Submitted:
15 March 2023
Posted:
21 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Design
2.1. Genetic Material
2.2. Field Study Area
2.3. Planting and Agronomic Management

2.4. Postharvest Study and Sampling
2.5. Metabolites Extraction
2.5.1. Sample Processing
2.6. GC-MS Analysis
2.6.1. Data Processing
2.6. Data Statistical Analysis and Visualization
3. Results
3.1. Metabolic Profiling of White Yam Tuber During Dormancy
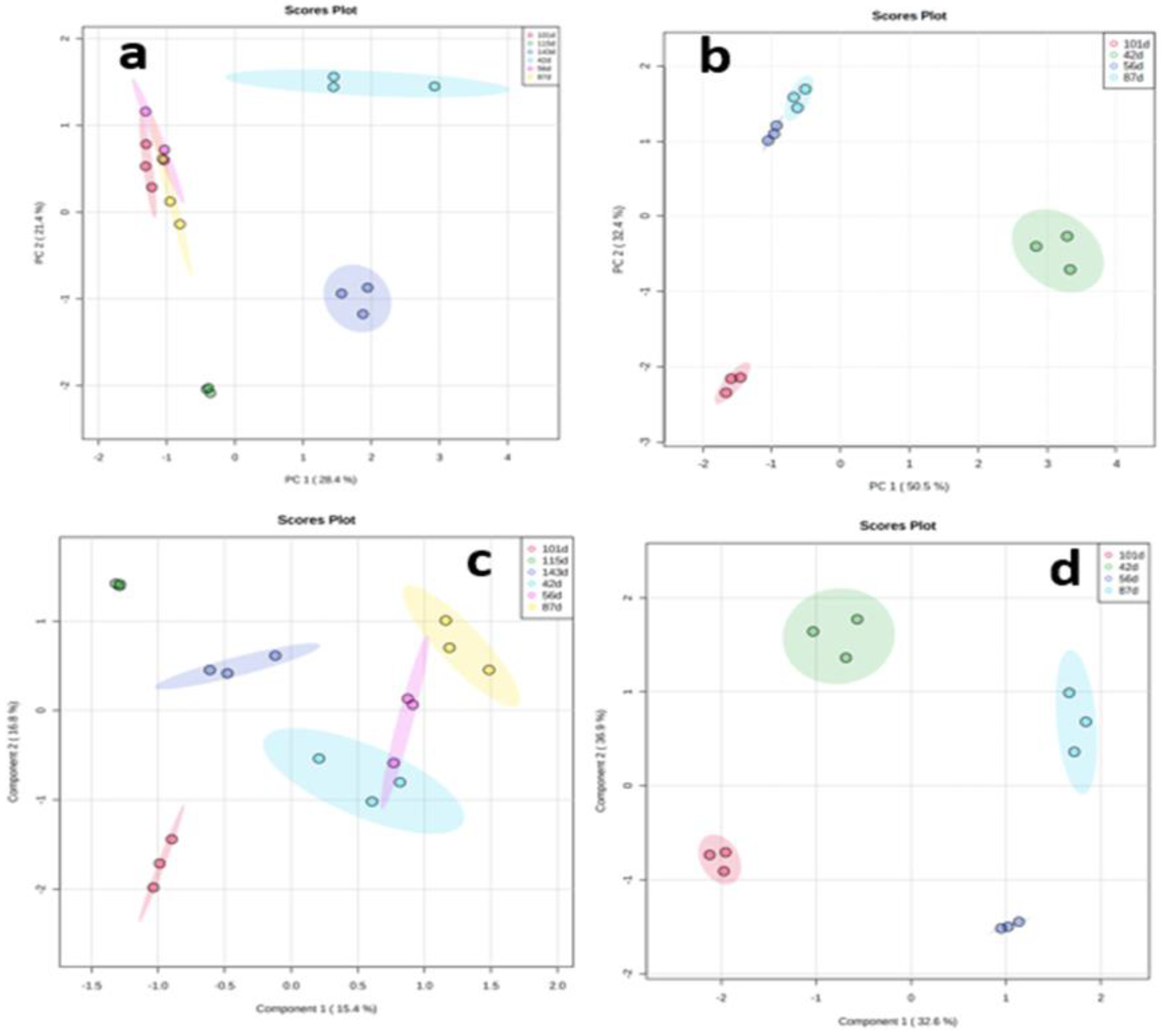
3.2. Principal Component Analysis (PCA) and Partial Least Square. Discriminant Analysis (PLS – DA) of Metabolites
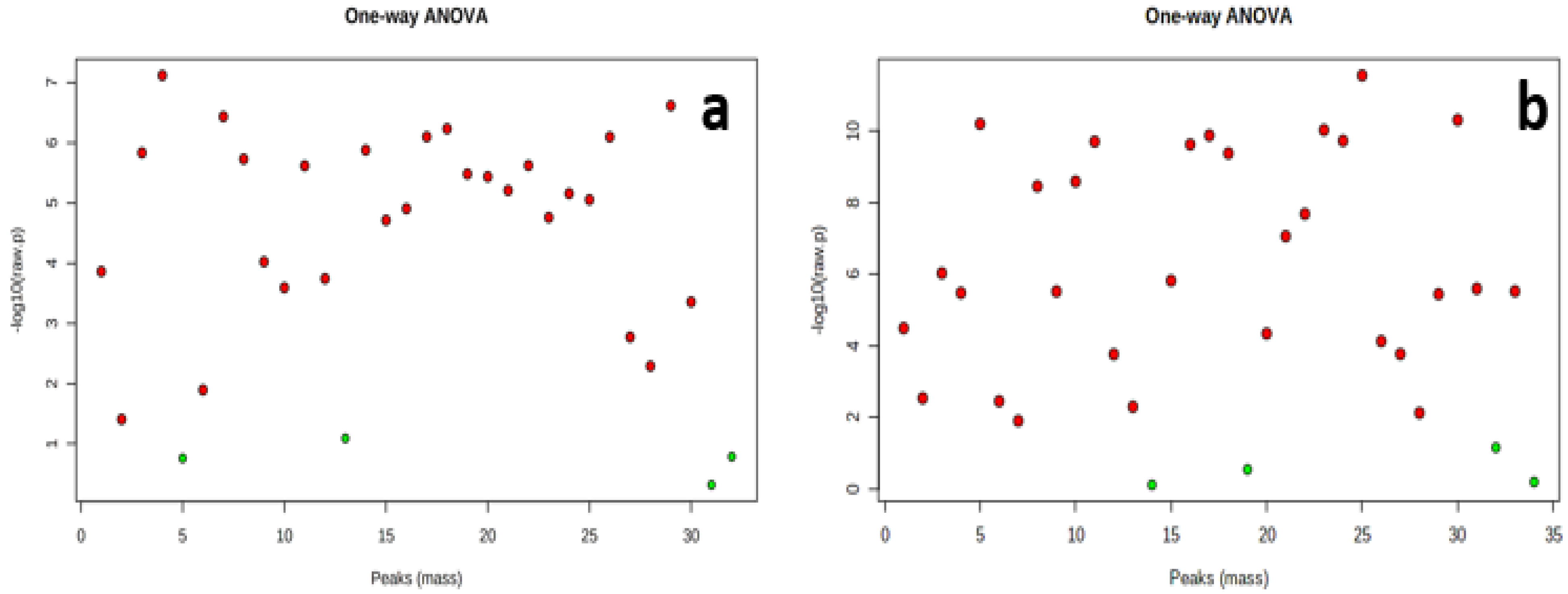
3.4. Identification of Differentially Accumulated Metabolites Across the Studied Tuber Dormancy Stages

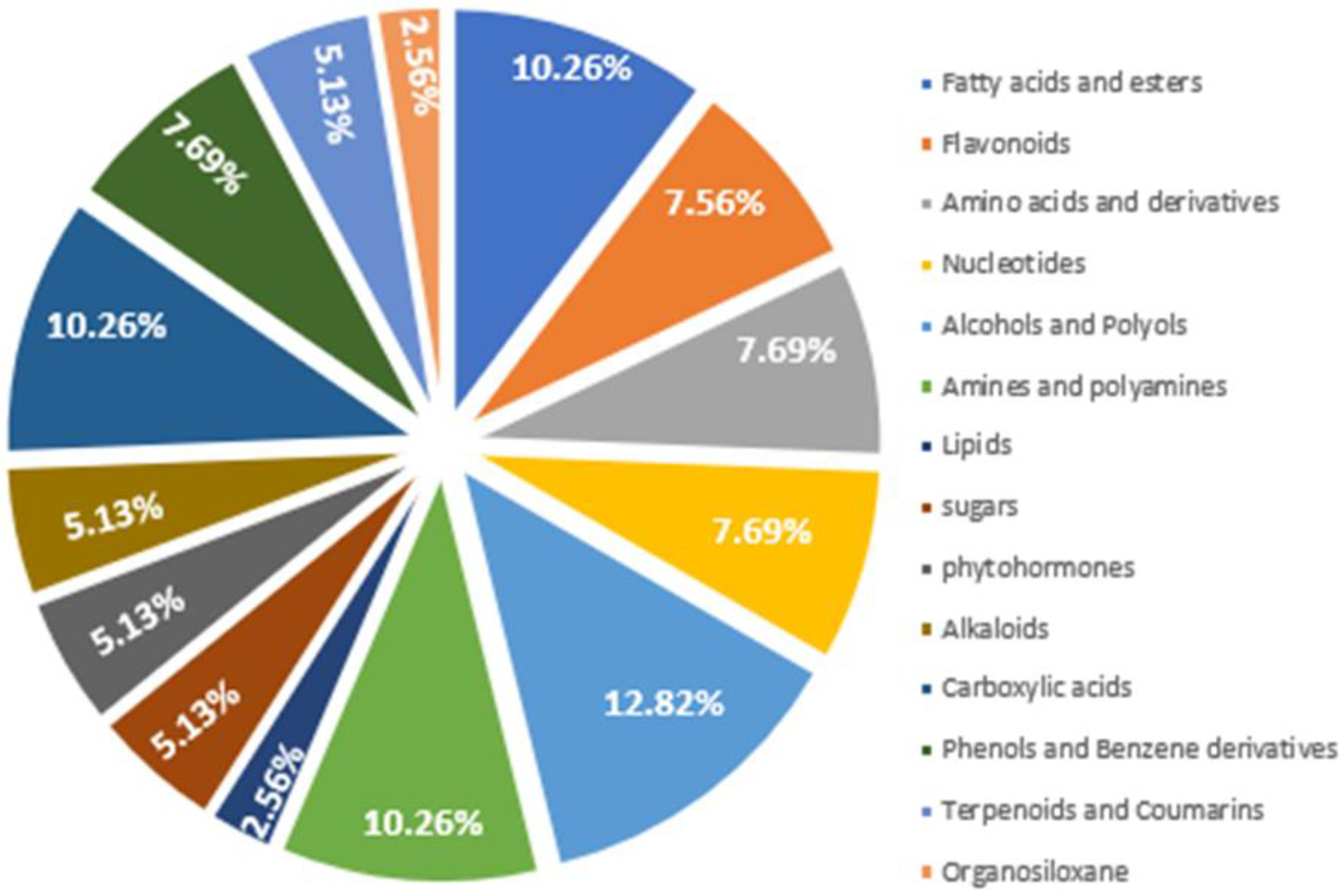
3.3. Metabolites Mapping and Chemical Functional Groups Identification
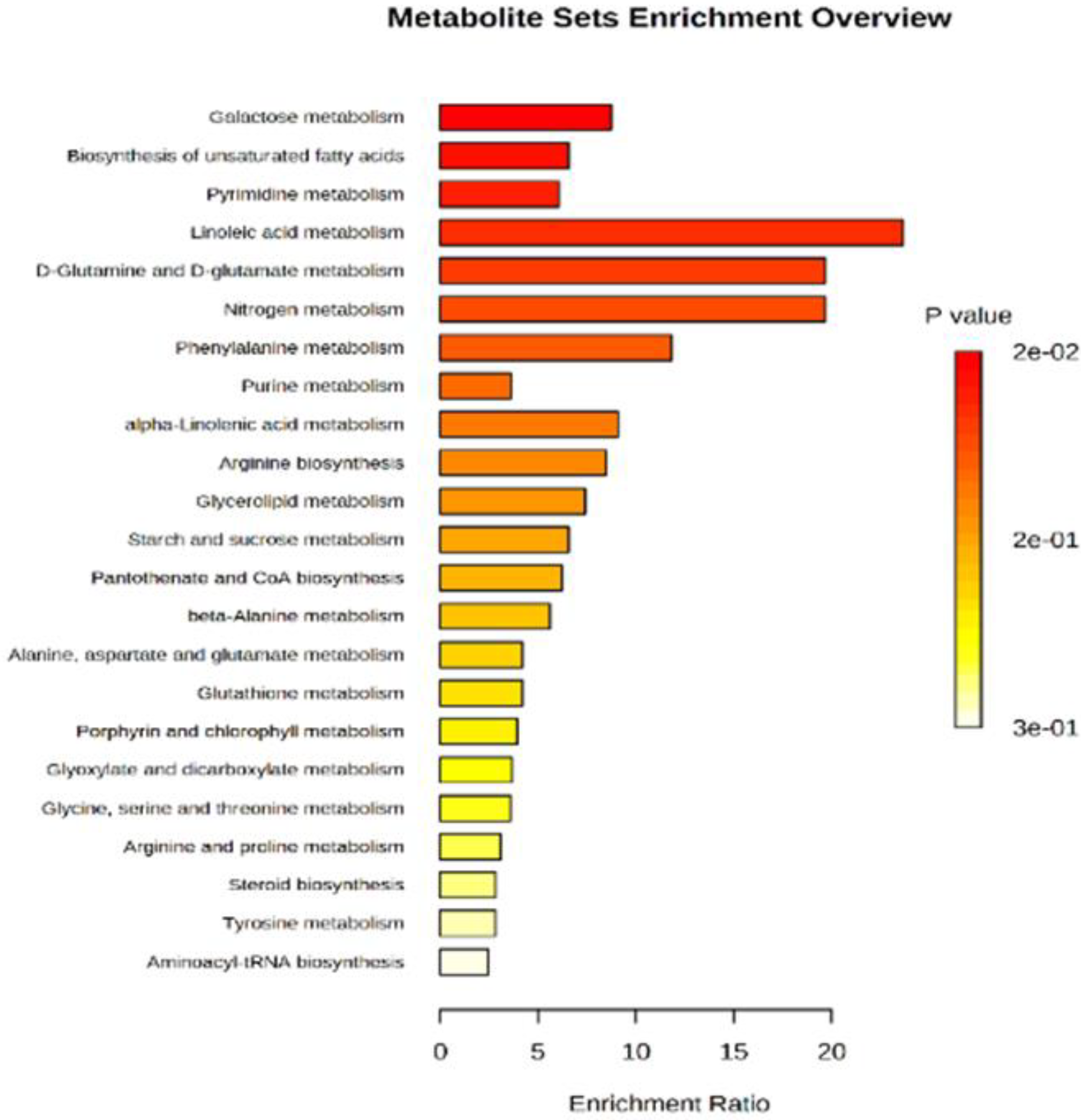
3.5. Metabolites Sets Enrichment Analysis and Pathways Annotation
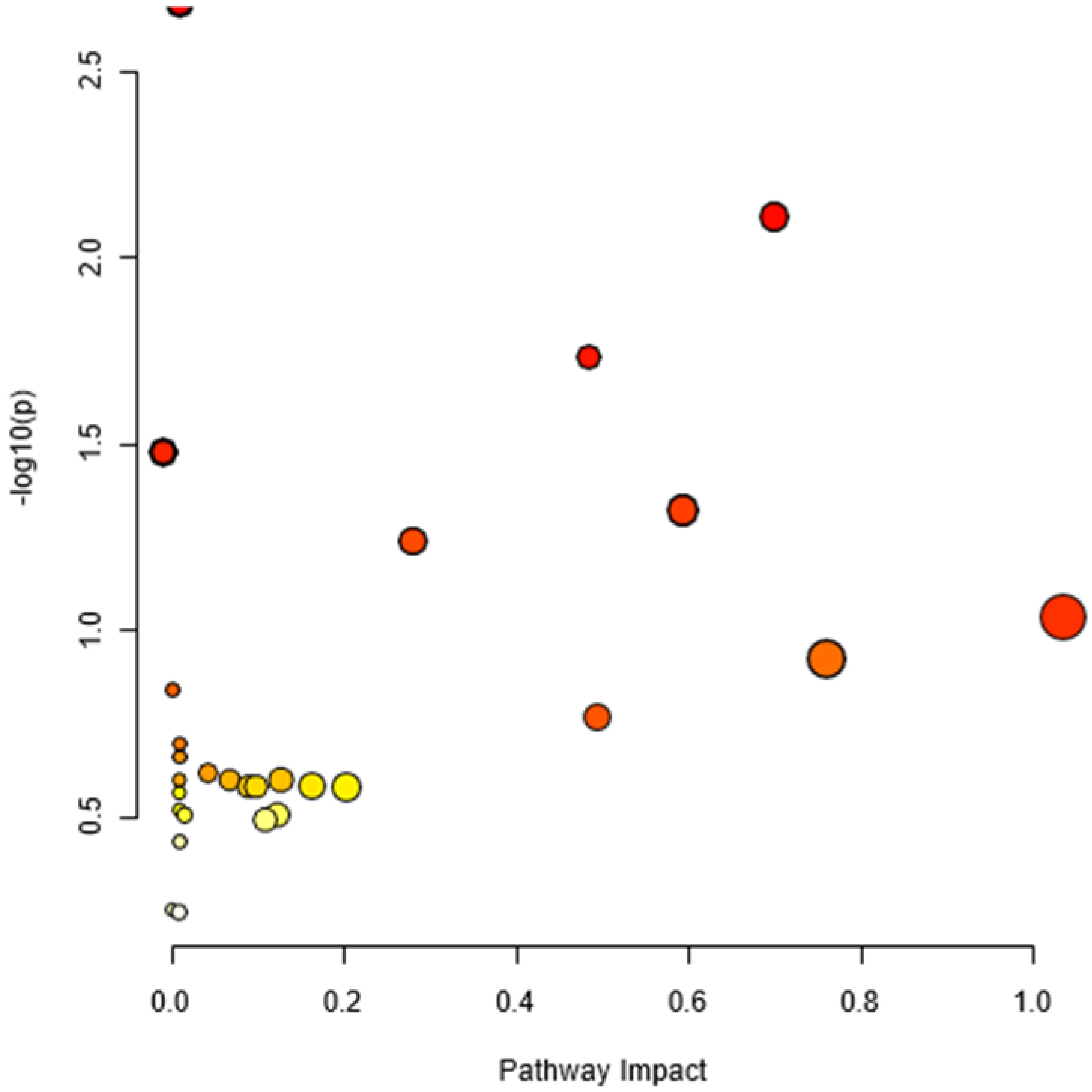
3.6. Pathway Topology Analysis
4. Discussions
4.1. Efficiency of Metabolomics in Deciphering Molecular Mechanisms in a Biological System
4.2. Differentially Accumulated Metabolites Determined the Phenotypic Variation in Dormancy Duration of the Two White Yam Genotypes
4.3. Metabolic Regulation of Yam Tuber Dormancy
4.4. Amines and Amino Acids Metabolisms
4.5. Energy Metabolism
4.6. Nucleotides Metabolism and Cellular Processes
4.7. Secondary Metabolites and Phytohormones Metabolism
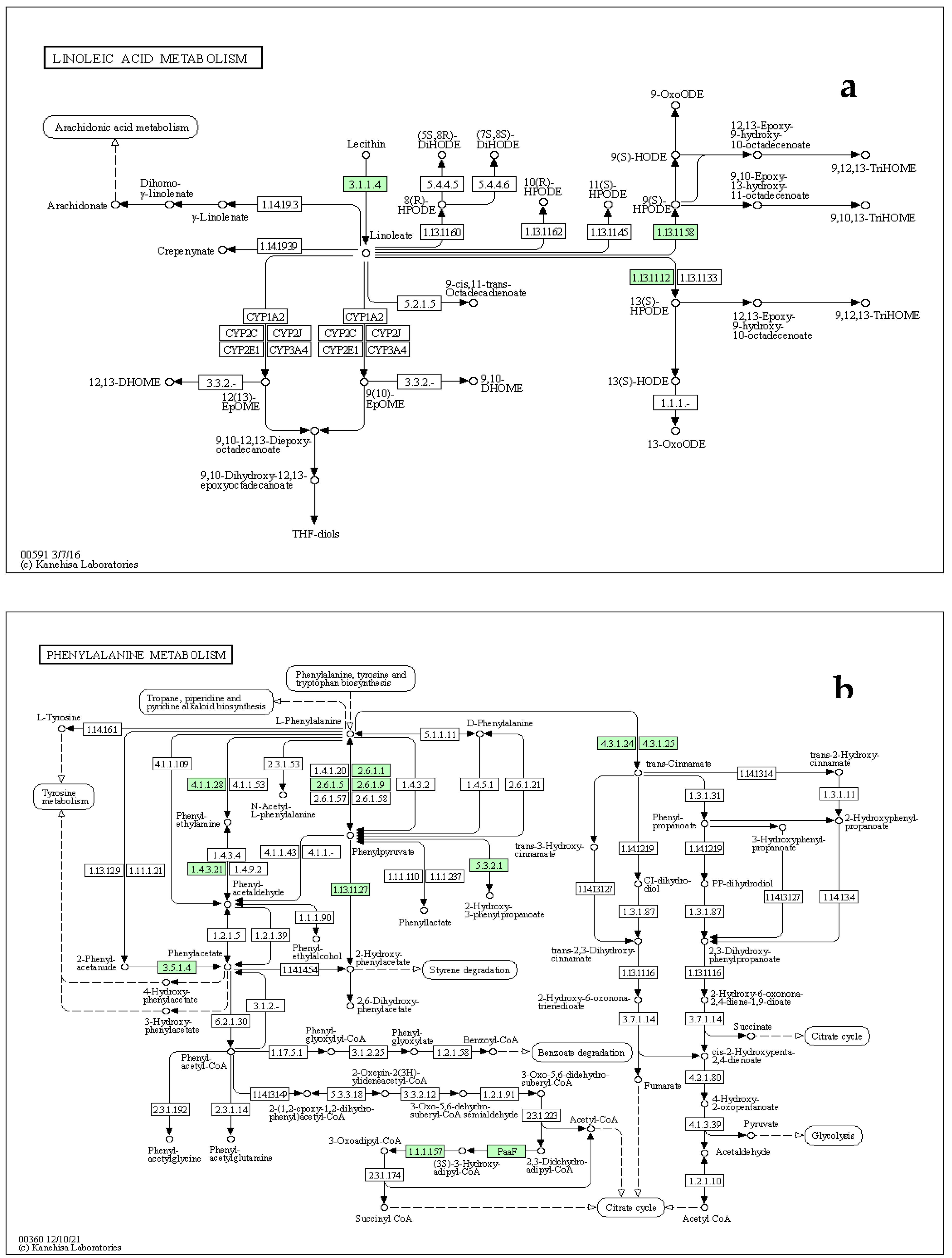
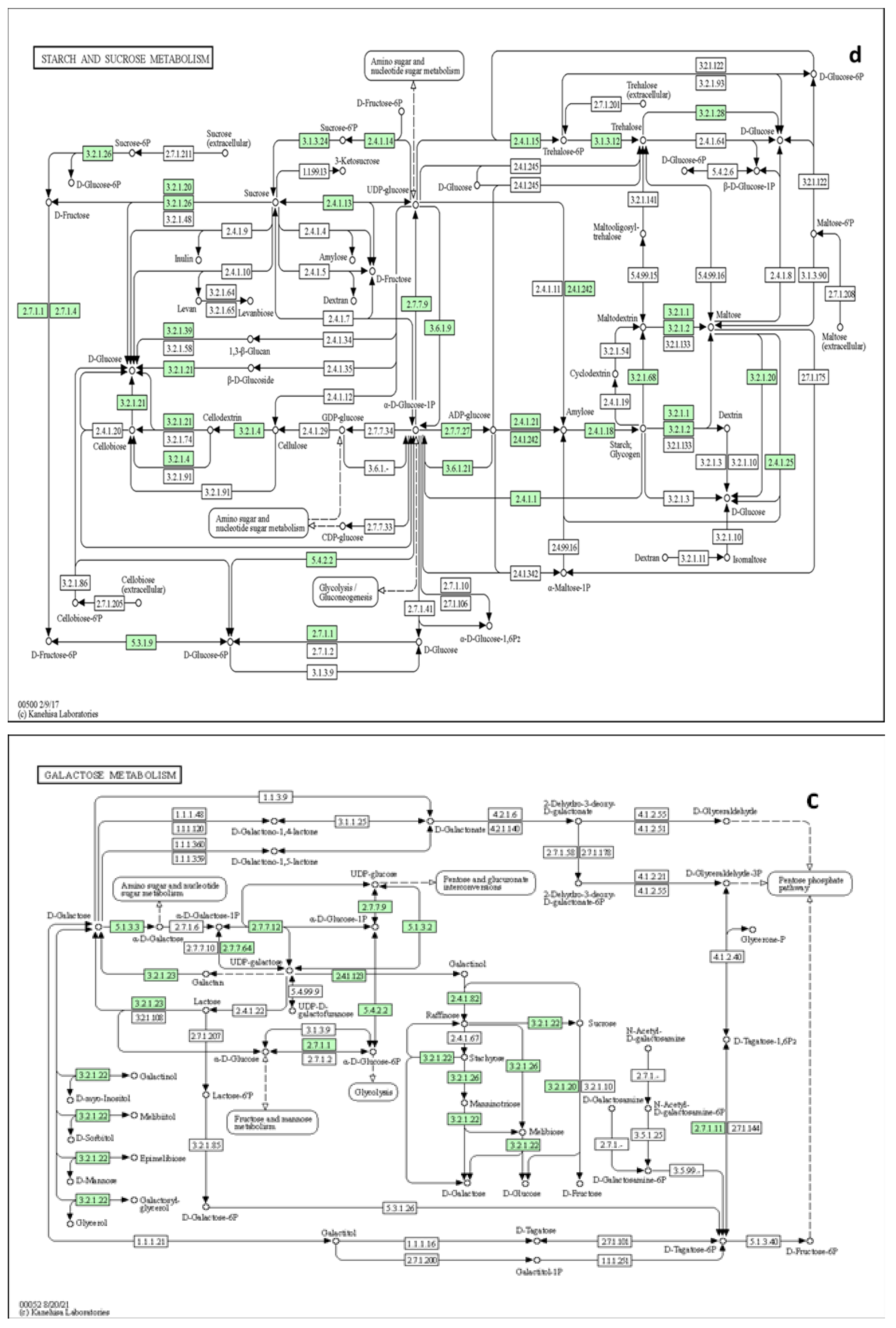
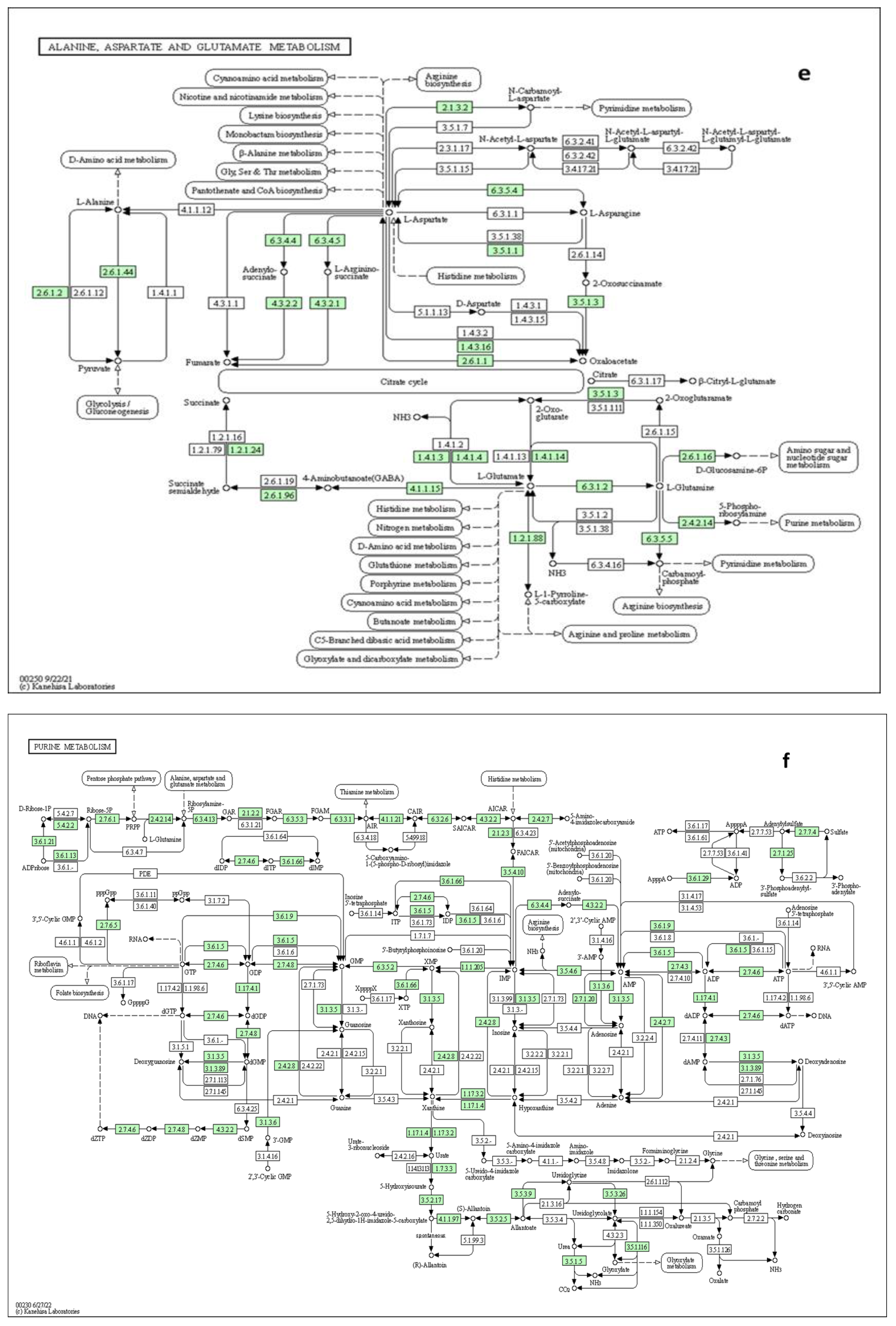
4.8. Pathways Elucidations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, E.J.; Wilkin, P.; Sarasan, V.; Fraser, P.D. Metabolite profiling of Dioscorea (yam) species reveals underutilised biodiversity and renewable sources for high-value compounds. Scientific reports 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea Genus (Yam)—An appraisal of nutritional and therapeutic potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef]
- Mignouna, H.D.; Abang, M.M.; Asiedu, R. Harnessing modern biotechnology for tropical tuber crop improvement: Yam (Dioscorea spp.) molecular breeding. African Journal of Biotechnology 2003, 2, 478–485. [Google Scholar]
- Lev, L.S.; Shriver, A.L. A trend analysis of yam production, area, yield, and trade (1961-1996). Colloques-CIRAD 1998, 11–20. [Google Scholar]
- Dansi, A.; Dantsey-Barry, H.; Dossou-Aminon, I.; N’kpenu, E.; Agré, A.; Sunu, Y.; Kombaté, K.; Loko, Y.; Dansi, M.; Assogba, P. Varietal diversity and genetic erosion of cultivated yams (Dioscorea cayenensis Poir-D. rotundata Lam complex and D. alata L.) in Togo. Int. J. Biodivers. Conserv 2013, 5, 223–239. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://wwwfaoorg/ faostat/en/#data/QC. 2020.
- Nwogha, J.S.; Wosene, A.G.; Raveendran, M.; Oselebe, H.O.; Obidiegwu, J.E.; Amirtham, D. Physiological and Molecular basis of dormancy in yam tuber: A way forward towards genetic manipultion of dormancy in yam tubers. Global Journal of science frontier research 2022b, XXll, 47–73. [Google Scholar]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohy- drates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Yolou; Elie Idossou1 ADECHOKAN Hibath Audrey, A.; Didier, M.Z.J. Evaluation of yam (Dioscorea cayenensis–Dioscorea rotundata) seed germination grown in Centre Benin. International Journal 2015, 3, 277–284.
- Ile, E.; Craufurd, P.; Battey, N.; Asiedu, R. Phases of dormancy in yam tubers (Dioscorea rotundata). Annals of Botany 2006, 97, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Passam, H. Dormancy of yams in relation to storage. Yams. Ignames. 1982, 285–293. [Google Scholar]
- Hamadina, E.I.; Craufurd, P.Q. Changes in Free Phenolics Contents during Tuber Development, Dormancy and Sprouting in White Yam (Dioscorea rotundata Poir.). International Journal of Plant Research 2015, 5, 34–41. [Google Scholar]
