Submitted:
16 March 2023
Posted:
16 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Peptides
2.3. ELISA
2.4. Measurements of KD via Surface Plasmon Resonance (SPR)
3. Results
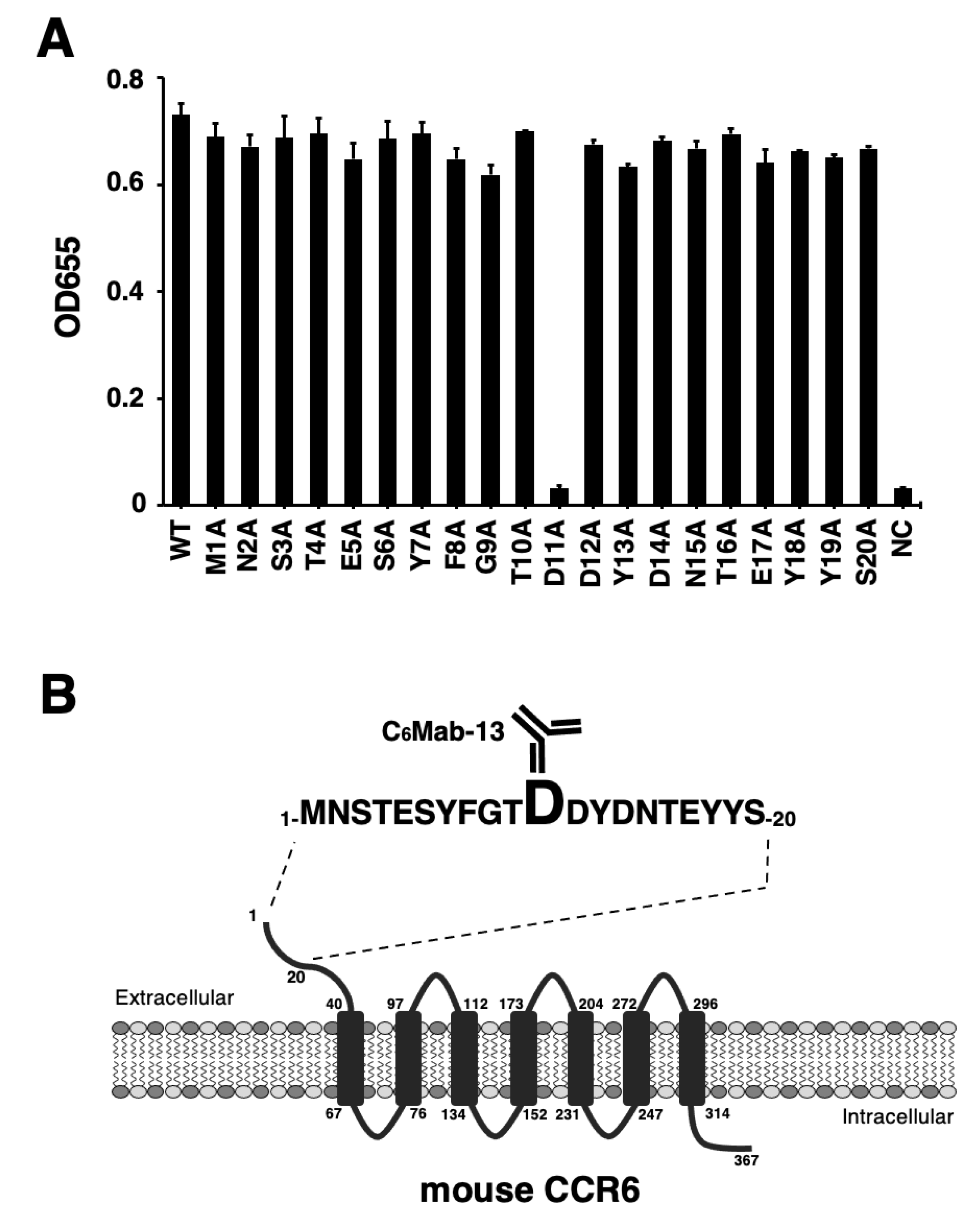
3.1. Epitope Identification of C6Mab-13 by ELISA using 1×Alanine-Substituted mCCR6 Peptides
| Peptides | Sequences | C6Mab-13 reactivity |
| p1_20 (WT) | MNSTESYFGTDDYDNTEYYS | +++ |
| M1A | ANSTESYFGTDDYDNTEYYS | +++ |
| N2A | MASTESYFGTDDYDNTEYYS | +++ |
| S3A | MNATESYFGTDDYDNTEYYS | +++ |
| T4A | MNSAESYFGTDDYDNTEYYS | +++ |
| E5A | MNSTASYFGTDDYDNTEYYS | +++ |
| S6A | MNSTEAYFGTDDYDNTEYYS | +++ |
| Y7A | MNSTESAFGTDDYDNTEYYS | +++ |
| F8A | MNSTESYAGTDDYDNTEYYS | +++ |
| G9A | MNSTESYFATDDYDNTEYYS | +++ |
| T10A | MNSTESYFGADDYDNTEYYS | +++ |
| D11A | MNSTESYFGTADYDNTEYYS | - |
| D12A | MNSTESYFGTDAYDNTEYYS | +++ |
| Y13A | MNSTESYFGTDDADNTEYYS | +++ |
| D14A | MNSTESYFGTDDYANTEYYS | +++ |
| N15A | MNSTESYFGTDDYDATEYYS | +++ |
| T16A | MNSTESYFGTDDYDNAEYYS | +++ |
| E17A | MNSTESYFGTDDYDNTAYYS | +++ |
| Y18A | MNSTESYFGTDDYDNTEAYS | +++ |
| Y19A | MNSTESYFGTDDYDNTEYAS | +++ |
| S20A | MNSTESYFGTDDYDNTEYYA | +++ |
3.2. Epitope Identification of C6Mab-13 by SPR using 1×Alanine-Substituted mCCR6 Peptides
| Peptides | ka (/ms) | kd (/s) | KD (M) |
| p1_20 (WT) | 6.84 × 103 | 3.77 × 10-3 | 5.52 × 10-7 |
| M1A | 6.94 × 103 | 4.15 × 10-3 | 5.99 × 10-7 |
| N2A | 7.86 × 103 | 4.23 × 10-3 | 5.38 × 10-7 |
| S3A | 7.62 × 103 | 4.53 × 10-3 | 5.94 × 10-7 |
| T4A | 7.92 × 103 | 4.55 × 10-3 | 5.75 × 10-7 |
| E5A | 8.20 × 103 | 4.64 × 10-3 | 5.65 × 10-7 |
| S6A | 9.05 × 103 | 5.25 × 10-3 | 5.81 × 10-7 |
| Y7A | 8.16 × 103 | 3.45 × 10-3 | 4.23 × 10-7 |
| F8A | 1.43 × 103 | 1.23 × 10-2 | 8.55 × 10-6 |
| G9A | ND | ND | ND |
| T10A | 1.31 × 104 | 3.15 × 10-2 | 2.40 × 10-6 |
| D11A | ND | ND | ND |
| D12A | 7.43 × 103 | 7.09 × 10-3 | 9.55 × 10-7 |
| Y13A | 1.43 × 103 | 1.30 × 10-2 | 9.12 × 10-6 |
| D14A | 6.87 × 103 | 1.05 × 10-2 | 1.53 × 10-6 |
| N15A | 6.19 × 103 | 5.61 × 10-3 | 9.06 × 10-7 |
| T16A | 6.23 × 103 | 5.17 × 10-3 | 8.30 × 10-7 |
| E17A | 6.38 × 103 | 6.67 × 10-3 | 1.05 × 10-6 |
| Y18A | 5.23 × 103 | 5.56 × 10-3 | 1.06 × 10-6 |
| Y19A | 5.75 × 103 | 6.02 × 10-3 | 1.05 × 10-6 |
| S20A | 4.68 × 103 | 5.96 × 10-3 | 1.27 × 10-6 |
