Submitted:
31 March 2023
Posted:
04 April 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1 Interaction Energy Decomposition Analysis
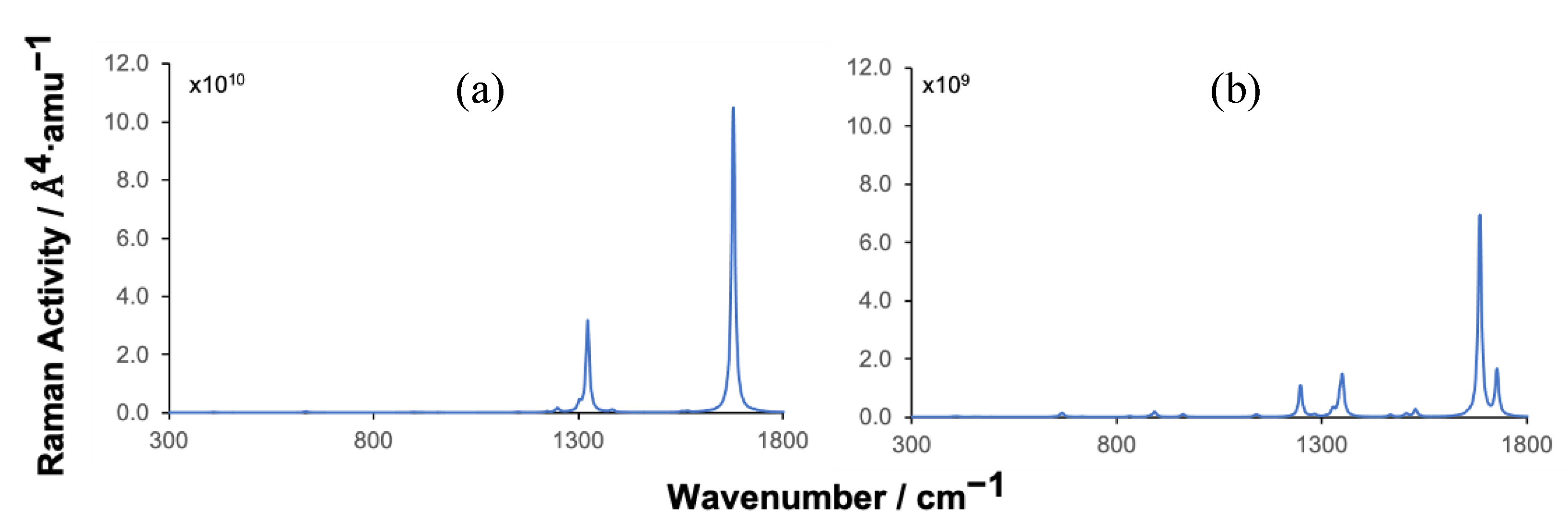
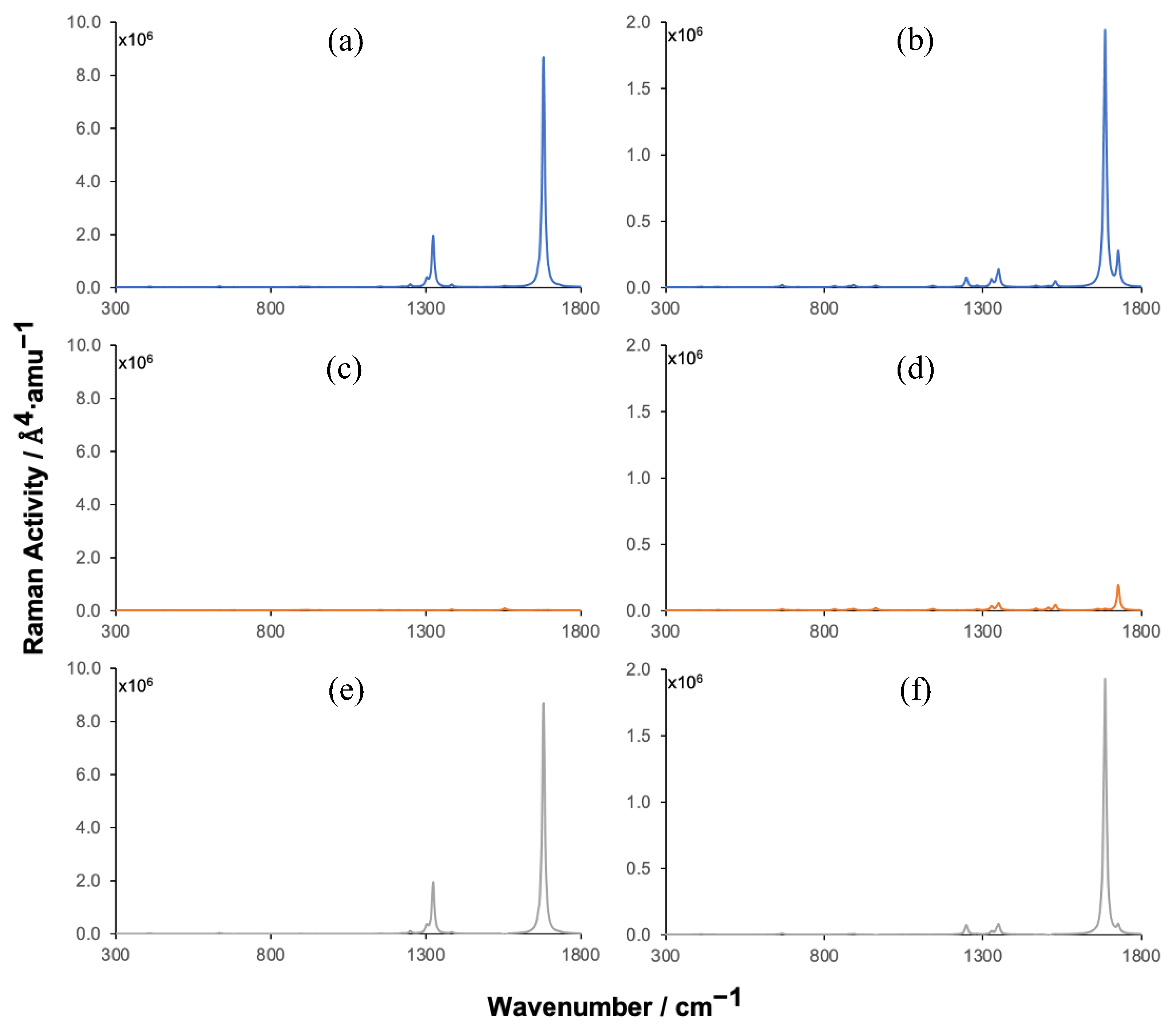
2.2. Simulation of Raman Spectra
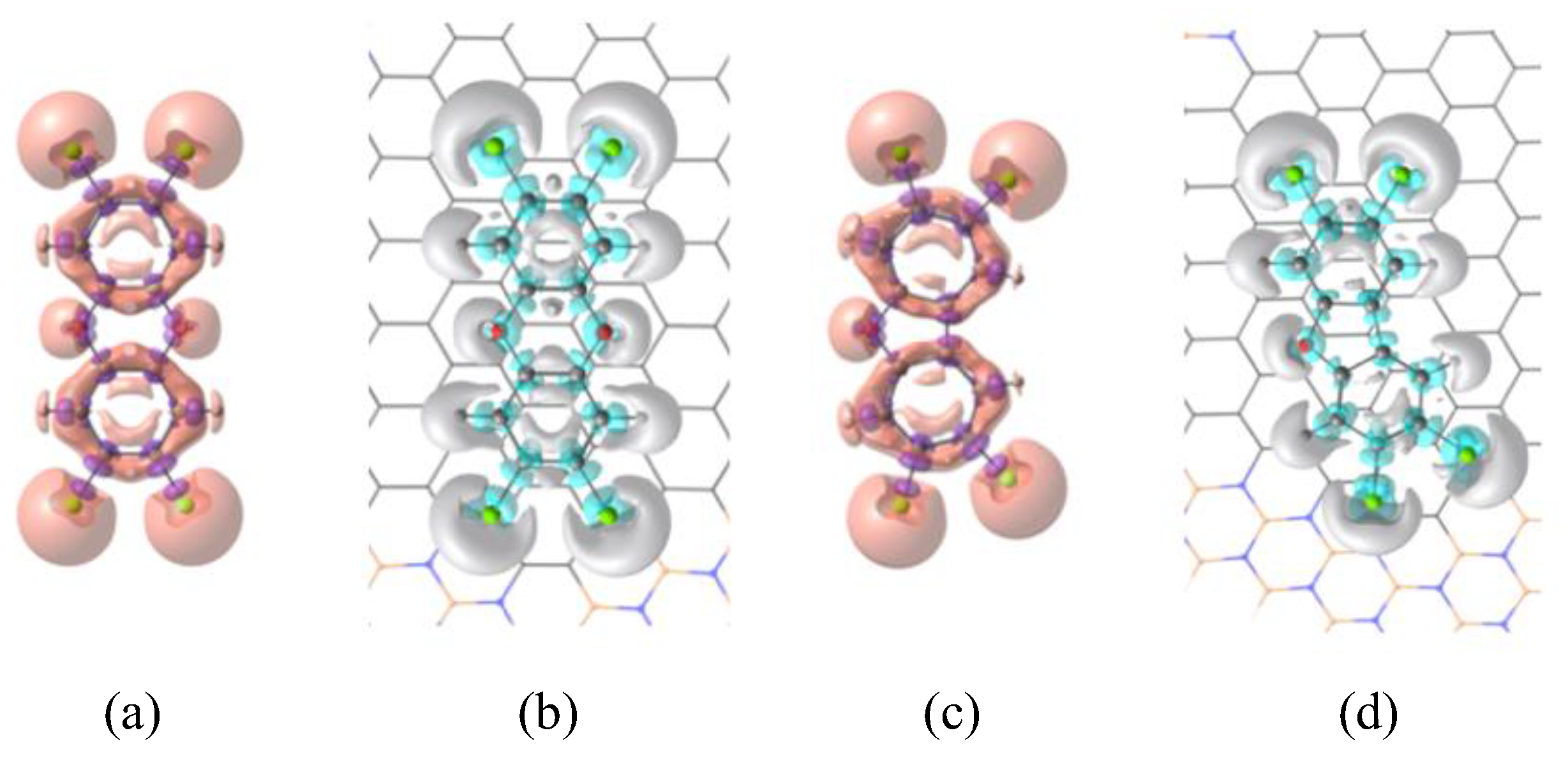
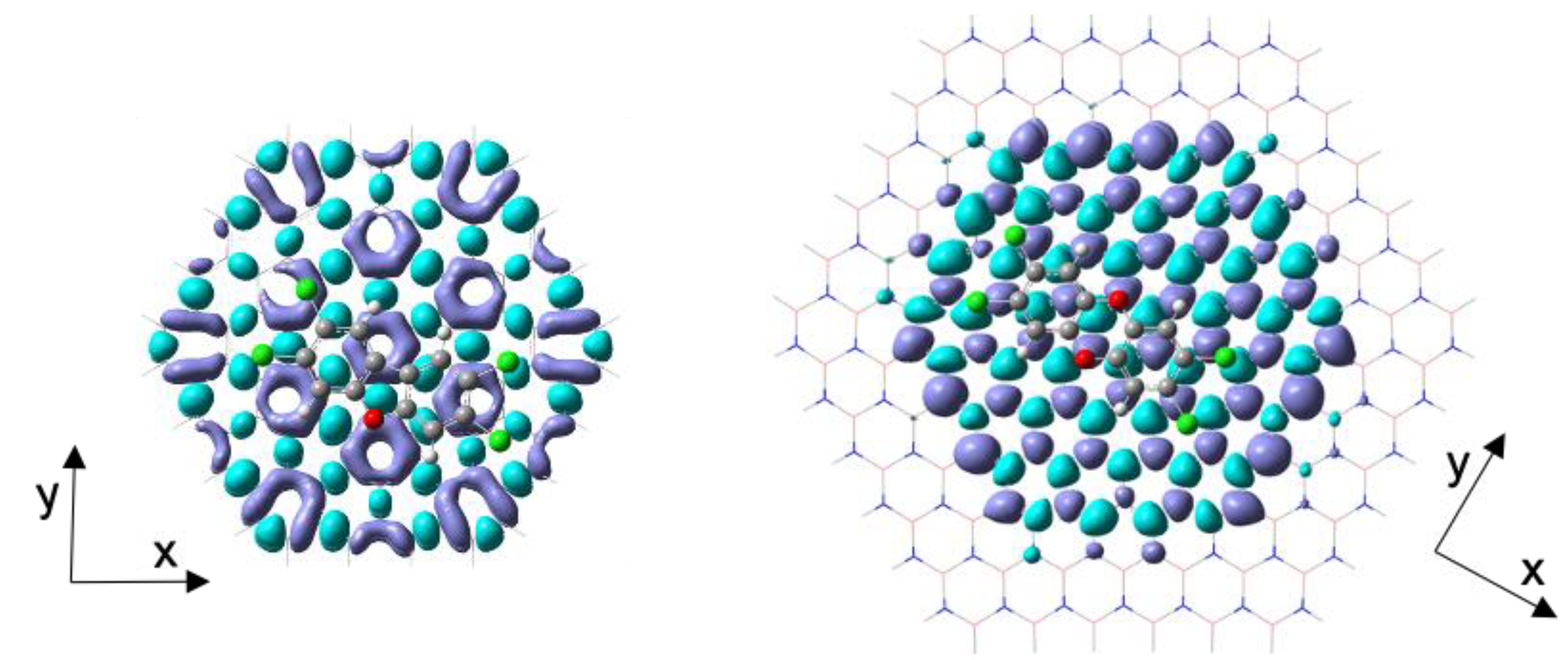
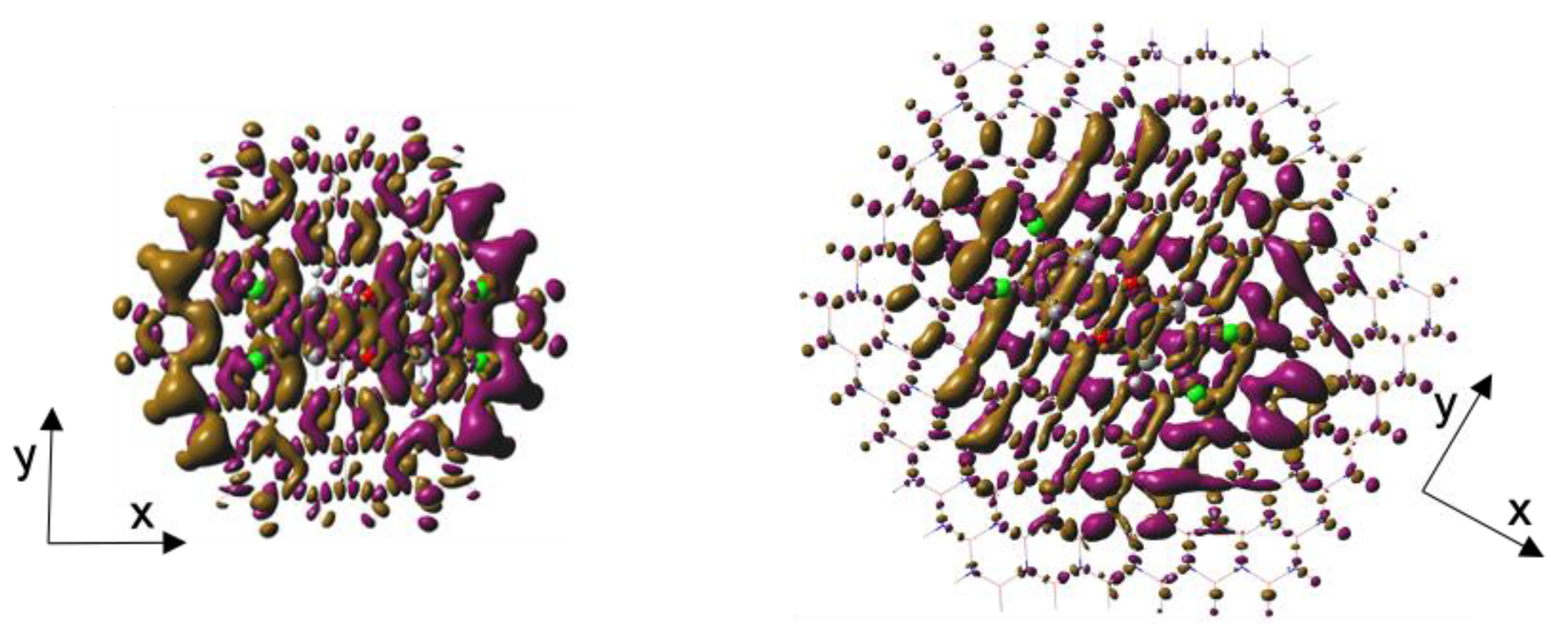
2.3. Real Space Representation of Electric Polarizabilities