- Shangguan, L.; Chen, M.; Fang, X.; Xie, Z.; Gong, P.; Huang, Y.; Wang, Z.; Fang, J. Comparative transcriptome analysis provides insight into regulation pathways and temporal and spatial expression characteristics of grapevine (Vitis vinifera) dormant buds in different nodes. BMC Plant Biology 2020, 20, 1–20. [Google Scholar] [CrossRef]
- Kaczmarska, K.T.; Chandra-Hioe, M.V.; Zabaras, D.; Frank, D.; Arcot, J. Effect of germination and fermentation on carbohydrate composition of Australian sweet lupin and soybean seeds and flours. Journal of Agricultural and Food Chemistry 2017, 65, 10064–10073. [Google Scholar] [CrossRef] [PubMed]
- González-Orenga, S.; Ferrer-Gallego, P.P.; Laguna, E.; López-Gresa, M.P.; Donat-Torres, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Insights on salt tolerance of two endemic Limonium species from Spain. Metabolites 2019, 9, 294. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.-d.; Xie, Q.; He, Z.-h. Two faces of one seed: Hormonal regulation of dormancy and germination. Molecular plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lyv, Y.; Zheng, W.; Yang, C.; Li, Y.; Wang, X.; Chen, R.; Wang, C.; Luo, J.; Qu, L. Comparative metabolomics reveals two metabolic modules affecting seed germination in rice (Oryza sativa). Metabolites 2021, 11, 880. [Google Scholar] [CrossRef]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Zhou, W.; Dai, Y.; Qi, Y.; Du, J.; Yang, F.; Liu, J. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Scientific Reports 2017, 7, 12620. [Google Scholar] [CrossRef]
- Kralj Cigić, I.; Rupnik, S.; Rijavec, T.; Poklar Ulrih, N.; Cigić, B. Accumulation of agmatine, spermidine, and spermine in sprouts and microgreens of alfalfa, fenugreek, lentil, and daikon radish. Foods 2020, 9, 547. [Google Scholar] [CrossRef]
- Sepúlveda, G.; de Jiménez, E.S. Polyamine distribution among maize embryonic tissues and its relation to seed germination. Biochemical and biophysical research communications 1988, 153, 881–887. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.; Rehman, H.u.; Hussain, M. Seed priming with polyamines improves the germination and early seedling growth in fine rice. Journal of New Seeds 2008, 9, 145–155. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Zhang, X.-Q.; Ma, X.; Huang, L.-K.; Yan, Y.-H. Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, C.; He, F.; Li, Z.; Guan, Y.; Hu, Q.; Hu, J. Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biology 2017, 17, 1–16. [Google Scholar] [CrossRef]
- Sibian, M.S.; Saxena, D.C.; Riar, C.S. Effect of germination on chemical, functional and nutritional characteristics of wheat, brown rice and triticale: A comparative study. Journal of the Science of Food and Agriculture 2017, 97, 4643–4651. [Google Scholar] [CrossRef]
- Gipson, A.B.; Morton, K.J.; Rhee, R.J.; Simo, S.; Clayton, J.A.; Perrett, M.E.; Binkley, C.G.; Jensen, E.L.; Oakes, D.L.; Rouhier, M.F. Disruptions in valine degradation affect seed development and germination in Arabidopsis. The Plant Journal 2017, 90, 1029–1039. [Google Scholar] [CrossRef]
- Desmaison, A.M.; Tixier, M. Amino acids content in germinating seeds and seedlings from Castanea sativa L. Plant physiology 1986, 81, 692–695. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, Y.; Zhan, Y.; Cao, X.; Liu, C.; Zhang, Y.; Gai, S. Metabolomics analysis reveals Embden Meyerhof Parnas pathway activation and flavonoids accumulation during dormancy transition in tree peony. BMC Plant Biology 2020, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Jiao, H.J.; Faust, M. Changes in metabolic enzyme activities during thidiazuron-induced lateral budbreak of apple. HortScience 1991, 26, 171–173. [Google Scholar] [CrossRef]
- Halaly, T.; Pang, X.; Batikoff, T.; Crane, O.; Keren, A.; Venkateswari, J.; Ogrodovitch, A.; Sadka, A.; Lavee, S.; Or, E. Similar mechanisms might be triggered by alternative external stimuli that induce dormancy release in grape buds. Planta 2008, 228, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, H.; Wang, X.; Wang, B.; Zheng, X.; Shi, X.; Liu, W.; Liu, F. Respiratory changes during dormancy of grape buds. Sci Agric Sin 2013, 46, 1201–1207. [Google Scholar]
