Submitted:
10 April 2023
Posted:
10 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
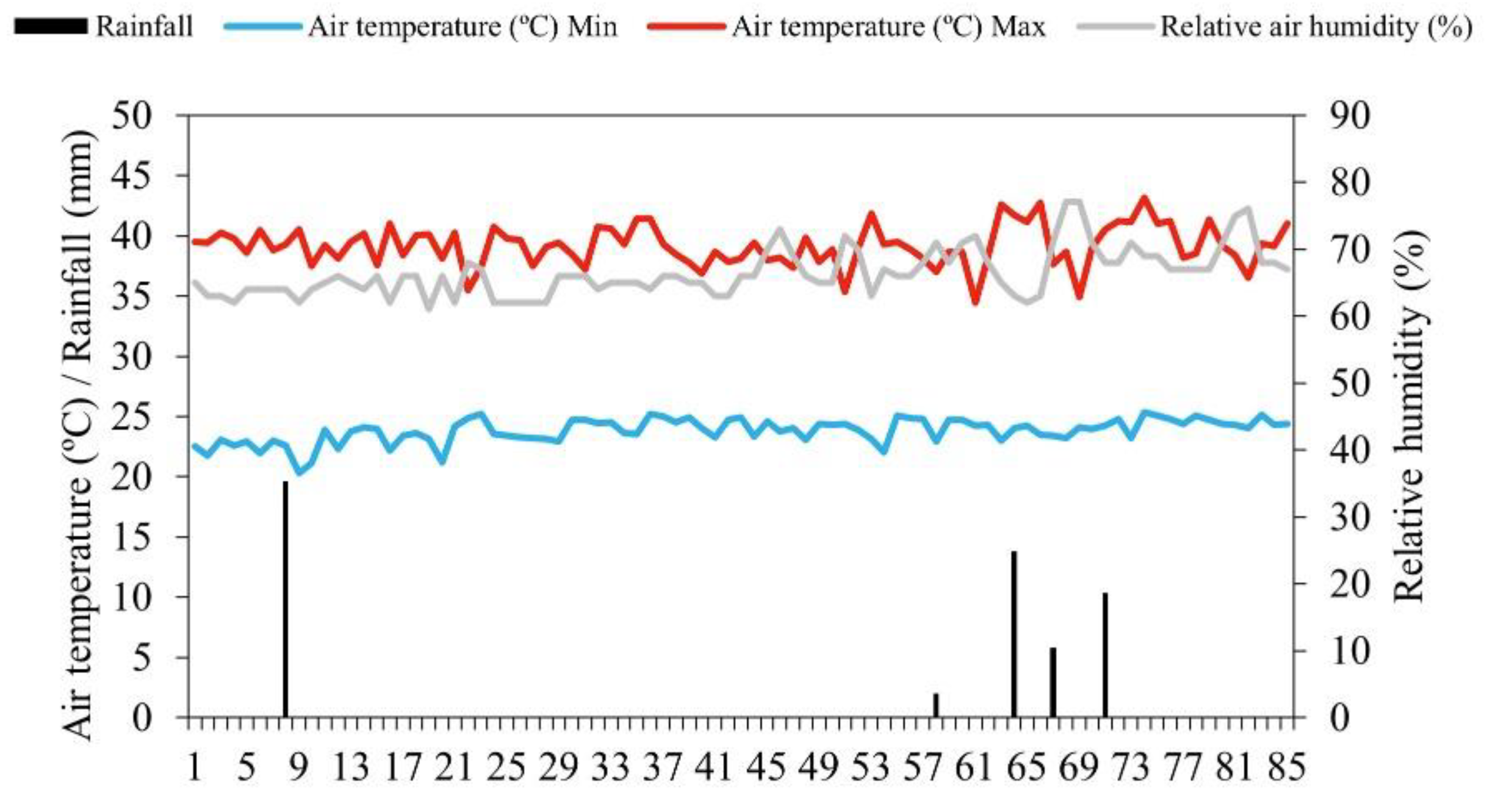
2.1. Location and characterisation of the experimental area
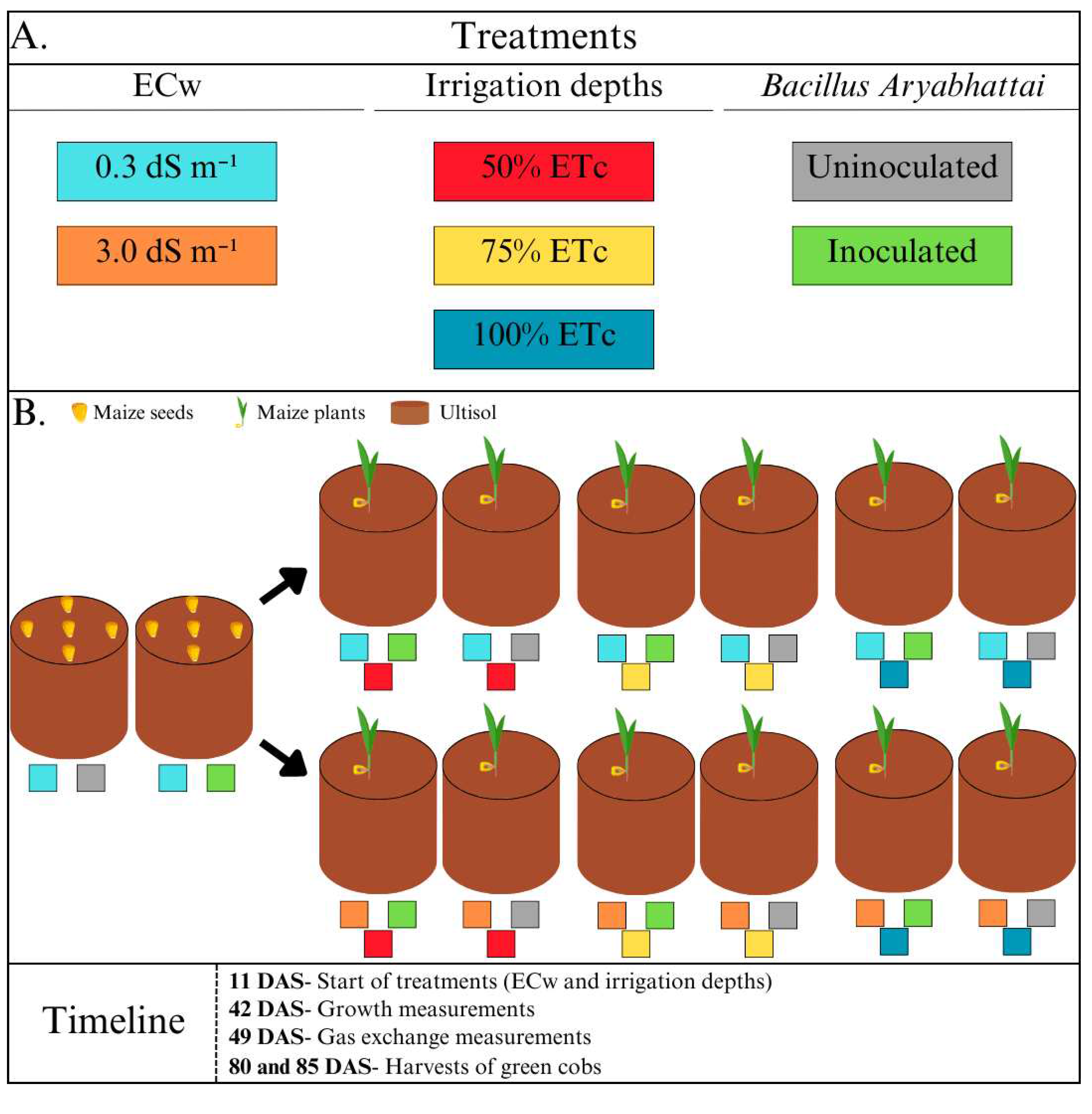
2.2. Experimental design and treatments
2.3. Irrigation management
- ETc – Crop evapotranspiration, in mm day-1;
- ECA - Evaporation measured in the class A pan, in mm/day-1;
- Kp - Class A pan coefficient, dimensionless;
- Kc - Crop coefficient, dimensionless.
- It - Irrigation time (min);
- ETc - Crop evapotranspiration for the period (mm);
- Sd - Spacing between emitters;
- Af - Application efficiency (0.92);
- q- Flow rate (L h-1).