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev 2003, 14, 409–426. [Google Scholar] [CrossRef]
- Baba, M.; Imai, T.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Hieshima, K.; Nomiyama, H.; Yoshie, O. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem 1997, 272, 14893–14898. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. The CCL20-CCR6 Axis in Cancer Progression. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Meitei, H.T.; Jadhav, N.; Lal, G. CCR6-CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun Rev 2021, 20, 102846. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, S.; Xing, M.; Hong, S.; Liu, L.; Ding, X.J.; Sun, X.Y.; Luo, Y.; Wang, C.X.; Zhang, M.; et al. Current evidence on the role of lipid lowering drugs in the treatment of psoriasis. Front Med (Lausanne) 2022, 9, 900916. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Adams, D.H. Chemokines and Chemokine Receptors as Therapeutic Targets in Inflammatory Bowel Disease; Pitfalls and Promise. J Crohns Colitis 2018, 12, S641–s652. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Takata, H.; Takiguchi, M. Functional expression of chemokine receptor CCR6 on human effector memory CD8+ T cells. Eur J Immunol 2007, 37, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Filì, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007, 204, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.S.; Körner, H. The CCR6-CCL20 axis in humoral immunity and T-B cell immunobiology. Immunobiology 2019, 224, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Varona, R.; Villares, R.; Carramolino, L.; Goya, I.; Zaballos, A.; Gutiérrez, J.; Torres, M.; Martínez, A.C.; Márquez, G. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest 2001, 107, R37–45. [Google Scholar] [CrossRef]
- Matti, C.; D'Uonnolo, G.; Artinger, M.; Melgrati, S.; Salnikov, A.; Thelen, S.; Purvanov, V.; Strobel, T.D.; Spannagel, L.; Thelen, M.; et al. CCL20 is a novel ligand for the scavenging atypical chemokine receptor 4. J Leukoc Biol 2020, 107, 1137–1154. [Google Scholar] [CrossRef]
- Meyrath, M.; Reynders, N.; Uchański, T.; Chevigné, A.; Szpakowska, M. Systematic reassessment of chemokine-receptor pairings confirms CCL20 but not CXCL13 and extends the spectrum of ACKR4 agonists to CCL22. J Leukoc Biol 2021, 109, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, R.; Eri, R. Modulation of the CCR6-CCL20 Axis: A Potential Therapeutic Target in Inflammation and Cancer. Medicina (Kaunas) 2018, 54. [Google Scholar] [CrossRef] [PubMed]