- Deng, S.; Xiao, Q.; Xu, C.; Hong, J.; Deng, Z.; Jiang, D.; Luo, S. Metabolome profiling of stratified seeds provides insight into the regulation of dormancy in Davidia involucrata. Plant Diversity 2022, 44, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Arc, E.; Chibani, K.; Grappin, P.; Jullien, M.; Godin, B.a.; Cueff, G.; Valot, B.; Balliau, T.; Job, D.; Rajjou, L. Cold stratification and exogenous nitrates entail similar functional proteome adjustments during Arabidopsis seed dormancy release. Journal of proteome research 2012, 11, 5418–5432. [Google Scholar] [CrossRef] [PubMed]
- Szczotka, Z.; Pawłowski, T.; Krawiarz, K. Proteins and polyamines during dormancy breaking of European beech (Fagus sylvatica L.) seeds. Acta Physiologiae Plantarum 2003, 25, 423–435. [Google Scholar] [CrossRef]
- Silva, A.C.d.; Suassuna, J.F.; Melo, A.S.d.; Costa, R.R.; Andrade, W.L.d.; Silva, D.C.d. Salicylic acid as attenuator of drought stress on germination and initial development of sesame. Revista Brasileira de Engenharia Agrícola e Ambiental 2017, 21, 156–162. [Google Scholar] [CrossRef]
- de Souza Vidigal, D.; Willems, L.; van Arkel, J.; Dekkers, B.J.; Hilhorst, H.W.; Bentsink, L. Galactinol as marker for seed longevity. Plant Science 2016, 246, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Baena-González, E.; Lunn, J.E. SnRK1 and trehalose 6-phosphate–two ancient pathways converge to regulate plant metabolism and growth. Current Opinion in Plant Biology 2020, 55, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, J.-Y.; Roh, J.; Marchive, C.; Kim, S.-K.; Meyer, C.; Sun, Y.; Wang, W.; Wang, Z.-Y. TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Current Biology 2016, 26, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, S.W.; Henry, C.; Griffiths, C.A.; Paul, M.J.; Feil, R.; Lunn, J.E.; Stitt, M.; Lagrimini, L.M. The role of Tre6P and SnRK1 in maize early kernel development and events leading to stress-induced kernel abortion. BMC Plant Biology 2017, 17, 1–17. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E. A tale of two sugars: Trehalose 6-phosphate and sucrose. Plant physiology 2016, 172, 7–27. [Google Scholar] [CrossRef]
- D. TIAMA1, N.S.; R. E. TRAORE1, M.Y.; P. BATIONO-KANDO; J. ZOUNDJIHEKPON2; SAWADOGO, M.; ZONGO1, J.D. Effect of chemical fertilizers on production of yams (nyù) of passore in farmers’ environment. Agronomie Africaine 2018, 30, 99–105. [Google Scholar]
- Enfissi, E.M.; Barneche, F.; Ahmed, I.; Lichtlé, C.; Gerrish, C.; McQuinn, R.P.; Giovannoni, J.J.; Lopez-Juez, E.; Bowler, C.; Bramley, P.M. Integrative transcript and metabolite analysis of nutritionally enhanced DE-ETIOLATED1 downregulated tomato fruit. The Plant Cell 2010, 22, 1190–1215. [Google Scholar] [CrossRef]
- Oliveros, J.C. VENNY. An interactive tool for comparing lists with Venn Diagrams. http://bioinfogp. cnb. csic. es/tools/venny/index. html 2007.
- S., N.J.; Abtew, W.G.; Raveendran, M.; Oselebe, H.O.; Obidiegwu, J.E.; Chilaka, C.A.; Amirtham, D.D. Role of Non-Structural Sugar Metabolism in Regulating Tuber Dormancy in White Yam (Dioscorea rotundata). Agriculture (Switzerland) 2023, 13. https://doi.org/.
- Han, C.; Zhen, S.; Zhu, G.; Bian, Y.; Yan, Y. Comparative metabolome analysis of wheat embryo and endosperm reveals the dynamic changes of metabolites during seed germination. Plant Physiology and Biochemistry 2017, 115, 320–327. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Dong, S.; Liu, Y.-L.; Li, Z.-H. The Opposite Roles of White Light in Regulating Germination of Fresh and Aged Seed in Tobacco. Plants 2021, 10, 2457. [Google Scholar] [CrossRef]
- Yang, M.; Yang, J.; Su, L.; Sun, K.; Li, D.; Liu, Y.; Wang, H.; Chen, Z.; Guo, T. Metabolic profile analysis and identification of key metabolites during rice seed germination under low-temperature stress. Plant Science 2019, 289, 110282. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, O.; Aizat, W.M.; Baharum, S.N.; Azizan, K.A.; Noor, N.M. Metabolomics analysis of developing Garcinia mangostana seed reveals modulated levels of sugars, organic acids and phenylpropanoid compounds. Scientia Horticulturae 2018, 233, 323–330. [Google Scholar] [CrossRef]