2.4. Computational Details
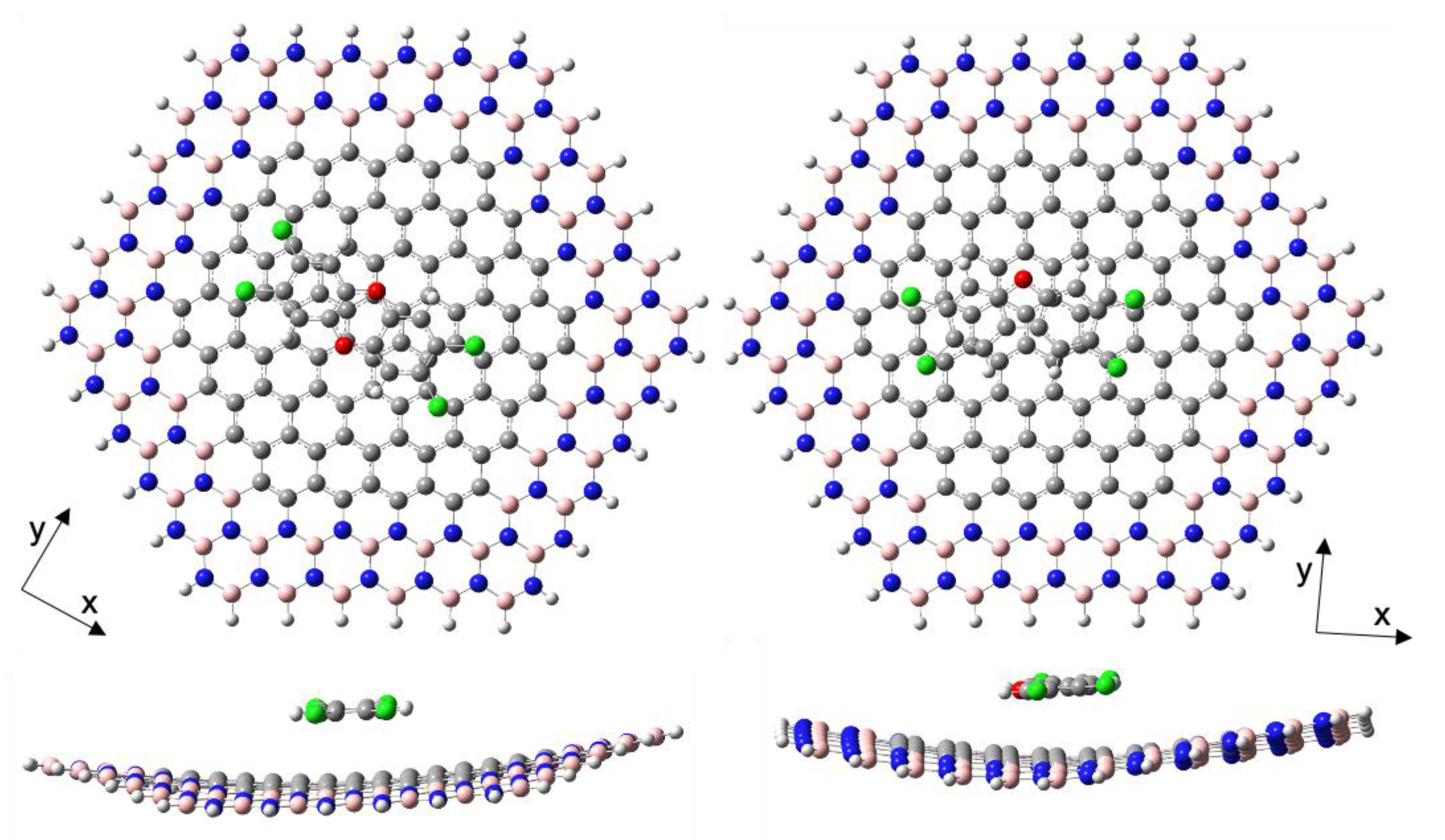
3. Results
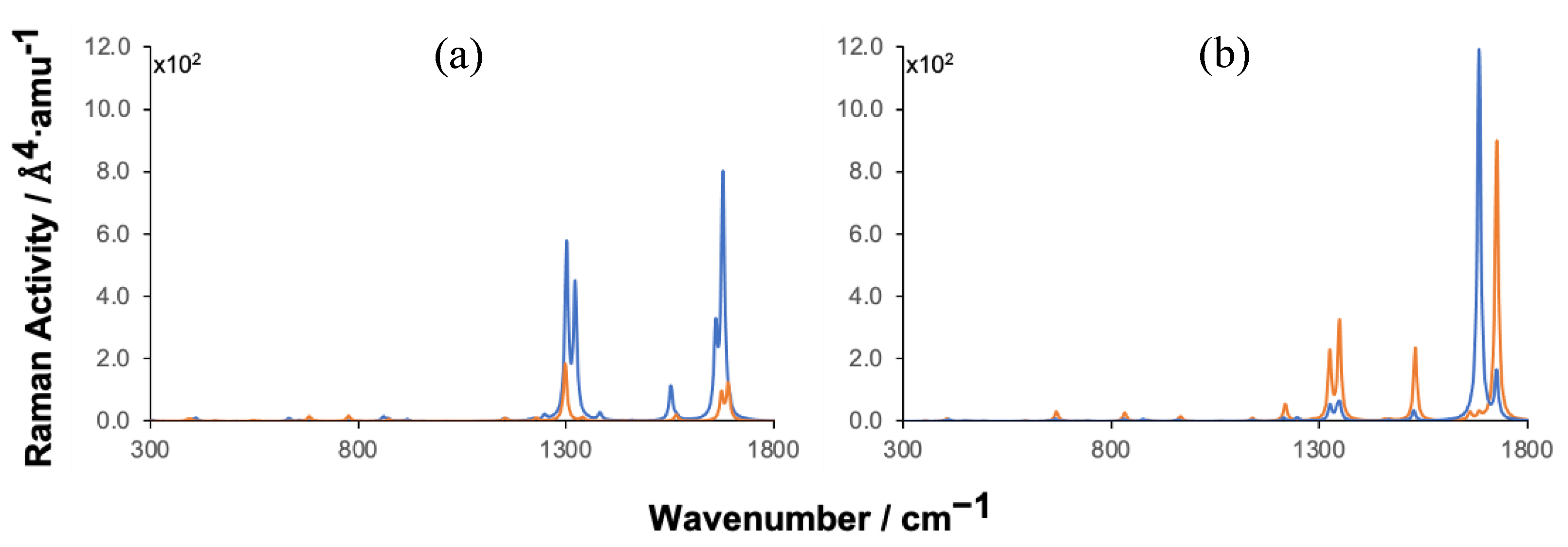
3.2 Static Raman Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Den Berg, M.; Birnbaum, L.; Bosveld, A. T. C.; Brunström, B.; Cook, P.; Feeley, M.; Giesy, J. P.; Hanberg, A.; Hasegawa, R.; Kennedy, S. W.; Kubiak, T.; Larsen, J. C.; Van Leeuwen, F. X. R.; Liem, A. K. D.; Nolt, C.; Peterson, R. E.; Poellinger, L.; Safe, S.; Schrenk, D.; Tillitt, D.; Tysklind, M.; Younes, M.; Wærn, F.; Zacharewski, T. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 1998, 106, 775. [CrossRef]
- Van den Berg, M.; Birnbaum, L.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; Rose, M.; Safe, S.; Schrenk, D.; Tohyama, C.; Tritscher, A.; Tuomisto, J.; Tysklind, M.; Walker, N.; Peterson, R. E. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223. [CrossRef]
- World Health Organization, Polychlorinated dibenzo-para-dioxins and dibenzofurans. Environ. Health Criteria. 1989, 88, 1.
- Polychlorinated Dibenzo-para-dioxins and Polychlorinated Dibenzofurans. IARC Monogr. Eval. Carcinog. Risks Hum. Suppl. 1997, 69, 1.
- Schecter, A. (Ed), Dioxin and Health, Plenum Press. Springer. New York, USA 1994.
