3) 2Cl2]. Science 1982, 218, 1075-1082. [CrossRef]
- Brabec, V.; Hrabina, O.; Kasparkova, J. Cytotoxic platinum coordination compounds. DNA binding agents. Coord Chem Rev 2017, 351, 2-31. [CrossRef]
- Wagner, A. D.; Grothe, W. Transcatheter Arterial Chemo Embolization Haerting, J.; Kleber, G.; Grothey, A.; Fleig, W. E. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006, 24, 2903-2909. [CrossRef]
- Enzinger, P. C.; Ilson, D. H.; Kelsen, D. P. Chemotherapy in esophageal cancer. Semin Oncol 1999, 26, 12-20.
- Fennell, D. A.; Summers, Y.; Cadranel, J.; Benepal, T.; Christoph, D. C.; Lal, R.; Das, M.; Maxwell, F.; Visseren-Grul, C.; Ferry, D. Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat Rev 2016, 44, 42-50. [CrossRef]
- Du Bois, A.; Lück, H. J.; Meier, W.; Adams, H. P.; Möbus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schröder, W.; Olbricht, S.; Nitz, U.; Jackisch, C.; Emons, G.; Wagner, U.; Kuhn, W.; Pfisterer, J.; Arbeitsgemeinschaft Gynäkologische Onkologie Ovarian Cancer Study Group. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 2003, 95, 1320-1329. [CrossRef]
- de Vries, G.; Rosas-Plaza, X.; van Vugt, M. A. T. M.; Gietema, J. A.; de Jong, S. Testicular cancer: Determinants of cisplatin sensitivity and novel therapeutic opportunities. Cancer Treat Rev 2020, 88, 102054. [CrossRef]
- Lamont, E. B.; Vokes, E. E. Chemotherapy in the management of squamous-cell carcinoma of the head and neck. The Lancet Oncol 2001, 2, 261-269. [CrossRef]
- Santabarbara, G.; Maione, P.; Rossi, A.; Gridelli, C. Pharmacotherapeutic options for treating adverse effects of Cisplatin chemotherapy. Expert Opin Pharmacother 2016, 17, 561-570. [CrossRef]
- Ranganath, P.; Einhorn, L.; Albany, C. Management of chemotherapy induced nausea and vomiting in patients on multiday cisplatin based combination chemotherapy. Biomed Res Int 2015, 2015, 943618. [CrossRef]
- DeConti, R. C.; Toftness, B. R.; Lange, R. C.; Creasey W. A. Clinical and pharmacological studies with cis-diamminedichloroplatinum (II). Cancer Res 1973, 33, 1310-1315.
- Nishiyama, N.; Okazaki, S.; Cabral, H.; Miyamoto, M.; Kato, Y.; Sugiyama, Y.; Nishio, K.; Matsumura, Y.; Kataoka, K. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res 2003, 63, 8977-8983.
- Uchino, H.; Matsumura, Y.; Negishi, T.; Koizumi, F.; Hayashi, T.; Honda, T.; Nishiyama, N.; Kataoka, K.; Naito, S.; Kakizoe, T. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer 2005, 93, 678-687. [CrossRef]
- Saisyo, A.; Nakamura, H.; Fang, J.; Tsukigawa, K.; Greish, K.; Furukawa, H.; Maeda, H. pH-sensitive polymeric cisplatin-ion complex with styrene-maleic acid copolymer exhibits tumor-selective drug delivery and antitumor activity as a result of the enhanced permeability and retention effect. Colloids Surf B Biointerfaces 2016, 138, 128-137. [CrossRef]
- Islam, W.; Kimura, S.; Islam, R.; Harada, A.; Ono, K.; Fang, J.; Niidome, T.; Sawa, T.; Maeda, H. EPR-effect enhancers strongly potentiate tumor-targeted delivery of nanomedicines to advanced cancers: Further extension to enhancement of the therapeutic effect. J Pers Med 2021, 11, 487. [CrossRef]
- Yorozu, K.; Kaibori, M.; Kimura, S.; Ichikawa, M.; Matsui, K.; Kaneshige, S.; Kobayashi, M.; Jimbo, D.; Torikai, Y.; Fukuzawa, Y.; Okamoto, Y. Experience with Photodynamic Therapy Using Indocyanine Green Liposomes for Refractory Cancer. J Pers Med 2022, 12, 1039. [CrossRef]
- DasGupta, R.; Yap, A.; Yaqing, E. Y.; Chia, S. Evolution of precision oncology-guided treatment paradigms. WIREs Mech Dis 2023, 15, e1585. [CrossRef]
- Borgeaud, M.; Kim, F.; Friedlaender, A.; Lococo, F.; Addeo, A.; Minervini, F. The Evolving Role of Immune-Checkpoint Inhibitors in Malignant Pleural Mesothelioma. J Clin Med 2023, 12, 1757. [CrossRef]
- Boulikas, T.; Vougiouka, M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs. Oncol Rep 2004, 11, 559-595. [CrossRef]
- Dewit, L. Combined treatment of radiation and cisdiamminedichloroplatinum (II): a review of experimental and clinical data. Int J Radiat Oncol Biol Phys 1987, 13, 403-426. [CrossRef]
- Ichikawa, M.; Matsunaga, W.; Ishikawa, T.; Gotoh, A. Lentiviral vector-mediated gene transfer combined with cisplatin enhances tumor suppression in human bladder cancer cell lines. Personalized Medicine Universe 2019, 8, 15-19. [CrossRef]
- Aapro, M. S.; Walko, C. M. Aprepitant: drug–drug interactions in perspective. Ann Oncol 2010, 21, 2316-2323. [CrossRef]
- Grunberg, S. M. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: dosing, efficacy, and tolerability analysis. Ann Oncol 2007, 18, 233-240. [CrossRef]
- Brock, P. R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M. J.; Laithier, V.; Ronghe, M.; Dall'Igna, P.; Hiyama, E.; Brichard, B.; Skeen, J.; Mateos, M. E.; Capra, M.; Rangaswami, A. A.; Ansari, M.; Rechnitzer, C.; Veal, G. J.; Covezzoli, A.; Brugières, L.; Perilongo, G.; Czauderna, P.; Morland, B.; Neuwelt, E. A. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 2018, 378, 2376-2385. [CrossRef]
- Araújo, R. S.; Cristina Oliveira, M.; Cardoso, V. N.; Keefe, D. M.; Stringer, A. M. The effect of free and encapsulated cisplatin into long-circulating and pH-sensitive liposomes on IEC-6 cells during wound healing in the presence of host–microbiota. J Pharm Pharmacol 2022, 74, 711-717. [CrossRef]
- Boulikas, T. Clinical overview on Lipoplatin™: a successful liposomal formulation of cisplatin. Expert Opin Investig Drugs 2009, 18, 1197-1218. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).