- Yan, S.; Huang, W.; Gao, J.; Fu, H.; Liu, J. Comparative metabolomic analysis of seed metabolites associated with seed storability in rice (Oryza sativa L.) during natural aging. Plant Physiology and Biochemistry 2018, 127, 590–598. [Google Scholar] [CrossRef]
- Feenstra, A.D.; Alexander, L.E.; Song, Z.; Korte, A.R.; Yandeau-Nelson, M.D.; Nikolau, B.J.; Lee, Y.J. Spatial mapping and profiling of metabolite distributions during germination. Plant physiology 2017, 174, 2532–2548. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Lin, R. The role of light in regulating seed dormancy and germination. Journal of Integrative Plant Biology 2020, 62, 1310–1326. [Google Scholar] [CrossRef]
- Horikoshi, H.M.; Sekozawa, Y.; Kobayashi, M.; Saito, K.; Kusano, M.; Sugaya, S. Metabolomics analysis of'Housui'Japanese pear flower buds during endodormancy reveals metabolic suppression by thermal fluctuation. Plant Physiology and Biochemistry 2018, 126, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Weidner, S. Biological activity of grapevine phenolic compounds. Grapevine molecular physiology & biotechnology 2009, 389–405. [Google Scholar]
- Wang, L.; Ruan, Y.-L. Regulation of cell division and expansion by sugar and auxin signaling. Frontiers in plant science 2013, 4, 163. [Google Scholar] [CrossRef]
- Hartig, K.; Beck, E. Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biology 2006, 8, 389–396. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. Journal of experimental botany 2007, 58, 2339–2358. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, S.; Ye, L.; Hu, H.; Li, C.; Zhang, G. The effects of GA and ABA treatments on metabolite profile of germinating barley. Food Chemistry 2016, 192, 928–933. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, L.; Zhao, H.; Ye, Q. Proteomic analysis of the early development of the Phalaenopsis amabilis flower bud under low temperature induction using the iTRAQ/MRM approach. Molecules 2020, 25, 1244. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Alcazar, R. Potential applications of polyamines in agriculture and plant biotechnology. Polyamines: Methods and Protocols 2018, 489–508. [Google Scholar]
- Ma, Z.; Bykova, N.V.; Igamberdiev, A.U. Cell signaling mechanisms and metabolic regulation of germination and dormancy in barley seeds. The Crop Journal 2017, 5, 459–477. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.-P. Amino acid catabolism in plants. Molecular plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections between amino acid metabolisms in plants: Lysine as an example. Frontiers in Plant Science 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. International Journal of Molecular Sciences 2022, 23, 5186. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. The Plant Cell 2005, 17, 3470–3488. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.P.; Ivakov, A.; Feil, R.; Duan, G.Y.; Walther, D.; Giavalisco, P.; Piques, M.; Carillo, P.; Hubberten, H.-M.; Stitt, M. The sucrose–trehalose 6-phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signalling by Tre6P. Journal of experimental botany 2014, 65, 1051–1068. [Google Scholar] [CrossRef]
- Muller, K.; Linkies, A.; Vreeburg, R.A.; Fry, S.C.; Krieger-Liszkay, A.; Leubner-Metzger, G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant physiology 2009, 150, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Calle, V.; Barrero-Sicilia, C.; Carbonero, P.; Iglesias-Fernandez, R. R. Mannans and endo-β-mannanases (MAN) in Brachypodium distachyon: Expression profiling and possible role of the BdMAN genes during coleorhiza-limited seed germination. J. Exp. Bot. 2015, 66, 3753–3764. [Google Scholar] [CrossRef]