- Steenland, K.; Bertazzi, P.; Baccarelli, A.; Kogevinas, M.; Dioxin revisited: developments since the 1997 IARC classification of dioxin as a human carcinogen. Environ. Health Perspect. 2004, 112, 1265. [CrossRef]
- Biswas, G.; Srinivasan, S.; Anandatheerthavarada, H. K.; Avadhani, N. G. Dioxin-mediated tumor progression through activation of mitochondria-to-nucleus stress signaling. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 186. [CrossRef]
- Wielgosiński, G. The Reduction of Dioxin Emissions from the Processes of Heat and Power Generation, J. Air & Waste Manage. Assoc. 2011, 61, 511.
- Rappe, C. Dioxins, patterns and source identification, Fresenius´ J. Anal. Chem. 1994, 348, 63. [CrossRef]
- Merrill, M. L.; Emond, C.; Kimi, M. J.; Antignaci, J.-P.; Bizeci, B. L.; Birnbaum, K. C. L. S.; Barouki, R. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ. Health Perspect. 2013, 121, 162. [CrossRef]
- Regnier, S. M.; Sargis, R. M. Adipocytes under assault: environmental disruption of adipose physiology. Biochim. Biophys. Acta, Mol. Basis Dis. 2014, 1842, 520. [CrossRef]
- Schrenk, D.; Chopra, M. Dioxins and Polychlorinated Biphenyls in Foods. In Chemical Contaminants and Residues in Food, Woodhead Publishing Series in Food Science, Technology and Nutrition, D. Schrenk, A. Cartus (eds), Elsevier. Cambridge, USA 2017.
- Beck, H.; Droß, A.; Eckart, K.; Mathar, W.; Wittkowski, R. PCDDs, PCDFs and related compounds in paper products. Chemosphere 1989, 19, 655. [CrossRef]
- Wiberg, K.; Lundström, K.; Glas, B.; Rappe, C. PCDDs and PCDFs in consumers’ paper products. Chemosphere 1989, 19, 735. [CrossRef]
- Sany, S. B. T.; Narimani, L.; Soltanian, F. K.; Hashim, R.; Rezayi, M.; Karlend, D. J.; Mahmud, H. N. M. E. An overview of detection techniques for monitoring dioxin-like compounds: latest technique trends and their applications. RSC Adv. 2016, 6, 55415. [CrossRef]
- Schecter, A.; Birnbaum, L.; Ryan, J. J.; Constable, J. D. Dioxins: an overview. Environ. Res. 2006, 101, 419. [CrossRef]
- Behnisch, P. A.; Hosoe, K.; Sakai, S.-i. Bioanalytical screening methods for dioxins and dioxin-like compounds - a review of bioassay/biomarker technology. Environ. Int. 2001, 27, 413. [CrossRef]
- Torres, M. A.; Barros, M. P.; Campos, S. C. G.; Pinto, E.; Rajamani, S.; Sayre, R. T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol. Environ. Saf. 2008, 71, 1. [CrossRef]
- Eichbaum, K.; Brinkmann, M.; Buchinger, S.; Reifferscheid, G.; Hecker, M.; Giesy, J. P.; Engwall, M.; van Bavel, B.; Hollert, H. In vitro bioassays for detecting dioxin-like activity - Application potentials and limits of detection, a review. Sci. Total Environ. 2014, 487, 37. [CrossRef]
- Beníšek, M.; Kukučka, P.; Giulio Mariani, G.; Suurkuusk, G.; Gawlik, B. M.; Locoro, G.; Giesy, J. P.; Bláha, L. Dioxins and dioxin-like compounds in composts and digestates from European countries as determined by the in vitro bioassay and chemical analysis. Chemosphere 2015, 122, 168. [CrossRef]
- Du, B.; Liu, A.; Huang, Y. Uncertainty evaluation of the determination of toxic equivalent quantity of polychlorinated dibenzo-p-dioxins and dibenzofurans in soil by isotope dilution high resolution gas chromatography and high resolution mass spectrometry. Chin. J. Chromatogr. 2014, 32, 967. [CrossRef]
- L'Homme, B.; Scholl, G.; Eppe, G.; Focant, J. F. Validation of a gas chromatography-triple quadrupole mass spectrometry method for confirmatory analysis of dioxins and dioxin-like polychlorobiphenyls in feed following new EU Regulation 709/2014. J. Chromatogr. A 2015, 1376, 149. [CrossRef]
- Baughman, R.; Meselson, M. An analytical method for detecting TCDD (dioxin): levels of TCDD in samples from Vietnam. Environ. Health Perspect. 1973, 5, 27. [CrossRef]
- Schecter, A.; Tiernan, T. Occupational exposure to polychlorinated dioxins, polychlorinated furans, polychlorinated biphenyls, and biphenylenes after an electrical panel and transformer accident in an office building in Binghamton. Environ. Health Perspect. 1985, 60, 305. [CrossRef]
- Schecter, A.; Ryan, J. J.; Päpke, O.; Ball, M.; Lis, A. Elevated dioxin levels in the blood of male and female Russian workers with and without chloracne 25 years after phenoxyherbicide exposure: The UFA “Khimprom” incident. Chemosphere 1993, 27, 253. [CrossRef]
- Wei, Y.; Kong, L.-T.; Yang, R.; Wang, L.; Liu, J.-H.; Huang, X.-J. Electrochemical impedance determination of polychlorinated biphenyl using a pyrenecyclodextrin-decorated single-walled carbon nanotube hybrid. Chem. Commun. 2011, 47, 5340. [CrossRef]
- Wang, M.; Meng, G.; Huang, Q.; Li, M.; Lia, Z.; Tang, C. Fluorescence detection of trace PCB101 based on PITC immobilized on porous AAO membrane. Analyst 2011, 136, 278. [CrossRef]
- Zhu, C.; Meng, G.; Huang, Q. Vertically aligned Ag nanoplate-assembled film as a sensitive and reproducible SERS substrate for the detection of PCB-77. J. Hazard. Mater. 2012, 211, 389. [CrossRef]