2.4. Agroecological maize production system (plant material, inoculation, and fertilisation)
2.5. Variables under analysis
2.5.1. Growth
2.5.2. Leaf gas exchange
2.5.3. Yield
2.6. Data analysis
3. Results and Discussion
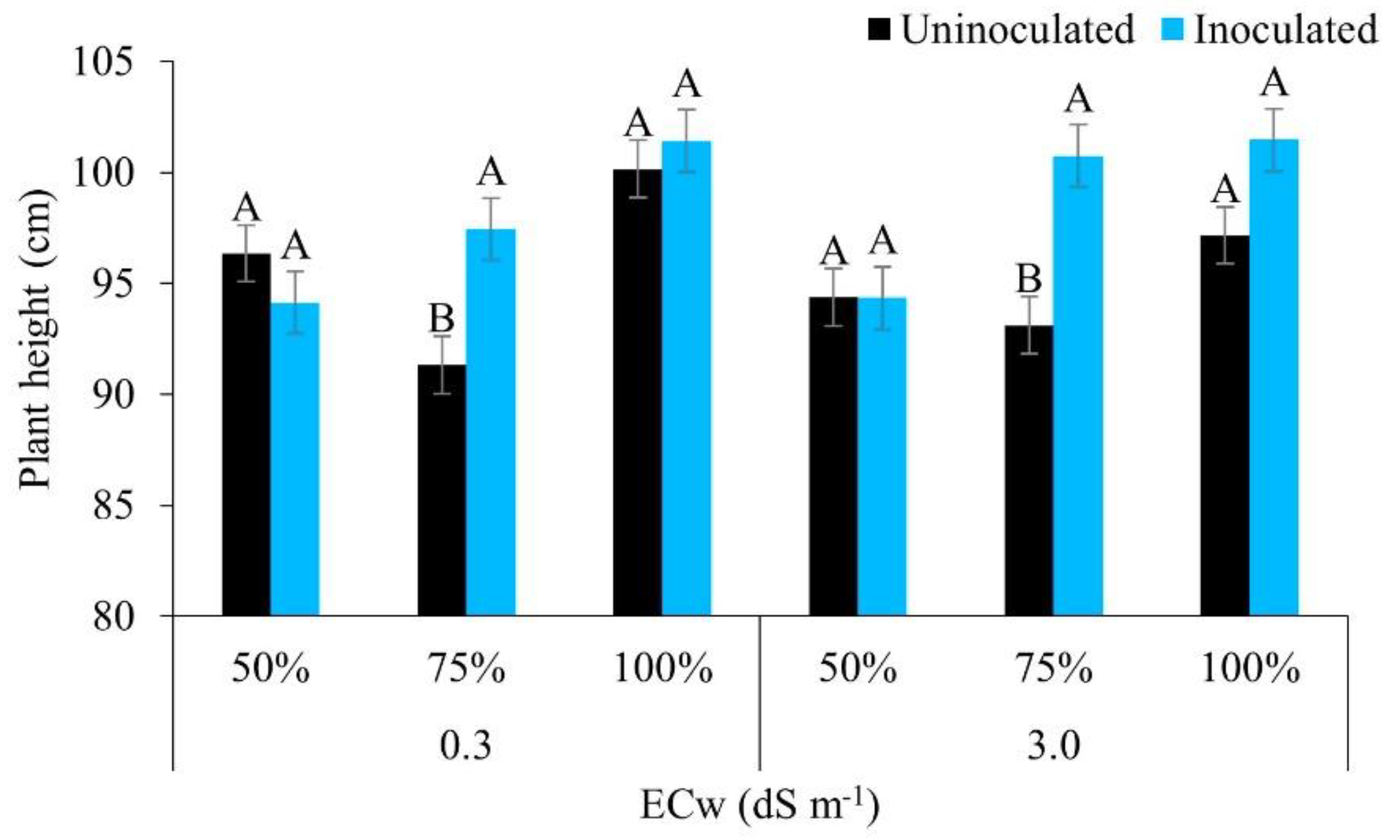
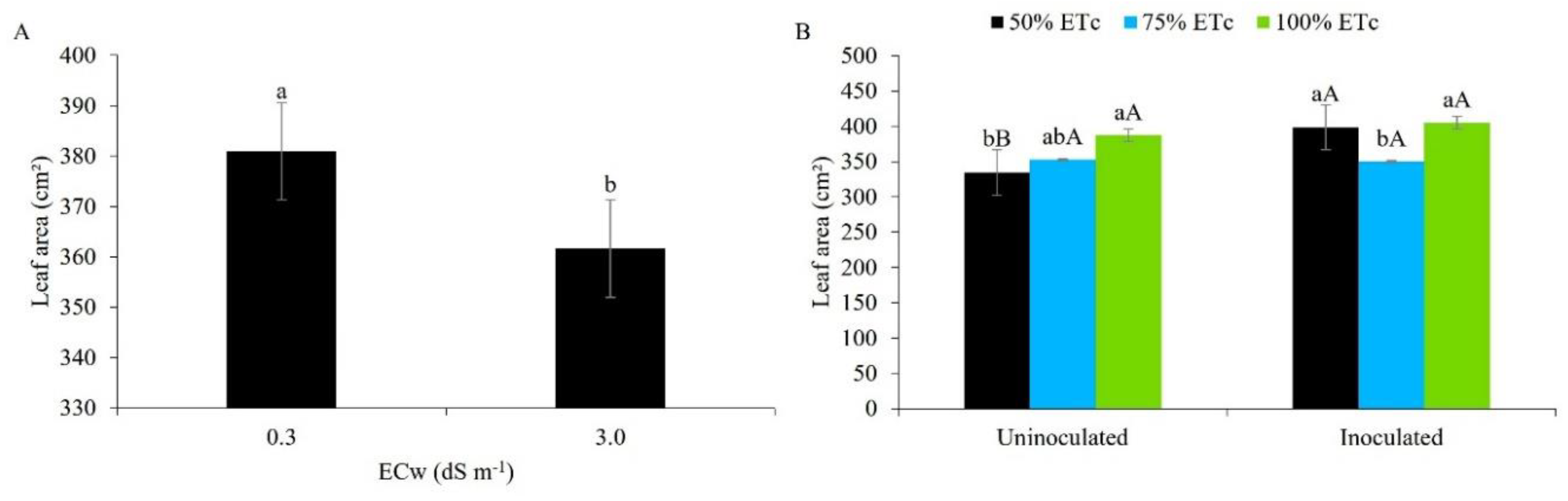
3.1. Growth
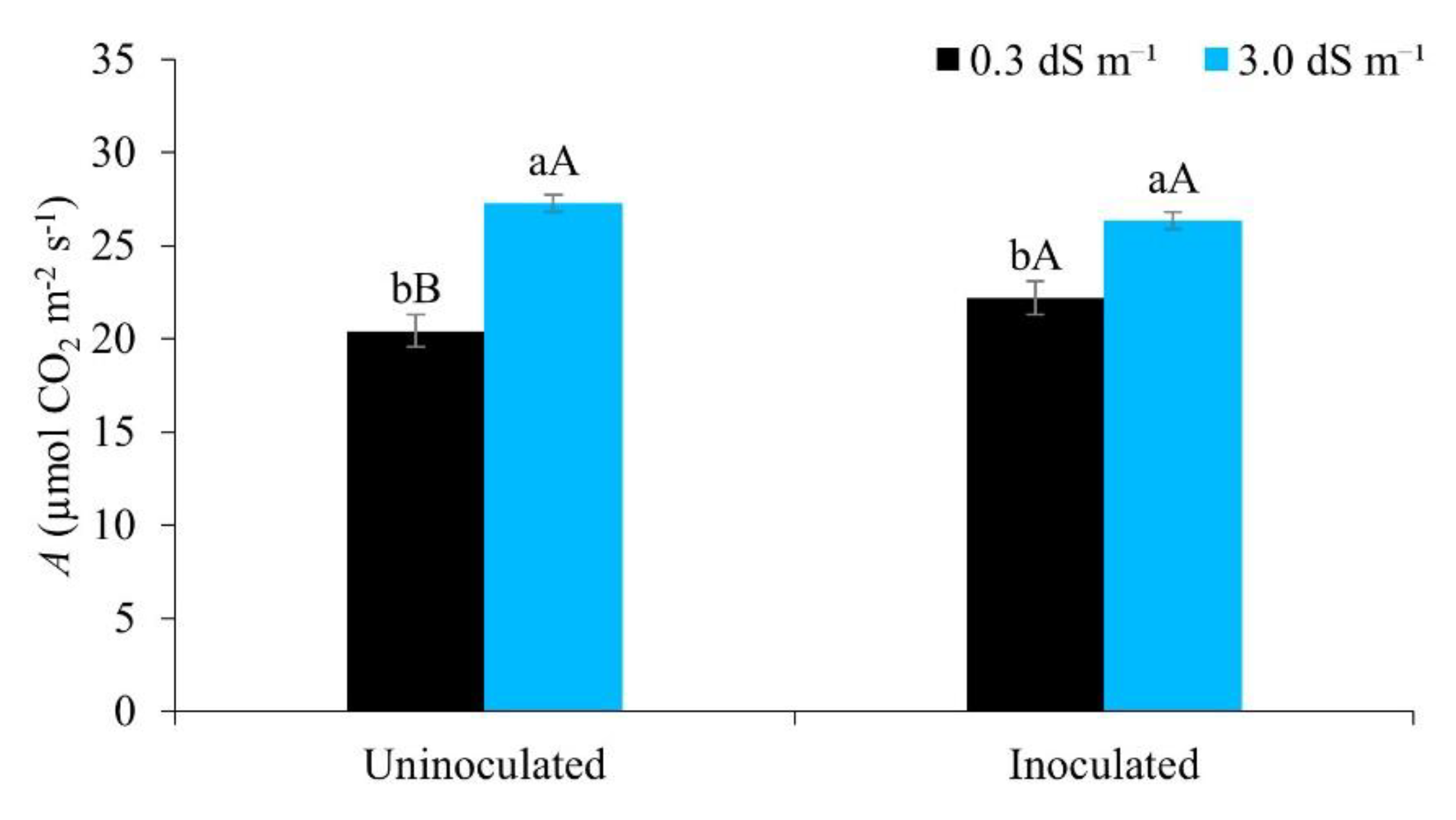
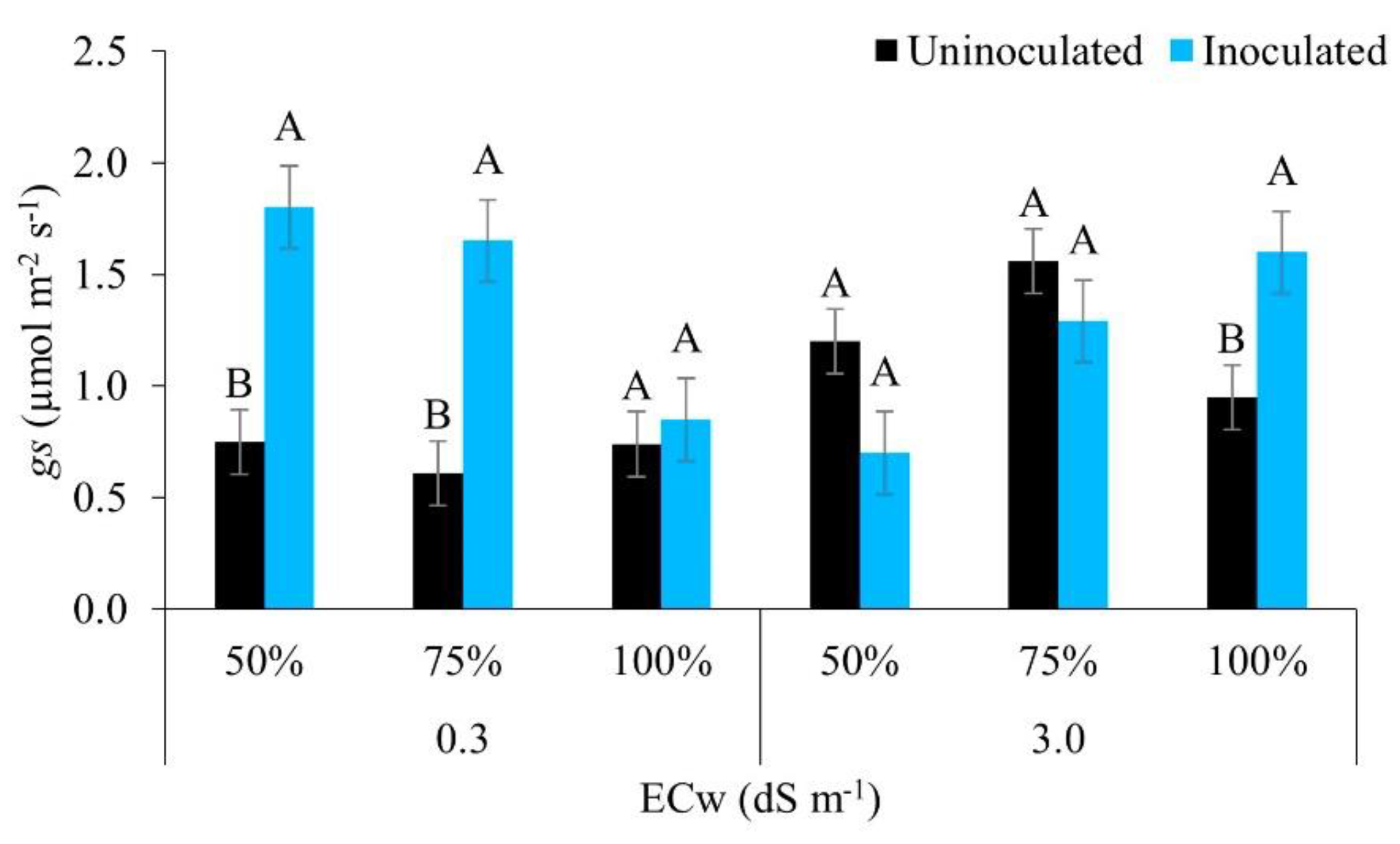
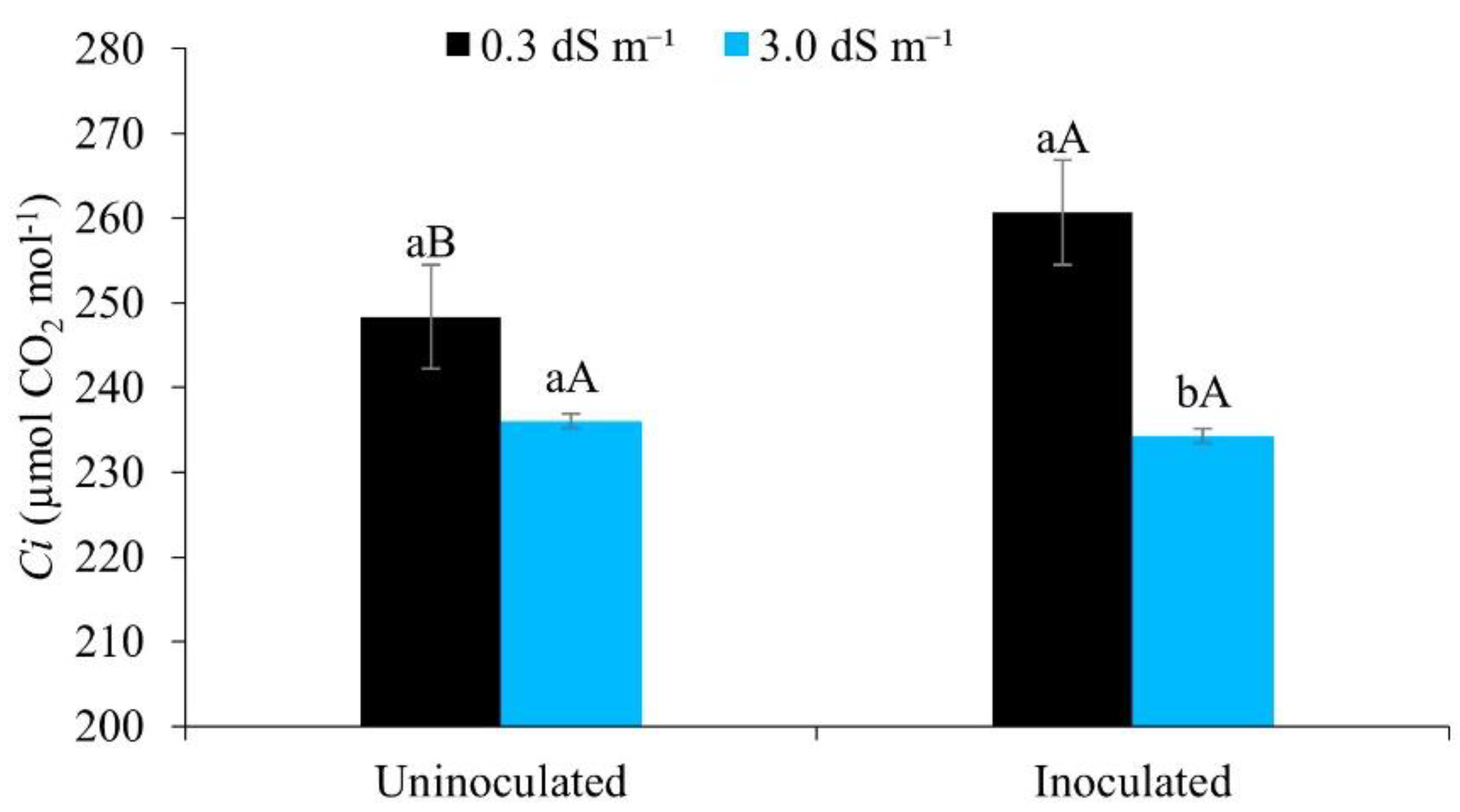