- Li, W.-Y.; Chen, B.-X.; Chen, Z.-J.; Gao, Y.-T.; Chen, Z.; Liu, J. Reactive oxygen species generated by NADPH oxidases promote radicle protrusion and root elongation during rice seed germination. International Journal of Molecular Sciences 2017, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Falco, S.; Guida, T.; Locke, M.; Mauvais, J.; Sanders, C.; Ward, R.; Webber, P. Transgenic canola and soybean seeds with increased lysine. Bio/technology 1995, 13, 577–582. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Wang, X.; Liu, Q.; Yuan, D.; Pan, G.; Sun, S.S.; Tu, J. Development of high-lysine rice via endosperm-specific expression of a foreign LYSINE RICH PROTEIN gene. BMC Plant Biology 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Jia, M.; Wu, H.; Clay, K.L.; Jung, R.; Larkins, B.A.; Gibbon, B.C. Identification and characterization of lysine-rich proteins and starch biosynthesis genes in the opaque2mutant by transcriptional and proteomic analysis. BMC plant biology 2013, 13, 1–14. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Wang, X.; Chen, H.; Chen, F.; Shen, S. Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 2007, 7, 3358–3368. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Okamoto, M.; Koshiba, T.; Kamiya, Y.; Nambara, E. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: Epigenetic and genetic regulation of transcription in seed. The plant journal 2005, 41, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Gianinetti, A.; Finocchiaro, F.; Bagnaresi, P.; Zechini, A.; Faccioli, P.; Cattivelli, L.; Valè, G.; Biselli, C. Seed dormancy involves a transcriptional program that supports early plastid functionality during imbibition. Plants 2018, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Liu, X.; Sun, F.; Cao, J.; Huo, N.; Wuda, B.; Xin, M.; Hu, Z.; Du, J.; Xia, R. Wheat miR9678 affects seed germination by generating phased siRNAs and modulating abscisic acid/gibberellin signaling. The Plant Cell 2018, 30, 796–814. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Honda, C.; Moriguchi, T. Involvement of polyamine in floral and fruit development. Japan Agricultural Research Quarterly: JARQ 2006, 40, 51–58. [Google Scholar] [CrossRef]
- Nambeesan, S.; Avtar K., Handa; Mattoo, A.K. Polyamines and Regulation of Ripening and Senescence. In Postharvest Biology and Technology of Fruit, Vegetables and Flowers; Paliyath, G., Murr, D.P., Handa, A.K., Lurie, S., Eds.; Wiley-Blackwell, 2016. [Google Scholar]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, Y.; Ma, C.; Qin, J.; Nguyen, T.H.N.; Liu, D.; Gan, H.; Ding, S.; Luo, Z.-B. Phenylalanine as a nitrogen source induces root growth and nitrogen-use efficiency in Populus× canescens. Tree physiology 2018, 38, 66–82. [Google Scholar] [CrossRef]
- Sato, K.; Yamane, M.; Yamaji, N.; Kanamori, H.; Tagiri, A.; Schwerdt, J.G.; Fincher, G.B.; Matsumoto, T.; Takeda, K.; Komatsuda, T. Alanine aminotransferase controls seed dormancy in barley. Nature communications 2016, 7, 11625. [Google Scholar] [CrossRef]
- Das, A.; Kim, D.-W.; Khadka, P.; Rakwal, R.; Rohila, J.S. Unraveling key metabolomic alterations in wheat embryos derived from freshly harvested and water-imbibed seeds of two wheat cultivars with contrasting dormancy status. Frontiers in Plant Science 2017, 8, 1203. [Google Scholar] [CrossRef]
- Verslues , P.E., and Sharma , Sandeep. Proline Metabolism and Its Implications for Plant- Environment Interaction Authors : Published By : The American Society of Plant Biologists Proline Metabolism and Its Implications for Plant-Environment Interactio. In The Arabidopsis Book, 2010(8), https://doi.org/10.1199/tab.0140, Ed.; The American Society of Plant Biologists: 2010. [CrossRef]
- Lechowska, K.; Wojtyla, Ł.; Quinet, M.; Kubala, S.; Lutts, S.; Garnczarska, M. Endogenous Polyamines and Ethylene Biosynthesis in Relation to Germination of Osmoprimed Brassica napus Seeds under Salt Stress Katarzyna. International Journal of Molecular Sciences 2022, 23. [Google Scholar]
- Llebrés, M.-T.; Pascual, M.-B.; Debille, S.; Trontin, J.-F.; Harvengt, L.; Avila, C.; Cánovas, F.M. The role of arginine metabolic pathway during embryogenesis and germination in maritime pine (Pinus pinaster Ait.). Tree physiology 2018, 38, 471–484. [Google Scholar] [CrossRef]
- Dilworth, M.F.; Dure III, L. Developmental biochemistry of cotton seed embryogenesis and germination: X. Nitrogen flow from arginine to asparagine in germination. Plant Physiology 1978, 61, 698–702. [Google Scholar] [CrossRef]