- Silva, E.; Mascini, M.; Centi, S.; Turner, A. P. F. Detection of polychlorinated biphenyls (PCBs) in milk using a disposable immunomagnetic electrochemical sensor. Anal. Lett. 2007, 40, 1371. [CrossRef]
- Kurosawa, S.; Aizawa, H.; Park, J.-W. Quartz crystal microbalance immunosensor for highly sensitive 2,3,7,8-tetrachlorodibenzo-p-dioxin detection in fly ash from municipal solid waste incinerators. Analyst 2005, 130, 1495. [CrossRef]
- Chobtang, J.; De Boer, I. J. M.; Hoogenboom, R. L. A. P.; Haasnoot, W.; Kijlstra, A.; Meerburg, B. G. The need and potential of biosensors to detect dioxins and dioxin-like polychlorinated biphenyls along the milk, eggs and meat food chain. Sensors 2011, 11, 11692. [CrossRef]
- Centi, S.; Silva, E.; Laschi, S.; Palchetti, I.; Mascini, M. Polychlorinated biphenyls (PCBs) detection in milk samples by an electrochemical magnetoimmunosensor (EMI) coupled to solid-phase extraction (SPE) and disposable low-density arrays. Anal. Chim. Acta 2007, 594, 9. [CrossRef]
- Wang, S.; Sun, B.; Feng, J.; An, F.; Li, N.; Wang, H.; Tian, M. Development of affinity between target analytes and substrates in surface enhanced Raman spectroscopy for environmental pollutant detection. Analytical Methods 2020, 12, 5657. [CrossRef]
- Fang, X.; Song, Y.; Huang, Y.; Yang, G.; Han, C.; Li, H.; Qu, L. Two-dimensional MXene modified AgNRs as a surface-enhanced Raman scattering substrate for sensitive determination of polychlorinated biphenyls. Analyst 2020, 145, 7421. [CrossRef]
- Patrizi, B.; De Cumis, M.S.; Viciani, S.; D’Amato, F. Dioxin and related compound detection: Perspectives for optical monitoring. Int. J. Mol. Sci. 2019, 20, 2671. [CrossRef]
- Rindzevicius, T.; Barten, J.; Vorobiev, M.; Schmidt, M.S.; Castillo, J. J.; Boisen, A. Detection of surface-linked polychlorinated biphenyls using surface-enhanced Raman scattering spectroscopy. Vib. Spectrosc. 2017, 90, 1. [CrossRef]
- Potara, M.; Farcau, C.; Botiz, I.; Astilean, S. Detection of Environmental Pollutants by Surface-Enhanced Raman Spectroscopy, In RSC Detection Science, 2017, pp. 479.
- Hou, M.; Huang, Y.; Ma, L.; Zhang, Z. Sensitivity and Reusability of SiO2 NRs@ Au NPs SERS Substrate in Trace Monochlorobiphenyl Detection. Nanoscale Res. Lett. 2015, 10, 444. [CrossRef]
- Chen, S.N.; Li, X.; Han, S.; Liu, J. H.; Zhao, Y.Y. Synthesis of surface-imprinted Ag nanoplates for detecting organic pollutants in water environments based on surface enhanced Raman scattering. RSC Adv. 2015, 5, 99914. [CrossRef]
- Jing, L.; Shi, Y.-E.; Cui, J.; Zhang, X.; Zhan, J. Hydrophobic gold nanostructures via electrochemical deposition for sensitive SERS detection of persistent toxic substances. RSC Adv. 2015, 5, 13443. [CrossRef]
- Lu, Y.; Yao, G.; Sun, K.; Huang, Q. β-Cyclodextrin coated SiO2@Au@Ag core-shell nanoparticles for SERS detection of PCBs. Phys. Chem. Chem. Phys. 2014, 17, 21149. [CrossRef]
- Bao, Z.Y.; Liu, X.; Chen, Y.; Wu, Y.; Chan, H. L. W.; Dai, J.; Lei, D. Y. Quantitative SERS detection of low-concentration aromatic polychlorinated biphenyl-77 and 2,4,6-trinitrotoluene. J. Hazard. Mater. 2014, 280, 706. [CrossRef]
- Chen, B.; Meng, G.; Zhou, F.; Huang, Q.; Zhu, C.; Hu, X.; Kong, M. Ordered arrays of Au-nanobowls loaded with Ag-nanoparticles as effective SERS substrates for rapid detection of PCBs. Nanotechnology, 2014, 25, 145605. [CrossRef]
- Li, D.-W.; Zhai, W.-L.; Li, Y.-T.; Long, Y.-T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2014, 181, 23. [CrossRef]
- Xia Han, X.; Rodriguez, R. S.; Haynes, C. L.; Ozaki, Y.; Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers 2021, 1, 87. [CrossRef]
- Tripp, R.A.; Dluhy, R.A.; Zhao, Y. Novel nanostructures for SERS biosensing, Nano Today 2008, 3, 31.
- Hudson, S. D.; Chumanov, G. Bioanalytical applications of SERS (surface-enhanced Raman spectroscopy), Anal Bioanal. Chem. 2009, 394, 679.
- El-Ansary, A.; Faddah, L. M. Nanoparticles as biochemical sensors, Nanotechnol. Sci. Appl. 2010, 3, 65.
- Sharma, B.; Frontiera, R. R.; Henry, A.-I.; Ringe, E.; Van Duyne, R. P. SERS: Materials, applications, and the future, Materials Today 2012, 15, 16.
- Halvorson, R. A.; Vikesland, P. J. Surface-Enhanced Raman Spectroscopy (SERS) for Environmental Analyses, Environ. Sci. Technol. 2010, 44, 7749. [CrossRef]
- Ong, T. T. X.; Blanch, E. W.; Jones, O. A. H. Surface Enhanced Raman Spectroscopy in environmental analysis, monitoring and assessment, Sci. Total Environ. 2020, 720, 137601. [CrossRef]
- Terry, L. R.; Sanders, S.; Potoff, R. H.; Kruel, J. W.; Jain, M.; Guo, H. Applications of surface-enhanced Raman spectroscopy in environmental detection, Anal. Sci. Adv. 2022, 3, 113.
- Bodelón, G.; Pastoriza-Santos, I. Recent Progress in Surface-Enhanced Raman Scattering for the Detection of Chemical Contaminants in Water, Front. Chem. 2020, 8, 478.
- Guo, H.; He, L.; Xing, B. Applications of surface-enhanced Raman spectroscopy in the analysis of nanoparticles in the environment, Environ. Sci.: Nano 2017, 4, 2093.