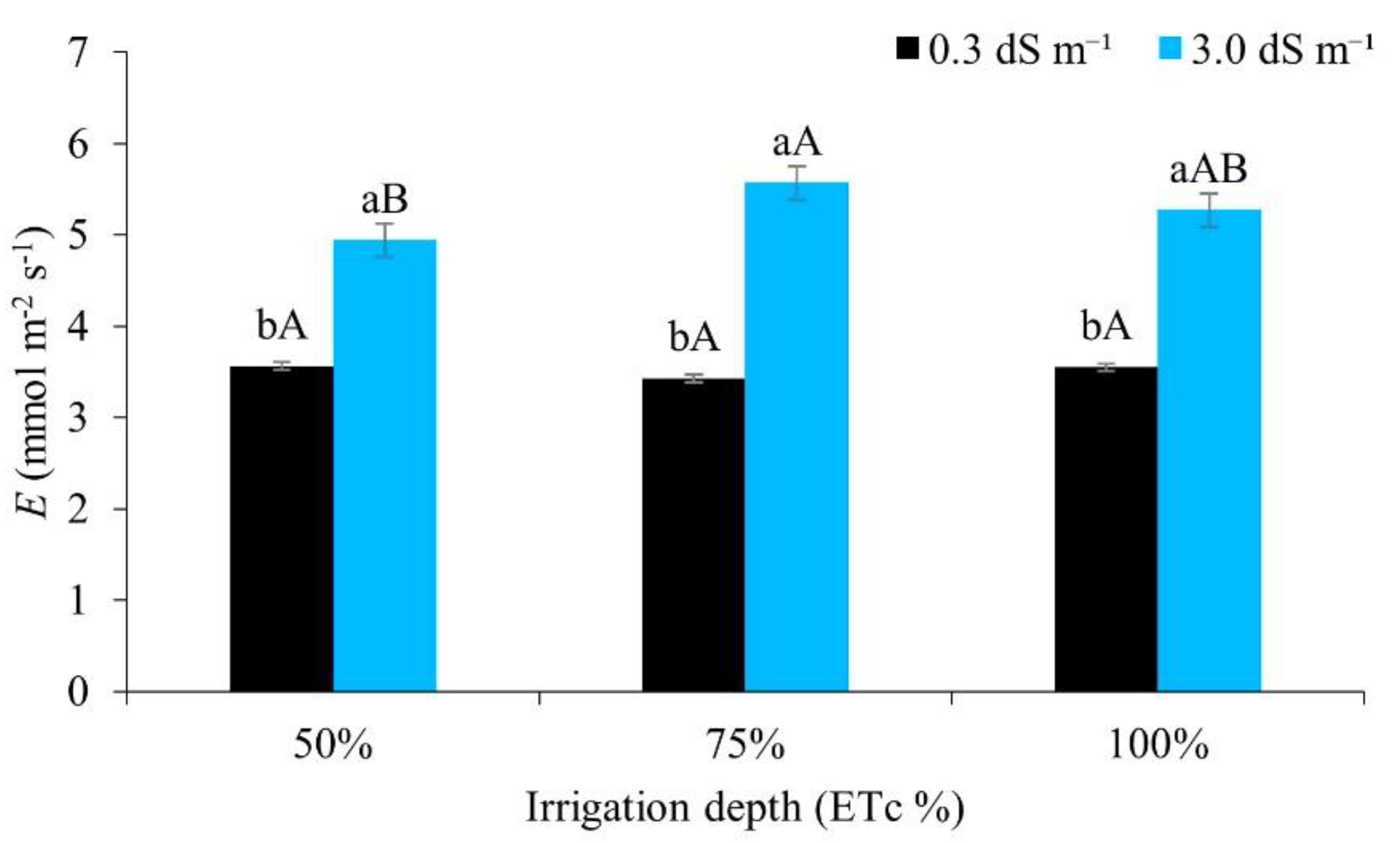
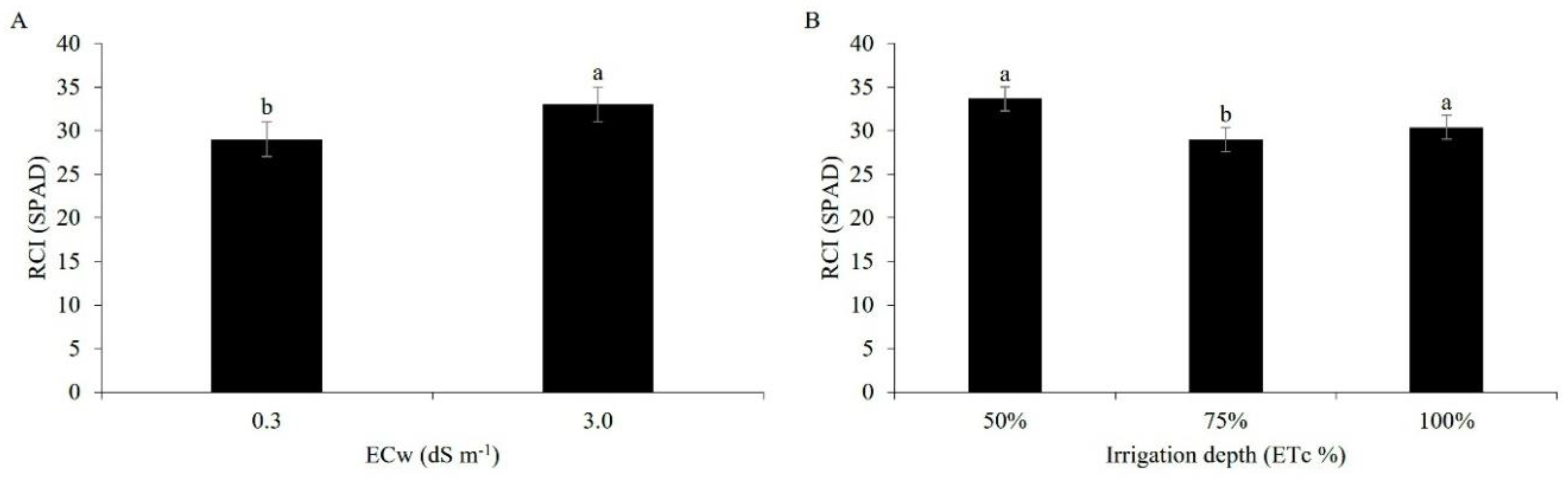
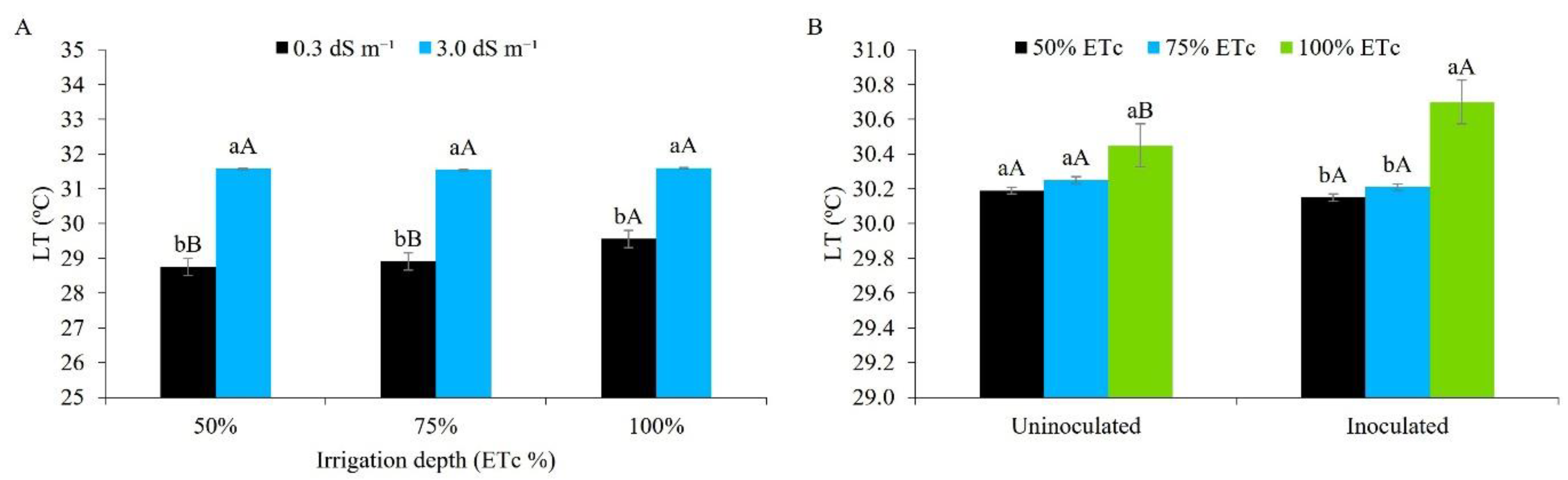
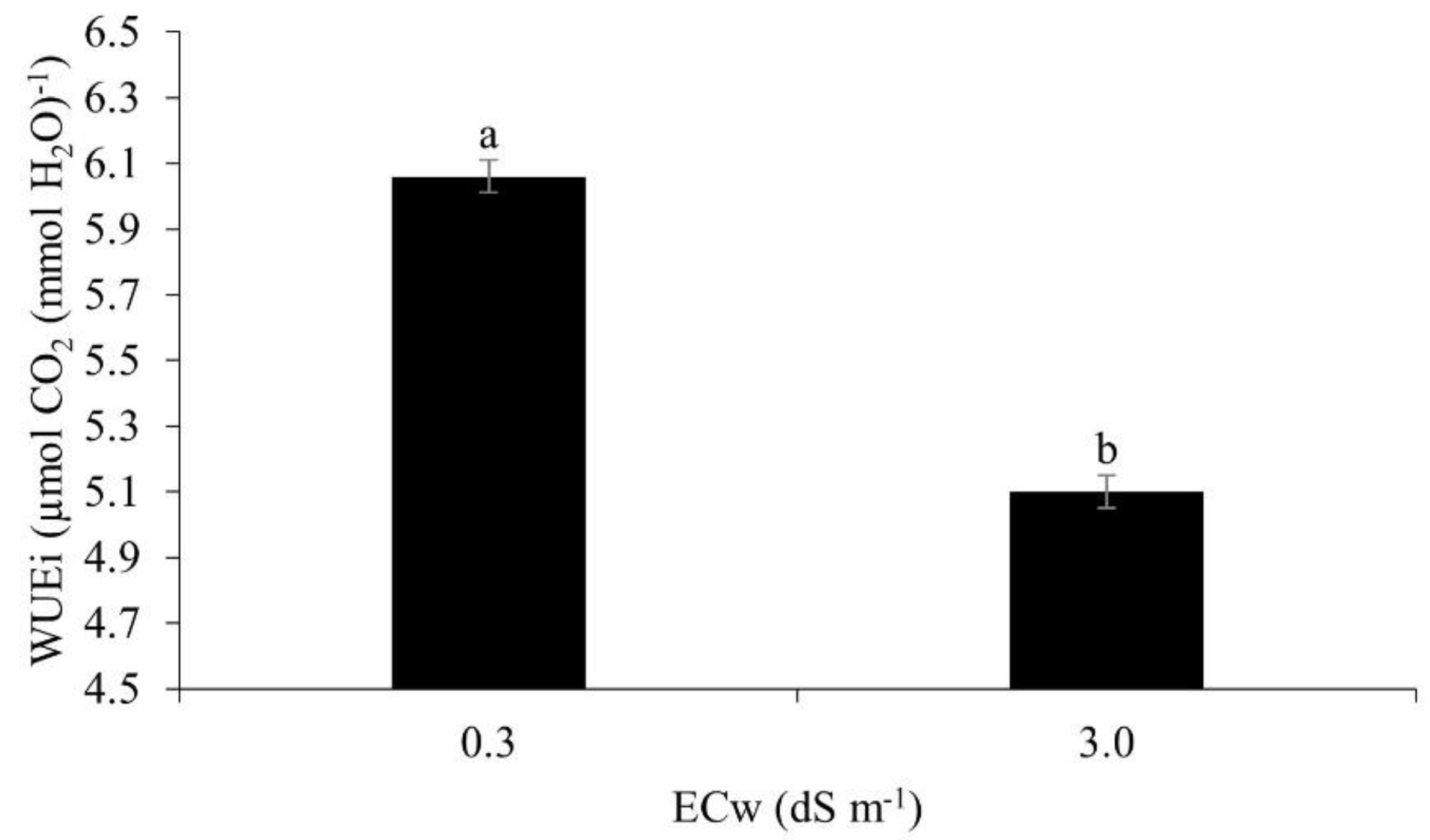
3.2. Leaf gas exchange