- Shalaby, A. Changes in biogenic amines in mature and germinating legume seeds and their behavior during cooking. Food/Nahrung 2000, 44, 23–27. [Google Scholar] [CrossRef]
- MacGregor, R.D.; Sarah, L. Kendall; Hannah Florance; Fabio Fedi; Karen Moore; Konrad Paszkiewicz; Smirnoff, N.; Penfield, S. Seed production temperature regulation of primary dormancy occurs through control of seed coat phenylpropanoid metabolism. New Phytol. 2014, 205, 642–652. [Google Scholar] [CrossRef]
- E., W.J. Seed coat and water absorption properties of seed of near-isogenic snap bean lines differing in seed coat color. Journal of the American Society for Horticultural Science 1977, 102, 478–480. [CrossRef]
- Corbineau, F.; M.A. Picard; Côme, D. Germinability of leek seeds and its improvement by osmopriming. Acta Horticulture 1993. [CrossRef]
- MM., E. Dormancy in seeds of Charlock: III. Occurrence and mode ofaction ofan inhibitor associated with dormancy. Journal of Experimental Botany 1968, 19, 601–610. [CrossRef]
- Tobe, K.; Zhang, L.; Yu Qiu, G.; Shimizu, H.; Omasa, K. Characteristics of seed germination in five non-halophytic Chinese desert shrub species. Journal of Arid Environments 2001, 47, 191–201. [Google Scholar] [CrossRef]
- Wingler, A.; Henriques, R. Sugars and the speed of life—Metabolic signals that determine plant growth, development and death. Physiologia Plantarum 2022, 174, e13656. [Google Scholar] [CrossRef]
- Anderson, G.H.; Veit, B.; MR, H. The Arabidopsis AtRaptor genes are essential for post-embryonic plant growth. BMC Biol 2005, 3. [Google Scholar] [CrossRef]
- Tixier, A.; Gambetta, G.A.; Godfrey, J.; Orozco, J.; Zwieniecki, M.A. Non-structural carbohydrates in dormant woody perennials; the tale of winter survival and spring arrival. Frontiers in Forests and Global Change 2019, 2. [Google Scholar] [CrossRef]
- Tarancon, C.; González-Grando, E.; Oliveros, J.C.; Nicolas, M.; Cubas, P. A conserved carbon starvation response underlies bud dormancy in woody and herbaceous species. Frontiers in Plant Science 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Canam, T.; Kang, K.Y.; Unda, F.; Mansfield, S.D. Sucrose phosphate synthase expression influences poplar phenology. Tree Physiology 2009, 29, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, C.; Teulat-Merah, B.; MorÈRe-Le Paven, M.C.; Leprince, O.; Ly Vu, B.; Viau, L.; Ledroit, L.; Pelletier, S.; Payet, N.; Satour, P.; Lebras, C.; Gallardo, K.; Huguet, T.; Limami, A.M.; et al. Quantitative trait loci analysis reveals a correlation between the ratio of sucrose/raffinose family oligosaccharides and seed vigour in Medicago truncatula. Plant Cell Environ. 2011, 34, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fernie, A.R. On the role of the tricarboxylic cid cycle in plant productivity. Journal of Integrative Plant Biology 2018, 60, 1199–1216. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; LJ, S. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Fernie AR; E, M. Malate. Jack of all trades or master of a few? Phytochemistry 2009, 70, 828–883. [CrossRef]
- Kim, H.U. Lipid metabolism in plants. Plants 2020, 9, 1–4. [Google Scholar] [CrossRef]
- Mitio, H.; Sekozawa, Y.; Kobayashi, M.; Saito, K.; Kusano, M.; Sugaya, S. Metabolomics analysis of ' Housui ' Japanese pear fl ower buds during endodormancy reveals metabolic suppression by thermal fl uctuation. Plant Physiology and Biochemistry 2018, 126, 134–141. [Google Scholar]
- Qiu, X.M.; Sun, Y.Y.; Ye, X.Y.; Li, Z.G. Signaling Role of Glutamate in Plants. Frontiers in Plant Science 2020, 10, 1–11. [Google Scholar] [CrossRef]
- M, K.; Araújo, W.L.; AR, F.; Galili, G. In Posidonia oceanica cadmium induces changes in DNA methylation and chromatin patterning. In Posidonia oceanica cadmium induces changes in DNA methylation and chromatin patterning 2012, 63, 695–709. [Google Scholar]
- Nieuwland, J., Menges, M., and Murray, J. A. H. . ''The plant cyclins,” in Cell Cycle Control and Plant Development, . In Plant J, Inze, D., Ed.; Oxford: Blackwell Publishing: Oxford, 2007; Volume 41. Oxford: Blackwell Publishing: Oxford.