- Liu, X.; Guo, J.; Li, Y.; Wang, B.; Yang, S.; Chen, W.; Wu, X.; Guo, J.; Ma, X. SERS substrate fabrication for biochemical sensing: towards point-of-care diagnostics, J. Mat. Chem. B 2021, 9, 8378. [CrossRef]
- Xie, X.; Pu, H.; Sun, D.-W. Recent advances in nanofabrication techniques for SERS substrates and their applications in food safety analysis, Crit. Rev. Food Sci. Nutr. 2018, 58, 2800. [CrossRef]
- Ouyang, L.; Ren, W.; Zhu, L.; Irudayaraj, J. Prosperity to challenges: recent approaches in SERS substrate fabrication, Rev. Anal. Chem. 2016, 36, 20160027. [CrossRef]
- Ge, K.; Hu, Y.; Li, G. Recent Progress on Solid Substrates for Surface-Enhanced Raman Spectroscopy Analysis, Biosensors 2022, 12, 941.
- Guselnikova, O.; Lim, H.; Kim, H.-J.; Kim, S. H.; Gorbunova, A.; Eguchi, M.; Postnikov, P.; Nakanishi, T.; Asahi, T.; Na, J.; Yamauch, Y. New Trends in Nanoarchitectured SERS Substrates: Nanospaces, 2D Materials, and Organic Heterostructures, Small 2022, 18, 2107182.
- Chen, M.; Liu, D.; Du, X.; Lo, K. H.; Wang, S.; Zhou, B.; Pan, H. 2D materials: Excellent substrates for surface-enhanced Raman scattering (SERS) in chemical sensing and biosensing, Trends Anal. Chem. 2020, 130, 115983.
- Jebakumari, K. A. E.; Murugasenapathi, N. K.; Palanisamy, T. Engineered Two-Dimensional Nanostructures as SERS Substrates for Biomolecule Sensing: A Review, Biosensors 2023, 13, 102.
- Liu, Y.; Qin, Z.; Deng, J.; Zhou, J.; Jia, X.; Wang, G.; Luo, F. The Advanced Applications of 2D Materials in SERS, Chemosensors 2022, 10, 455.
- Luo, W.; Xiong, W.; Han, Y.; Yan, X.; Mai, L. Application of two-dimensional layered materials in surface-enhanced Raman spectroscopy (SERS), Phys. Chem. Chem. Phys. 2022, 24, 26398. [CrossRef]
- Kannan, P. K.; Shankar, P.; Blackman, C.; Chung, C.-H. Recent Advances in 2D Inorganic Nanomaterials for SERS Sensing, Adv. Mater. 2019, 31, 1803432.
- Zhang, N.; Tong, L.; Zahng, J. Graphene-Based Enhanced Raman Scattering toward Analytical Applications, Chem. Mater. 2016, 28, 6426.
- Silver, A.; Kitadai, H.; Liu, H.; Granzier-Nakajima, T.; Terrones, M.; Ling, X.; Huang, S. Chemical and Bio Sensing Using Graphene-Enhanced Raman Spectroscopy, Nanomaterials 2019, 9, 516.
- Xu, W.; Mao, N.; Zhang, J. Graphene: a platform for surface-enhanced Raman spectroscopy, Small 2013, 9, 1206.
- Cai, Q.; Mateti, S.; Watanabe, K.; Taniguchi, T.; Huang, S.; Chen, Y.; Li, L. H. Boron Nitride Nanosheet-Veiled Gold Nanoparticles for Surface-Enhanced Raman Scattering, ACS Appl. Mater. Interfaces 2016, 8, 15630. [CrossRef]
- Kim, G; Kim, M.; Hyun, C.; Hong, S.; Ma, K. Y.; Shin, H. S.; Lim, H. Hexagonal Boron Nitride/Au Substrate for Manipulating Surface Plasmon and Enhancing Capability of Surface-Enhanced Raman Spectroscopy, ACS Nano 2016, 10, 11156.
- Kim, N.-Y.; Leem, Y.-C.; Hong, S.-H.; Park, J.-H.; Yim, S.-Y. Ultrasensitive and Stable Plasmonic Surface-Enhanced Raman Scattering Substrates Covered with Atomically Thin Monolayers: Effect of the Insulating Property, ACS Appl. Mater. Interfaces 2019, 11, 6363. [CrossRef]
- Chugh, D.; Jagadish, C.; Tan, H. Large-Area Hexagonal Boron Nitride for Surface Enhanced Raman Spectroscopy, Adv. Mater. Technol. 2019, 4, 1900220.
- Basu, N.; Satya Bharathi, M. S.; Sharma, M.; Yadav, K.; Parmar, A. S.; Soma, V. R.; Lahiri, J. Large Area Few-Layer Hexagonal Boron Nitride as a Raman Enhancement Material, Nanomaterials 2021, 11, 622.
- Nie, S. M.; Emory, S. R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering, Science 1997, 275, 1102.
- Ramos-Berdullas, N.; López-Carballeira, D.; Pérez-Juste, I.; Mandado, M. On the mechanism responsible of Raman enhancement on carbon allotropes surfaces: the role of molecule-surface vibrational coupling in SERS, J. Raman Spectrosc. 2015, 46, 1205. [CrossRef]
- López-Carballeira, D.; Ramos-Berdullas, N.; Pérez-Juste, I.; Mandado, M. Can single graphene nanodisks be used as Raman enhancement platforms?, RSC Adv. 2016, 6, 71397. [CrossRef]
- Ramos-Berdullas, N.; Otero, N.; Mandado, M. Graphene Molecules as Platforms for SERS Detection: A Future Perspective, In Handbook of Graphene: Biosensors and Advanced Sensors, Palys, B. (ed.), Vol 6, pp 431, John Wiley & Sons Inc, Hoboken, 2019.
- Ci, L.; Song, L.; Jin, C.; Jariwala, D.; Wu, D.; Li, Y.; Srivastava, A.; Wang, Z.F.; Storr, K.; Balicas, L.; Liu, F.; Ajayan, P. M. Atomic layers of hybridized boron nitride and graphene domains, Nat. Mater. 2010, 10, 430.
- Gong, Y.; Shi, G.; Zhang, Z.; Zhou, W.; Jung, J.; Gao, W.; Ma, L.; Yang, Y.; Yang, S.; You, G.; Vajtai, R.; Xu, Q.; MacDonald, A. H.; Yakobson, B. I.; Lou, J.; Liu, Z.; Ajayan, P. M. Direct chemical conversion of graphene to boron- and nitrogen- and carbon-containing atomic layers, Nat. Commun. 2014, 5, 3193.
- Lorenzo-García, M.M.; Bonifazi, D. Renaissance of an Old Topic: From Borazines to BN-doped Nanographenes, Chimia 2017, 71, 550.