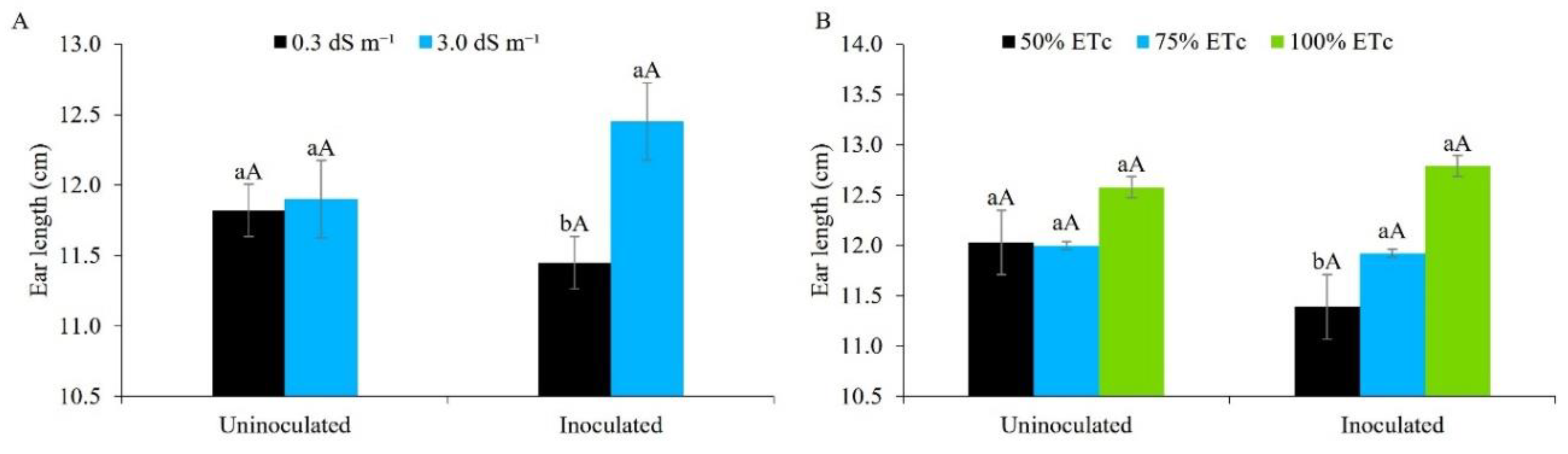
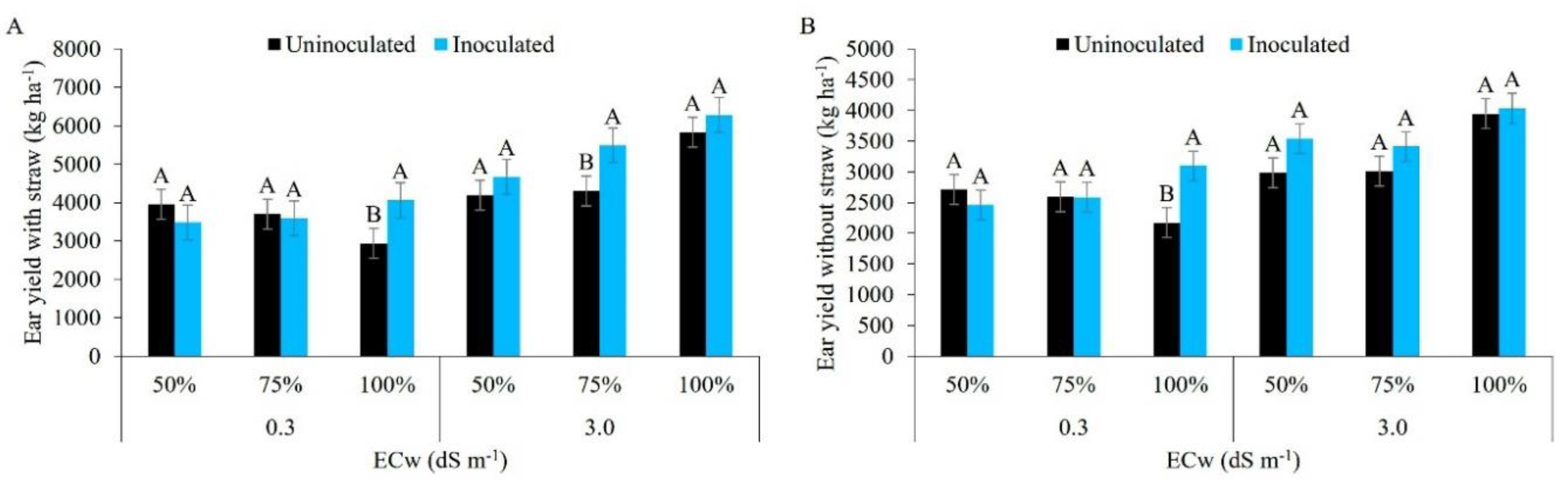
3.3. Yield
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fornasieri Filho, D. Manual da cultura do milho. Jaboticabal: FUNEP, 2007. 576 p.