- Zsigmond, L.; Tomasskovics, B.; Deák, V.; Rigó, G.; Szabados, L.; Bánhegyi, G.; Szarka, A. Plant Physiology and Biochemistry Enhanced activity of galactono-1, 4-lactone dehydrogenase and ascorbate e glutathione cycle in mitochondria from complex III de fi cient Arabidopsis. Plant Physiology et Biochemistry 2011, 49, 809–815. [Google Scholar] [CrossRef]
- Blokhina, O.; Fagerstedt, K.V. Reactive oxygen species and nitric oxide in plant mitochondria: Origin and redundant regulatory systems. Physiologia Plantarum 2010, 138, 447–462. [Google Scholar] [CrossRef]
- Paradiso, A.; Pinto, M.C.D.; Locato, V.; Gara, L.D. Galactone- c -lactone-dependent ascorbate biosynthesis alters wheat kernel maturation. Plant Biology 2012, 1435–8603. [Google Scholar] [CrossRef] [PubMed]
- Xool-tamayo, J.; Tamayo-ordoñez, Y.; Monforte-gonz, M.; Muñoz-s, A.; Felipe, V. Alkaloid Biosynthesis in the Early Stages of the Germination of Argemone mexicana L. (Papaveraceae). Plants https://doi.org/. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Smith, D.L. Flavonoids in Agriculture : Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations. Agronomy 2020, 10. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds ? New Phytologist 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant Science 2020, 296, 110–471. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373. [Google Scholar] [CrossRef]
- Cotelle, N.; Bernier, J.L.; Catteau, J.P.; Pommery, J.; Wallet, J.C.; Gaydou, E.M. Antioxidant properties of hydroxy-flavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Prinsi, B. Seed Germination Induced by Coumarin Is Mediated by a Lower Ability to Sustain the Energetic Metabolism. Plants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Chattha, F.A.; Munawar, M.A.; Nisa, M.; Kousar, S. Plant growth regulating activity of coumarins. Plant growth regulating activity of coumarins. In Coumarin-Based Heteroaromatics as Plant Growth Coumarin-Based Heteroaromatics as Plant Growth Regulators Regulators; University of the Punjab: Lahore, Pakistan, 2016; pp. 91–104. [Google Scholar]
- Chen, Z.; Ma, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of exogenous Ca2+ on phenolic accumulation and physiological changes in germinated wheat (Triticum aestivum L.) under UV-B radiation. Food Chem. 2019, 288, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Pergo, E.M., D. ; Abrahim, P.C.; Soares, K.A.; Kern, L.J.D.; Silva, E.V.; Iwamoto., E.L.I. Bidens pilosa L. exhibits high sensitivity to coumarin in comparison with three other weed species. J. Chem. Ecol. 2008, 34, 499–507. [CrossRef] [PubMed]
- Berrie, A.M.M.; Parker, W.; Knights, B.A.; Hendrie, M.R. Studies on lettuce seed germination-I. Coumarin induced dormancy. Phytochemistry 1968, 7, 567–573. [Google Scholar] [CrossRef]
- Goren, R.; Tomer, E. Effect of seselin and coumarin on growth, indoleacetic acid oxidase and peroxidase with special reference to cucumber radicles. Plant Physiol. 1971, 47, 312–316. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Kumar, D.A.; Touhidur Rahman Anik; Md. Mezanur Rahman; Keya, S.S.; Islam, M.R.; Rahman, M.A.; Sultana, S.; Ghosh, P.K.; Khan, S.; Ahamed, T.; et al. Ethanol Treatment Enhances Physiological and Biochemical Responses to Mitigate Saline Toxicity in Soybean. Plants 2022, 11.
- Ventura, I.; Brunello, L.; Iacopino, S.; Valeri, M.C.; Novi, G.; Dornbusch, T.; Perata, P.; Loreti, E. Arabidopsis phenotyping reveals the importance of alcohol dehydrogenase and pyruvate decarboxylase for aerobic plant growth. Scientific Reports 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Su, W.; Ren, Y.; Wang, D.; Su, Y.; Feng, J.; Zhang, C.; Tang, H.; Xu, L.; Muhammad, K.; Que, Y. The alcohol dehydrogenase gene family in sugarcane and its involvement in cold stress regulation. BMC Genomics 2020, 21, 1–17. [Google Scholar] [CrossRef]
- Skalák, J.; Vercruyssen, L.; Claeys, H.; Hradilová, J.; Černý, M.; Novák, O.; Plačková, L.; Saiz-Fernández, I.; Skaláková, P.; Coppens, F.; et al. Multifaceted activity of cytokinin in leaf development shapes its size and structure in Arabidopsis. Plant Journal 2019, 97, 805–824. [Google Scholar] [CrossRef]
- Mao, J.; Li, W.; Mi, B.; Mujitaba, M.; Ma, Z.; Zhang, Y.; Chen, B.; Caldero, A. Different exogenous sugars affect the hormone signal pathway and sugar metabolism in ‘‘ Red Globe ’’ (Vitis vinifera L.) plantlets grown in vitro as shown by transcriptomic analysis. Planta 2017, 246, 537–552. [Google Scholar] [CrossRef]
- Magome, H.; Nomura, T.; Hanada, A.; Takeda-Kamiya, N.; Ohnishi, T.; Shinma, Y.; Katsumata, T.; Kawaide, H.; Kamiya, Y.; Yamaguchi, S. CYP714B1 and CYP714B2 encode gibberellin 13-oxidases that reduce gibberellin activity in rice. PNAS www.pnas.org/cgi/doi/10.1073/pnas.1215788110 2013, 110, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The synthesis and role of β-alanine in plants. Frontiers in plant science 2019, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Sodek, L.; Licausi, F.; Hameed, M.W.; Dornelas, M.C.; Van Dongen, J.T. nalysis of alanine aminotransferase in various organs of soybean (Glycine max) and in dependence of different nitrogen fertilisers during hypoxic stress. Amino Acids 2010, 39, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Liu, X.; Liu, F.; Ren, Y.; Wang, Y.; Zhu, J.; Teng, X.; Duan, E.; Wang, F.; Zhang, H. FLOURY ENDOSPERM12 encoding alanine aminotransferase 1 regulates carbon and nitrogen metabolism in rice. Journal of Plant Biology 2019, 62, 61–73. [Google Scholar] [CrossRef]
- Tzin, V. Malitsky S.; Galili G, A.A. Expression of a bacterial bi-functional chorismate mutase/prephenate dehydratase modulates primary and secondary metabolism associated with aromatic amino acids in Arabidopsis. Plant J. 2009, 60, 156–167. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Current opinion in biotechnology 2019, 56, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef]
- Dennis, R. The role of primary carbohydrate metabolism in wheat grain dormancy and germination. 2019.