- Herrera-Reinoza, N.; dos Santos, A. C.; de Lima, L. H.; Landers, R.; de Siervo, A. Atomically Precise Bottom-Up Synthesis of h-BNC: Graphene Doped with h-BN Nanoclusters, Chem. Mat. 2021, 33, 2871. [CrossRef]
- Mandado, M.; Ramos-Berdullas, N. Confinement on the Optical Response in h-BNCs: Towards Highly Efficient SERS-active 2D Substrates, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 266, 120451. [CrossRef]
- Karamanis, P.; Otero, N.; Pouchan, C. Unleashing the quadratic nonlinear optical responses of graphene by confining white-graphene (h-BN) sections in its framework, J. Am. Chem. Soc. 2014, 113, 7464. [CrossRef]
- Karamanis, P.; Otero, N.; Pouchan, C. Electric property variations in nanosized hexagonal boron nitride/graphene hybrids, J. Phys. Chem. C 2015, 119, 11872. [CrossRef]
- Otero, N.; El-Kelany, K.E.; Pouchan, C.; Rérat, M.; Karamanis, P. Establishing the pivotal role of local aromaticity in the electronic properties of boron-nitride graphene lateral hybrids, Phys. Chem. Chem. Phys. 2016, 18, 25315. [CrossRef]
- Bevilacqua, A. C.; Köhler, M. H.; Azevedo, S.; Baierle, R. J. Stability, and optical and electronic properties of ultrathin h-BNC, Phys. Chem. Chem. Phys. 2017, 19, 5629. [CrossRef]
- Alvarado, R.; Ramos-Berdullas, N.; Mandado, M. On the adsorption affinity of graphene and white graphene sheets by dioxin-like pollutants, Int. J. Quantum Chem. 2021, 121, e26591. [CrossRef]
- Mandado, M.; Hermida-Ramón, J. M. Electron Density Based Partitioning Scheme of Interaction Energies, J. Chem. Theo. Comput. 2011, 7, 633. [CrossRef]
- Ramos-Berdullas, N.; Pérez-Juste, I.; C. Van Alsenoy, C.; Mandado, M. Theoretical study of the adsorption of aromatic units on carbon allotropes including explicit (empirical) DFT dispersion corrections and implicitly dispersion-corrected functionals: the pyridine case, Phys. Chem. Chem. Phys. 2015, 17, 575. [PubMed]
- Cárdenas, G.; Pérez-Barcia, A.; Mandado, M.; Nogueira, J. J. Characterization of cisplatin/membrane interactions by QM/MM energy decomposition analysis, Phys. Chem. Chem. Phys. 2021, 23, 20533. [CrossRef]
- Pérez-Barcia, A.; Cárdenas, G.; Nogueira, J. J.; Mandado, M. Effect of the QM Size, Basis Set, and Polarization on QM/MM Interaction Energy Decomposition Analysis, J. Chem. Inf. Model. 2023, 63, 882. [CrossRef]
- Le Ru, E.; Etchegoin, P. Principles of Surface Enhanced Raman Spectroscopy and related plasmonic effects, pp. 14-20, Elsevier, Amsterdam, 2009.
- Ramos-Berdullas, N.; López-Carballeira, D.; Mandado, M.; Pérez-Juste, I. Revisiting the mechanism and the influence of the excitation wavelength on the surface-enhanced Raman scattering of the pyridine–Ag20 system, Theo. Chem. Acc. 2015, 134, 60. [CrossRef]
- Krishtal, A.; Senet, P.; Yang, M.; Van Alsenoy, C. A Hirshfeld partitioning of polarizabilities of water clusters, J. Chem. Phys. 2006, 125, 034312. [CrossRef]
- Otero, N.; Van Alsenoy, C.; Pouchan, C.; Karamanis, P. Hirshfeld-based intrinsic polarizability density representations as a tool to analyze molecular polarizability, J. Comput. Chem. 2015, 36, 1831. [CrossRef]
- Geldof, D.; Krishtal, A.; Blockhuys, F.; Van Alsenoy, C. An Extension of the Hirshfeld Method to Open Shell Systems Using Fractional Occupations, J. Chem. Theo. Comput. 2011, 7, 1328. [CrossRef]
- Gaussian 16, Revision C.01, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian, Inc., Wallingford CT, 2016.
- Mandado, M.; Van Alsenoy, C. EDA-NCI: A program to perform energy decomposition analysis of non-covalent interactions, available at: https://github.com/marcos-mandado/EDA-NCI.
- GaussView, Version 6, Dennington, Roy; Keith, Todd A.; Millam, John M. Semichem Inc., Shawnee Mission, KS, 2016.
- Allouche, R. A. Gabedit - A graphical user interface for computational chemistry softwares, J. Comput. Chem. 2011, 32, 174. [CrossRef]
- Chemcraft - graphical software for visualization of quantum chemistry computations, available at: https://www.chemcraftprog.com.
- Boys, S. F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors, Mol. Phys. 1970, 19, 553.
- López-Carballeira, D.; Ramos-Berdullas, N.; Pérez-Juste, I.; Cagide-Fajín, J. L.; Cordeiro, M. N. D. S., Mandado, M. A computational study of the interaction of graphene structures with biomolecular units, Phys. Chem. Chem. Phys. 2016, 18, 15312.
- Norman, P.; Bishop, D. M.; Jensen, H. J. A.; Oddershede, J. Near-resonant absorption in the time-dependent self-consistent field and multiconfigurational self-consistent field approximations, J. Chem. Phys. 2001, 115, 10323. [CrossRef]
| TCDD | TCDF | ||||
| C96 | BNC96 | C96 | BNC96 | ||
| -21.3 | -21.1 | -19.6 | -19.0 | ||
| 52.5 | 51.2 | 48.7 | 46.9 | ||
| -5.0 | -5.1 | -4.2 | -4.3 | ||
| -59.5 | -59.7 | -56.2 | -55.9 | ||
| -33.4 | -34.7 | -31.4 | -32.3 | ||
| TCDD | TCDF | ||||
| Isolated | BNC96 | Isolated | BNC96 | ||
| 302.20 | 182.42 | 310.05 | 172.82 | ||
| 174.38 | 87.55 | 171.89 | 75.30 | ||
| 82.91 | 129.73 | 80.23 | 139.99 | ||
| 186.50 | 133.23 | 187.39 | 129.37 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