- Dantas Junior, E.E.; Chaves, L.H.G.; Fernandes, J.D. Lâminas de irrigação localizada e adubação potássica na produção de milho verde, em condições semiáridas. Revista Espacios 2016, 37, 1-9.
- Lopes, J.R.F.; Dantas, M.P.; Ferreira, F.E.P. Identificação da influência da pluviometria no rendimento do milho no semiárido brasileiro. Rev. Bras. Agric. Irrig. 2019, 13, 3610-3618.
- CONAB - Companhia Nacional de Abastecimento. Ministério da Agricultura, Pecuária e Abastecimento. Safra Brasileira de Grãos: Boletim de grãos 2019/2020. Available online: https://portaldeinformacoes.conab.gov.br/safra-estimativa-de-evolucao-graos.html. (accessed on 05 March 2023).
- Song, L.; Jin, J.; He, J. Effects of Severe Water Stress on Maize Growth Processes in the Field. Sustainability 2019,11, 5086. [CrossRef]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Narayan, S.C.; Rana, M.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944. [CrossRef]
- SUDENE - Superintendência do Desenvolvimento do Nordeste. Nova delimitação do semiárido. 2017. Available online: http://antigo.sudene.gov.br/images/arquivos/semiarido/arquivos/Rela%C3%A7%C3%A3o_de_Munic%C3%Adpios_Semi%C3%A1rido.pdf. (accessed on 09 February 2023).
- Frizzone, J.A.; Lima, S.C.R.V.; Lacerda, C.F.; Mateos, L. Socio-Economic Indexes for Water Use in Irrigation in a Representative Basin of the Tropical Semiarid Region. Water 2021, 13, 2643. [CrossRef]
- Marengo, J.A.; Bernasconi, M. Regional differences in aridity/drought conditions over Northeast Brazil: Present state and future projections. Clim. Chang. 2015, 129, 103–115. [CrossRef]
- Cavalcante Júnior, R.G.; Freitas, M.A.V.; Silva, N.F.; Azevedo Filho, F.R. Sustainable groundwater exploitation aiming at the reduction of water vulnerability in the Brazilian semi-arid region. Energies 2019, 12, 904. [CrossRef]
- Lessa, C.I.N.; de Lacerda, C.F.; Cajazeiras, C.C.d.A.; Neves, A.L.R.; Lopes, F.B.; Silva, A.O.d.; Sousa, H.C.; Gheyi, H.R.; Nogueira, R.d.S.; Lima, S.C.R.V.; Costa, R.N.T.; Sousa, G.G.d. Potential of Brackish Groundwater for Different Biosaline Agriculture Systems in the Brazilian Semi-Arid Region. Agriculture 2023, 13, 550.
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of Salinity on Seed Germination and Seedling Development of Sorghum (Sorghum bicolor (L.) Moench) Genotypes. Agronomy 2020, 10, 859. [CrossRef]
- Isayenkov, S.V., Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10. [CrossRef]
- Islam, A.T.M.T.; Koedsuk, T.; Ullah, H.; Tisarum, R.; Jenweerawat, S.; Cha-um, S.; Datta, A. Salt tolerance of hybrid baby corn genotypes in relation to growth, yield, physiological, and biochemical characters. S. Afr. J. Bot. 2022,147, 808-819. [CrossRef]
- Lacerda, C.F.; Ferreira, J.F.S.; Suarez, D.L.; Freitas, E.D.; Liu, X.; Ribeiro, A.A. Evidence of nitrogen and potassium losses in soil columns cultivated with maize under salt stress. Rev. Bras. Eng. Agrícola Ambient. 2018, 22, 553-557. [CrossRef]
- Sousa, H.C.; Sousa, G.G.; Lessa, C.I.N.; Lima, A.F.S.; Ribeiro, R.M.R.; Costa, F.H.R. Growth and gas exchange of corn under salt stress and nitrogen doses. Rev. Bras. Eng. Agrícola Ambient. 2021, 25, 174-181. [CrossRef]
- Stadnik, B.; Tobiasz-Salach, R.; Mazurek, M. Effect of silicon on oat salinity tolerance: Analysis of the egipegenetic and physiological response of plants. Agriculture 2023, 13, 81.
- Sousa, H.C.; Sousa, G.G.de.; Cambissa, P.B.C.; Lessa, C.I.N.; Goes, G.F.; Silva, F.D. B.da.; Abreu, F.da S.; Viana, T.V.de A. Gas exchange and growth of zucchini crop subjected to salt and water stress. Rev. Bras. Eng. Agrícola Ambient. 2022, 26, 815-822. [CrossRef]
- Goes, G.F.; Sousa, G.G.; Santos, S.O.; Silva Júnior, F. B.; Ceita, E. D’A. R.; Leite, K.N. Produtividade da cultura do amendoim sob diferentes supressões da irrigação com água salina. Irriga 2021, 26, 210-220.
- Shilev, S. Plant-Growth-Promoting Bacteria Mitigating Soil Salinity Stress in Plants. Appl. Sci. 2020, 10, 7326. [CrossRef]
- Armanhi, J.S.L.; Souza, R.S.C.; Biazotti, B.B.; Yassitepe, J.E.D.C.T.; Arruda, P. Modulating drought stress response of maize by a synthetic bacterial community. Front. Microbiol. 2021, 12, 747541. [CrossRef]
- Poudel, M.; Mendes, R.; Costa, L.A.S.; Bueno, C.G.; Meng, Y.; Folimonova, S.Y.; Garrett, K.A.; Martins, S.J. The role of plant-associated bacteria, fungi, and viruses in drought stress mitigation. Front. Microbiol. 2021, 12, 743512. [CrossRef]
- Kavamura, V.N.; Santos, S.N.; Silva, J.L.; Parma, M.M.; Ávila, L.A.; Visconti, A.; Zucchi, T.D.; TaketanI, R.G.; Andreote, F.D.; Melo, I.S. de. Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiol. Res. 2013, 168, 183-191. [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [CrossRef]
- Sousa, S.M.; De Oliveira, C.A.; Andrade, D.L.; Carvalho, C.G.De; Ribeiro, V.P.; Pastina, M.M.; Marriel, I.E.; Lana, U.G. de P.; Gomes, E.A.P. Tropical Bacillus Strains Inoculation Enhances Maize Root Surface Area, Dry Weight, Nutrient Uptake and Grain Yield. J. Plant Growth Regul. 2021, 40, 867-877. [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711-728.
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W. Manual de métodos de análise de solo. 3. ed. Brasília, DF: Embrapa, 2017. p.573.
- Souza, L.S.B. de; Moura, M.S.B. de; Sediyama, G.C.; Silva, T.G.F. da. Requerimento hídrico e coeficiente de cultura do milho e feijão-caupi em sistemas exclusivo e consorciado. Rev. Caatinga 2015, 28, 151-160.
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1985; p. 174.
- Medeiros, J. F. Qualidade da água de irrigação utilizada nas propriedades assistidas pelo "GAT" nos Estados do RN, PB, CE e avaliação da salinidade dos solos. Dissertação (Mestrado em Engenharia Agrícola) - Universidade Federal da Paraíba, Campina Grande, 1992. Available online: http://dspace.sti.ufcg.edu.br:8080/jspui/handle/riufcg/2896. (accessed on 08 January 2023).
- Silva, F.C. Manual de análises químicas de solos, plantas e fertilizantes. Brasília: Embrapa Comunicação para Transferência de Tecnologia, 1999; p.370.
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. US Department of Agriculture, Washington, USA, 1954; p.160.
- Fernandes, V.L.B. Recomendações de adubação e calagem para o estado do Ceará. Fortaleza: UFC, 1993; p.248.
- Silva, F. de A.S.; Azevedo, C.A.V. de. The Assistat Software Version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 2016, 11, 3733-3740.