| Query | Match | Chem-Fun Group | HMDB | PubChem | KEGG |
|---|---|---|---|---|---|
| 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | Alpha-Linolenic acid | Fatty acids | HMDB0001388 | 5280934 | C06427 |
| Ethylenediamine-N,N'-dipropionic acid | Edetic Acid | Flavonoids | HMDB0015109 | 6049 | C00284 |
| 1-Butanol, 2-methyl-, acetate | Methyl methacrylate | Esters | HMDB0032385 | 6658 | C19504 |
| Tetraacetyl-d-xylonic nitrile | Sarcosine | Amino Acids and Derivatives | HMDB0000271 | 1088 | C00213 |
| (+)-N-Acetylmuramic acid | UDP-N-acetylmuraminate | nucleotide-sugar | HMDB0011720 | 24755495 | C01050 |
| 1-Butanol, 3-methyl-, formate | 3-Methylbutyl formate | Fatty esters | HMDB0034163 | 8052 | C12293 |
| 1,3-Propanediol, 2-ethyl-2-(hydroxymethyl)- | 1,3-Butanediol | Alcohols and Polyols | HMDB0031320 | 7896 | C20335 |
| 1,8-Di(4-nitrophenylmethyl)-3,6-diazahomoadamantan-9-one | Tyramine | Amino Acids and Derivatives | HMDB0000306 | 5610 | C00483 |
| Benzeneethanamine, 2-fluoro-á,3,4-trihydroxy-N-isopropyl- | Phenylethylamine | Amines, Aromatic | HMDB0012275 | 1001 | C05332 |
| Desulphosinigrin | Delphinidin 3-O-sophoroside | Flavonoids | 47205615 | C16307 | |
| Glycerin | Glycerol | Alcohols and Polyols | HMDB0000131 | 753 | C00116 |
| 9,12-Octadecadienoic acid (Z,Z)- | Linoleic acid | Lipids | HMDB0000673 | 5280450 | C01595 |
| Dasycarpidan-1-methanol, acetate (ester) | Acetaminophen | Flavonoids | HMDB0001859 | 1983 | C06804 |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 3-Phenylpropyl 2-methylpropanoate | Alcohols | HMDB0034472 | 7662 | C02008 |
| Sucrose | Sucrose | Disaccharides | HMDB0000258 | 5988 | C00089 |
| 5-O-Methyl-d-gluconic acid dimethylamide | 3-Cresotinic acid | Phytohormone | HMDB0002390 | 6738 | C14088 |
| 2,6-Dioxa-tricyclo[3.3.2.0(3,7)]decan-9-one | Ibuprofen | Amines | HMDB0001925 | 3672 | C01588 |
| 1,3-Cyclohexanedione, 2,5,5-trimethyl- | 1,4-Cyclohexanedione | Alcohols | 10263 | C08063 | |
| 5-Methoxypyrrolidin-2-one | Galactosylglycerol | Alkaloids | HMDB0006790 | 656504 | C05401 |
| 2(3H)-Furanone, 5-heptyldihydro | Galactonolactone | Sugar Acids | HMDB0002541 | 5640 | C03383 |
| 2-Propanol, 1,1'-oxybis- | Propranolol | Amino Alcohols | HMDB0001849 | 4946 | C07407 |
| 10-Hydroxydecanoic acid | 12-Hydroxydodecanoic acid | Fatty Acids | HMDB0002059 | 79034 | C08317 |
| Succinamide | Putrescine | Biogenic Polyamines | HMDB0001414 | 1045 | C00134 |
| 2-Octenoic acid, 4,5,7-trhydroxy | Methyl acrylate | Carboxylic Acids | HMDB0033977 | 7294 | C19443 |
| 2-Hydroxy-3-methoxy-succinic acid, dimethyl ester | L-Malic acid | carboxylic acid | HMDB0000156 | 222656 | C00149 |
| Octanoic acid, 2-phenylethyl ester | Styrene | Benzene Derivatives | HMDB0034240 | 7501 | C19506 |
| 2-Cyclohexen-1-one, 4-(3-hydroxy-1-butenyl)-3,5,5-trimethyl- | Amoxycillin | Terpenoids | HMDB0030500 | 2171 | C06827 |
| 8-Hydroxy-7-methoxycoumarin | Oxybenzone | Coumarins | HMDB0015497 | 4632 | C14285 |
| Pyrimidine-4,6-diol, 5-methyl- | Uracil | Nucleic Acids and Derivatives | HMDB0000300 | 1174 | C00106 |
| Oxime-, methoxy-phenyl-_ | 3,4-Dihydroxyphenylacetaldehyde | phenols | HMDB0003791 | 119219 | C04043 |
| Adenine | Adenine | Nucleotides | HMDB0000034 | 190 | C00147 |
| 2,5-Cyclohexadiene-1,4-dione, dioxime | Quinone | Benzoquinones | HMDB0003364 | 4650 | C00472 |
| 2,5-Pyrrolidinedione, 3-(1-aminoethylidene)-4-methyl- | Isopyridoxal | Alkaloids | HMDB0004290 | 440899 | C06051 |
| Phthalic acid, hept-4-yl isobutyl ester | Thalidomide | Carboxylic Acids | HMDB0015175 | 92142 | C07910 |
| L-Glutamine | L-Glutamine | Amino Acids and Derivatives | HMDB0000641 | 5961 | C00064 |
| Methyl cis-13,16-Docosadienate | Methylitaconate | carboxylic acid | METPA0268 | C02295 | |
| 2-Hydroxyhippuric acid-3TMS | Salicyluric acid | Phytohormone | HMDB0000840 | 10253 | C07588 |
| 2,4-Di-tert-butylphenoxytrimethylsilane | Butenafine | Amines | HMDB0015223 | 2484 | C08067 |
| Pentasiloxane, dodecamethyl- | Dodecamethylpentasiloxane | organosiloxane | HMDB0062731 | 8853 |
| Total | Expected | Hits | Raw p | -log10(p) | Holm adjust | FDR | Impact | |
|---|---|---|---|---|---|---|---|---|
| Isoquinoline alkaloid biosynthesis | 6 | 0.08 | 2 | 2.26E-03 | 2.65E+00 | 2.15E-01 | 2.03E-01 | 0.00 |
| Galactose metabolism | 27 | 0.35 | 3 | 4.28E-03 | 2.37E+00 | 4.02E-01 | 2.03E-01 | 0.76 |
| Tyrosine metabolism | 18 | 0.23 | 2 | 2.11E-02 | 1.68E+00 | 1.00E+00 | 6.67E-01 | 0.04 |
| Biosynthesis of unsaturated fatty acids | 22 | 0.28 | 2 | 3.09E-02 | 1.51E+00 | 1.00E+00 | 7.33E-01 | 0.00 |
| Linoleic acid metabolism | 4 | 0.05 | 1 | 5.04E-02 | 1.30E+00 | 1.00E+00 | 8.15E-01 | 1.00 |
| Glyoxylate and dicarboxylate | 29 | 0.37 | 2 | 5.14E-02 | 1.29E+00 | 1.00E+00 | 8.15E-01 | 0.06 |
| metabolism | ||||||||
| Pyrimidine metabolism | 38 | 0.49 | 2 | 8.32E-02 | 1.08E+00 | 1.00E+00 | 1.00E+00 | 0.06 |
| Lysine biosynthesis | 9 | 0.12 | 1 | 1.10E-01 | 9.59E-01 | 1.00E+00 | 1.00E+00 | 0.47 |
| Nitrogen metabolism | 12 | 0.15 | 1 | 1.44E-01 | 8.41E-01 | 1.00E+00 | 1.00E+00 | 0.54 |
| Phenylalanine metabolism | 12 | 0.15 | 1 | 1.44E-01 | 8.41E-01 | 1.00E+00 | 1.00E+00 | 0.79 |
| Purine metabolism | 63 | 0.81 | 2 | 1.92E-01 | 7.17E-01 | 1.00E+00 | 1.00E+00 | 0.26 |
| Arginine biosynthesis | 18 | 0.23 | 1 | 2.09E-01 | 6.81E-01 | 1.00E+00 | 1.00E+00 | 0.00 |
| beta-Alanine metabolism | 18 | 0.23 | 1 | 2.09E-01 | 6.81E-01 | 1.00E+00 | 1.00E+00 | 0.00 |
| Citrate cycle (TCA cycle) | 20 | 0.26 | 1 | 2.29E-01 | 6.40E-01 | 1.00E+00 | 1.00E+00 | 0.33 |
| Zeatin biosynthesis | 21 | 0.27 | 1 | 2.39E-01 | 6.22E-01 | 1.00E+00 | 1.00E+00 | 0.00 |
| Carbon fixation in photosynthetic organ- | 21 | 0.27 | 1 | 2.39E-01 | 6.22E-01 | 1.00E+00 | 1.00E+00 | 0.06 |
| isms | ||||||||
| Glycerolipid metabolism | 21 | 0.27 | 1 | 2.39E-01 | 6.22E-01 | 1.00E+00 | 1.00E+00 | 0.26 |
| Phenylalanine, tyrosine and tryptophan | 22 | 0.28 | 1 | 2.49E-01 | 6.04E-01 | 1.00E+00 | 1.00E+00 | 0.08 |
| biosynthesis | ||||||||
| Starch and sucrose metabolism | 22 | 0.28 | 1 | 2.49E-01 | 6.04E-01 | 1.00E+00 | 1.00E+00 | 0.59 |
| Pyruvate metabolism | 22 | 0.28 | 1 | 2.49E-01 | 6.04E-01 | 1.00E+00 | 1.00E+00 | 0.24 |
| Alanine, aspartate and glutamate | 22 | 0.28 | 1 | 2.49E-01 | 6.04E-01 | 1.00E+00 | 1.00E+00 | 0.57 |
| metabolism | ||||||||
| Pantothenate and CoA biosynthesis | 23 | 0.30 | 1 | 2.59E-01 | 5.87E-01 | 1.00E+00 | 1.00E+00 | 0.00 |
| Cyanoamino acid metabolism | 26 | 0.33 | 1 | 2.87E-01 | 5.42E-01 | 1.00E+00 | 1.00E+00 | 0.00 |
| Glutathione metabolism | 27 | 0.35 | 1 | 2.97E-01 | 5.28E-01 | 1.00E+00 | 1.00E+00 | 0.01 |
| alpha-Linolenic acid metabolism | 27 | 0.35 | 1 | 2.97E-01 | 5.28E-01 | 1.00E+00 | 1.00E+00 | 0.11 |
| Arginine and proline metabolism | 28 | 0.36 | 1 | 3.06E-01 | 5.14E-01 | 1.00E+00 | 1.00E+00 | 0.16 |
| Glycine, serine and threonine metabolism | 33 | 0.42 | 1 | 3.50E-01 | 4.56E-01 | 1.00E+00 | 1.00E+00 | 0.00 |
| Aminoacyl-tRNA biosynthesis | 46 | 0.59 | 1 | 4.53E-01 | 3.44E-01 | 1.00E+00 | 1.00E+00 | 0.00 |
| Porphyrin and chlorophyll metabolism | 47 | 0.60 | 1 | 4.61E-01 | 3.37E-01 | 1.00E+00 | 1.00E+00 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