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807-838. [CrossRef]
- Kavamura, V.N. Bactérias associadas às cactáceas da Caatinga: promoção de crescimento de plantas sob estresse hídrico. Tese (Doutorado em Ciências) - Escola Superior de Agricultura “Luiz de Queiroz”, 2012. Available online: http://www.teses.usp.br/teses/disponiveis/11/11138/tde-25102012-095956/. (accessed on 10 February 2023).
- Oliveira, E.J.; Melo, H.C.de; Trindade, K.L.; Guedes, T.de M.; Sousa, C.M. Morphophysiology and yield of green corn cultivated under different water depths and nitrogen doses in the cerrado conditions of Goiás, Brazil. Res., Soc. Dev. 2020, 9, e6179108857.
- Taiz, L.; Zeiger, E.; Moller, I. M.; Murphy, A. Fisiologia e desenvolvimento vegetal. 6.ed. Porto Alegre: Artmed, 2017. 858p.
- Irmak, S.; Mohammed, A.T.; Kukal, M.S. Maize response to coupled irrigation and nitrogen fertilisation under center pivot, subsurface drip and surface (furrow) irrigation: Growth, development and productivity. Agric. Water Manag. 2020, 263, 107457.
- May, A.; Santos, M. de S.; Silva, E.H.F.M.da; Viana, R.da S.; Vieira Junior, N.A.; Ramos, N.P.; Melo, I.S. de. Effect of Bacillus aryabhattai on the initial establishment of pre-sprouted seedlings of sugarcane varieties. Res., Soc. Dev. 2021, 10, 1-9. [CrossRef]
- Qiu, Y; Fan, Y; Chen, Y; Hao, X; Li, S; Kang, S. Response of dry matter and water use efficiency of alfalfa to water and salinity stress in arid and semiarid regions of Northwest China. Agric. Water Manag. 2021, 254, 106934. [CrossRef]
- Ricardi, M.; Rosa, H.A. Desenvolvimento inicial do milho submetido a estresse salino. Rev. Cultiv. Saber 2018, 1, 174-184.
- Sá, F.; Ferreira Neto, M.; Lima, Y.; Paiva, E.; Prata, R.; Lacerda, C.; Brito, M. Growth, gas exchange and photochemical efficiency of the cowpea bean under salt stress and phosphorus fertilisation. Comum. Sci. 2018, 9, 668-679, 2018.
- Souza, M.V.P. de Sousa, G.G. de; Sales, J.R.S.; Freire, M.H. da C.; Silva, G.L.DA; Viana, T.V.de A. Água salina e biofertilisantes de esterco bovino e caprino na salinidade do solo, crescimento e fisiologia da fava. Rev. Bras. Cienc. Agrar. 2019, 14, 340-349.
- Oliveira, F.de A.; Medeiros, J.F.de.; Cunha, R.C.da; Souza, M.W.de L.; Lima, L.A. Uso de bioestimulante como agente amenizador do estresse salino na cultura do milho pipoca. Cienc. Agron. 2016, 47, 307-315.
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 9, 689-701. [CrossRef]
- Prado, R. M. Nutrição de plantas. 2. ed., São Paulo, SP: UNESP, 2020. 414 p.
- Jaghdani, S.J.; Janhns, P.; Trankner, M. The impact of magnesium deficiency on photosynthesis and photoprotection in Spinacia oleracea. Plant Stress 2021, 2, 1-11. [CrossRef]
- Lima, A.F.da S.; Santos, M.F.dos; Oliveira, M.L.; Sousa, G.G.de; Mendes Filho, P.F.; Luz, L.N.da. Physiological responses of inoculated and uninoculated peanuts under saline stress. Revista Ambiente & Água 2021, 16, e2643. [CrossRef]
- Cao, M.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Optimistic contributions of plant growth-promoting bacteria for sustainable agriculture and climate stress alleviation. Environ. Res. 2023, 217, 114924. [CrossRef]
- Vishnupradeep, R.; Bruno, L.B.; Taj, Z.; Karthik, C.; Challabathula, D.; Kumar, A., Freitas, H.; Rajkumar, M. Plant growth promoting bacteria improve growth and phytostabilization potential of Zea mays under chromium and drought stress by altering photosynthetic and antioxidant responses. Environ. Technol. Innov. 2022, 25, 102154. [CrossRef]
- Mishra, P.; Mishra, J.; Arora, N.K.; Plant growth promoting bacteria for combating salinity stress in plants–Recent developments and prospects: A review. Microbiol. Res. 2021, 252, 126861. [CrossRef]
- Sales, J.R.da S.; Magalhães, C.L.; Freitas, A.G.S.; Goes, G.F.; Sousa, H.C. Sousa, G.G. de. Índices fisiológicos de quiabeiro irrigado com água salina sob adubação organomineral. Rev. Bras. Eng. Agrícola Ambient. 2021, 25, 466-471.
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.E.; Ding, R. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci. Total Environ. 2022, 805, 150364. [CrossRef]
- Rodrigues, V.D.S.; Sousa, G.G.D.; Soares, S.D.C.; Leite, K.N.; Ceita, E.D.; Sousa, J.T.M.D. Gas exchanges and mineral content of corn crops irrigated with saline water. Rev. Ceres 2021, 68, 453-459.
- Nascimento, F.N; Bastos, E.A; Cardoso, M.J; Júnior, A.S.A; Ribeiro, V.Q. Parâmetros fisiológicos e produtividade de espigas verdes de milho sob diferentes lâminas de irrigação. Revista Brasileira de Milho e Sorgo 2015, 14, 167-181. [CrossRef]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Saif, T.; Abdelly, C.; Siddique, K.H.M.; Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 2019, 139, 171-178. [CrossRef]
- Abdel Latef, A.A.H., Abu Alhmad, M.F., Kordrostami, M.; Abo-Baker, A.B.A.E. Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum Reinforces Maize Growth by Improving Physiological Activities Under Saline Conditions. J Plant Growth Regul 2020, 39, 1293-1306. [CrossRef]
- Cavalcante, E.S.; Lacerda, C.F.; Mesquita, R.O.; de Melo, A.S.; da Silva Ferreira, J.F.; dos Santos Teixeira, A.; Lima, S.C.R.V.; da Silva Sales, J.R.; de Souza Silva, J.; Gheyi, H.R. Supplemental Irrigation with Brackish Water Improves Carbon Assimilation and Water Use Efficiency in Maize under Tropical Dryland Conditions. Agriculture 2022, 12, 544. [CrossRef]
- Costa-Gutierrez, S.B.; Raimondo, E.E.; Lami, M.J.; Vincent, P.A.; Espinosa-Urgel, M.; Cristóbal, R. E. Inoculation of Pseudomonas mutant strains can improve growth of soybean and corn plants in soils under salt stress. Rhizosphere 2020, 16, 100255. [CrossRef]
- Cavallet, L.E.; Pessoa, A.C.S.; Helmich, J.J.; Helmich, P.R.; Ost, C. f. Produtividade do milho em resposta à aplicação de nitrogênio e inoculação das sementes com Azospirillum spp. Rev. Bras. Eng. Agric. Ambient. 2000, 4, 129-132. [CrossRef]
- Freire, M.H.da C.; Viana, T.V.de A.; Sousa, G.G. de.; Azevedo, B.M.de.; Sousa, H.C.; Goes, G.F.; Lessa, C.I.N.; Silva, F.D.B.da. Organic fertilisation and salt stress on the agronomic performance of maize crop. Rev. Bras. Eng. Agrícola Ambient. 2022, 26, 848-854.
- Marrocos, S.T.P; Junior, J.N; Granjeiro, L.C; Ambrosio, M.M de Q; Da Cunha, A.P.A. Composição química e microbiológica de biofertilisantes em diferentes tempos de decomposição. Rev. Caatinga 2012, 25, 34-43.
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11.
- EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária. Boletim de Pesquisa e Desenvolvimento. Isolamento e Potencial Uso de Bactérias do Gênero Bacillus na Promoção de Crescimento de Plantas em Condições de Déficit Hídrico. 2019. Available online:https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1114738/1/bol192.pdf. (accessed on 01 March 2023).
- Araújo, V.L.V.P.; Lira Júnior, M.A.; Souza Júnior, V.S.; Araújo Filho, J.C.; Fracetto, F.J.C.; Andreote, F.D.; Pereira, A.P.A.; Mendes Júnior, J.P.; Barros, F.M.R.; Fracetto, G.G.M. Bacteria fromm tropical semiarid temporary ponds promote maize growth under water stress. Microbiol. Res. 2020, 24, 126564.
- Rodrigues, V.S.; Bezerra, F.M.L.; Sousa, G.G.; Fiusa, J.N.; Viana, T.V.A. Yield of maize crop irrigated with saline waters. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 101-105. [CrossRef]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183-1191.
- Arora, N.K., Tewari, S., Singh, R. Multifaceted plant-associated microbes and their mechanisms diminish the concept of direct and indirect PGPRs. In: Plant-microbe Symbiosis: Fundamentals and Advances; Arora, N.K., Ed.; Springer: New Delhi, India, 2013; pp. 411-449.
- Cavalcante Filho, H.A.; Helcias, R.P.; Pereira, R.G.; Cavalcante M.; Medeiros, P.V.Q.; Andrade, A.R.S. Desempenho de cultivares de milho para produção de milho verde, minimilho e forragem. Revista de Ciências Agroveterinárias 2022, 21, 449-455.
- Mazzuchelli, R.C.L.; Sossai, B.F.; Araujo, F.F. Inoculação de Bacillus subtilis e Azospirillum brasilense na cultura do milho. Colloq. Agrariae 2014, 10, 40-47.
- Silva, G.C.; Schmitz, R.; Silva, L.C.; Carpanini, G.G.; Magalhães, R.C. Desempenho de cultivares para produção de milho verde na agricultura familiar do sul de Roraima. Revista Brasileira de Milho e Sorgo 2015, 14, 273-282. [CrossRef]

| pH | OM | N | C | P | Ca | Mg | Na | Al | H + Al | K | ECse | ESP | C/N | V | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O | ---------- g kg-1 ---------- | mg kg-1 | ----------------------- cmolc dm-3 ----------------------- | dS m-1 | % | % | |||||||||
| 5.6 | 11.59 | 0.71 | 6.72 | 20 | 3.20 | 2.60 | 0.07 | 0.35 | 2.15 | 0.17 | 0.76 | 1 | 9 | 74 | |
| SD (g cm-3) | CS | FS | Silt | Clay | Textural Classification | ||||||||||
| Bulk | Particle | ---------------------------------------- g kg-1 -------------------------------------- | |||||||||||||
| 1.31 | 2.61 | 507 | 283 | 133 | 77 | Loamy Sand | |||||||||
| ECw (dS m-1) |
ETc (%) |
Total depth applied (mm) | |
|---|---|---|---|
| Uninoculated | Inoculated | ||
| 0.3 | 50 | 260.4 | 260.4 |
| 75 | 390.6 | 390.6 | |
| 100 | 520.8 | 520.8 | |
| 3.0 | 50 | 260.4 | 260.4 |
| 75 | 390.6 | 390.6 | |
| 100 | 520.8 | 520.8 | |
| ECw | Ca2+ | Mg2+ | K+ | Na+ | Cl- | HCO3- | pH | CE | SAR | Classification¹ |
|---|---|---|---|---|---|---|---|---|---|---|
| dS m-1 | ---------mmolc L-1-------- | ---mmol L-1--- | in H2O | dS m-1 | (mmolc L-1)0,5 | |||||
| 0.3 | 0.6 | 1.4 | 0.2 | 0.4 | 2.5 | 0.1 | 6.9 | 0.3 | 0.4 | C2S1 |
| 3.0 | 6.33 | 7.64 | 2.0 | 15.6 | 25 | 1.0 | 7.79 | 3.0 | 5.9 | C4S2 |
| Organic source | N | P | K+ | Ca2+ | Mg2+ |
|---|---|---|---|---|---|
| g L-1 | |||||
| Cattle manure | 0.96 | 0.47 | 0.59 | 1.10 | 0.25 |
| Cattle biofertiliser | 0.82 | 1.4 | 1.0 | 2.5 | 0.75 |
| Source of Variation | DF | Mean Square | |||
|---|---|---|---|---|---|
| PH | NL | SD | LA | ||
| Blocks | 5 | 29.67ns | 1.95ns | 1.53ns | 207.38ns |
| ECw | 1 | 0.06ns | 2.60ns | 87.96** | 5613.37* |
| Residual (ECw) | 5 | 27.97 | 0.61 | 2.74 | 412.05 |
| Irrigation depths (ID) | 2 | 21.30ns | 0.40ns | 56.08** | 10546.35** |
| Residual (ID) | 20 | 15.37 | 0.43 | 2.49 | 974.55 |
| Inoculation (INOC) | 1 | 9.56ns | 0.33ns | 35.25** | 10360.73** |
| Residual (INOC) | 30 | 16.57 | 0.54 | 3.05 | 1161.23 |
| ECw × ID | 2 | 160.75** | 0.25ns | 1.87ns | 2864.11ns |
| ECw × INOC | 1 | 149.91** | 0.004ns | 0.0007* | 2064.59ns |
| ID × INOC | 2 | 0.24* | 0.16ns | 0.27ns | 5769.978* |
| ECw × ID × INOC | 2 | 70.49* | 1.42ns | 4.56ns | 654.36ns |
| CV (%) - Ecw | 5.46 | 9.49 | 12.68 | 5.47 | |
| CV (%) - ID | 4.05 | 7.97 | 12.08 | 8.41 | |
| CV (%) - INOC | 4.20 | 8.95 | 13.37 | 9.18 | |
| Source of Variation | DF | Mean Square | ||||||
|---|---|---|---|---|---|---|---|---|
| A | gs | Ci | E | RCI | LT | WUEi | ||
| Blocks | 5 | 9.03ns | 0.67ns | 798.40ns | 0.43** | 10.05ns | 3.67ns | 0.006* |
| ECw | 1 | 361.19** | 0.15ns | 4504.68* | 36.83** | 191.12** | 74.72** | 11.07** |
| Residual (ECw) | 5 | 7.25 | 0.16 | 303.63 | 0.00** | 2.94 | 0.61 | 0.28 |
| Irrigation depths (ID) | 2 | 2.14ns | 0.26ns | 315.25ns | 0.25ns | 92.35* | 0.77* | 0.22ns |
| Residual (ID) | 20 | 8.90 | 0.14 | 101.83 | 0.11 | 23.43 | 0.15 | 0.15 |
| Inoculation (INOC) | 1 | 2.13ns | 3.60** | 336.02ns | 0.11ns | 39.45ns | 0.04* | 0.01ns |
| Residual (INOC) | 30 | 3.13 | 0.18 | 110.47 | 0.16 | 9.78 | 0.02 | 0.21 |
| ECw × ID | 2 | 2.86ns | 1.46** | 9.75ns | 0.58* | 66.17ns | 0.67* | 0.01ns |
| ECw × INOC | 1 | 6.97* | 2.48** | 595.02* | 0.47ns | 0.09ns | 0.04ns | 0.008ns |
| ID × INOC | 2 | 1.88ns | 0.04ns | 234.33ns | 0.27ns | 4.97ns | 0.10* | 0.006ns |
| ECw × ID × INOC | 2 | 0.78ns | 2.57** | 110.47ns | 0.18ns | 0.87ns | 0.02ns | 0.27ns |
| CV (%) - ECw | 11.20 | 11.85 | 6.34 | 0.77 | 5.54 | 2.59 | 9.60 | |
| CV (%) - ID | 12.41 | 10.04 | 3.67 | 7.88 | 15.62 | 1.32 | 7.03 | |
| CV (%) - INOC | 7.37 | 14.05 | 3.82 | 9.11 | 10.09 | 0.50 | 8.31 | |
| Source of Variation | DF | Mean Square | |||
|---|---|---|---|---|---|
| EL | ED | EYWS | EYWoS | ||
| Blocks | 5 | 3.09ns | 17.63ns | 6772473.13ns | 3162178.48ns |
| ECw | 1 | 5.15ns | 9.90ns | 40862788.82** | 14079648.00** |
| Residual (ECw) | 5 | 1.69 | 4.58 | 1711897.76 | 820739.41 |
| Irrigation depths (ID) | 2 | 1.35ns | 5.27ns | 3121961.40ns | 1275678.96* |
| Residual (ID) | 20 | 1.49 | 3.63 | 1464975.55 | 351695.38 |
| Inoculation (INOC) | 1 | 0.14ns | 0.38ns | 3533704.78** | 450274.80* |
| Residual (INOC) | 30 | 0.86 | 1.96 | 363835.20 | 309105.44 |
| ECw × ID | 2 | 0.34ns | 1.57ns | 5385095.95* | 1002466.33ns |
| ECw × INOC | 1 | 3.78* | 2.51ns | 1257793.16ns | 69497.37ns |
| ID × INOC | 2 | 6.11** | 1.65ns | 956622.93ns | 225383.24ns |
| ECw × ID × INOC | 2 | 1.27ns | 5.31ns | 1678902.68* | 1118325.91* |
| CV (%) - ECw | 10.92 | 6.24 | 29.91 | 29.74 | |
| CV (%) - ID | 10.24 | 5.56 | 27.67 | 19.47 | |
| CV (%) - INOC | 7.80 | 4.08 | 13.79 | 18.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