- Skovdahl, H.K.; Granlund, A.; Østvik, A.E.; Bruland, T.; Bakke, I.; Torp, S.H.; Damås, J.K.; Sandvik, A.K. Expression of CCL20 and Its Corresponding Receptor CCR6 Is Enhanced in Active Inflammatory Bowel Disease, and TLR3 Mediates CCL20 Expression in Colonic Epithelial Cells. PLoS One 2015, 10, e0141710. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Ludwiczek, O.; Holzmann, S.; Moschen, A.R.; Weiss, G.; Enrich, B.; Graziadei, I.; Dunzendorfer, S.; Wiedermann, C.J.; Mürzl, E.; et al. Increased expression of CCL20 in human inflammatory bowel disease. J Clin Immunol 2004, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Ichimura, Y.; Kubota, N.; Konishi, R.; Nakamura, Y.; Mizuno, S.; Takahashi, S.; Fujimoto, M.; Nomura, T.; Okiyama, N. The Role of PD-L1 on Langerhans Cells in the Regulation of Psoriasis. J Invest Dermatol 2022, 142, 3167–3174e3169. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Hiratsuka, K.; Nakano, T.; Naito, R.; Makino, T.; Iwamoto, H.; Yaegashi, H.; Shigehara, K.; Kadono, Y.; et al. Tumor-Associated Macrophages Induce Migration of Renal Cell Carcinoma Cells via Activation of the CCL20-CCR6 Axis. Cancers (Basel) 2019, 12. [Google Scholar] [CrossRef]
- Han, G.; Wu, D.; Yang, Y.; Li, Z.; Zhang, J.; Li, C. CrkL meditates CCL20/CCR6-induced EMT in gastric cancer. Cytokine 2015, 76, 163–169. [Google Scholar] [CrossRef]
- Yu, Q.; Lou, X.M.; He, Y. Preferential recruitment of Th17 cells to cervical cancer via CCR6-CCL20 pathway. PLoS One 2015, 10, e0120855. [Google Scholar] [CrossRef]
- Zhang, X.P.; Hu, Z.J.; Meng, A.H.; Duan, G.C.; Zhao, Q.T.; Yang, J. Role of CCL20/CCR6 and the ERK signaling pathway in lung adenocarcinoma. Oncol Lett 2017, 14, 8183–8189. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, X.; Zhu, J.; Zhang, L.; Chen, Y.; Zhang, B.; Li, Y.; Wang, M.; Zhang, Z.; Wang, C. lncRNA-u50535 promotes the progression of lung cancer by activating CCL20/ERK signaling. Oncol Rep 2019, 42, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kao, K.C.; Chiu, Y.L.; Jung, C.J.; Liu, C.J.; Cheng, S.J.; Chang, Y.L.; Ko, J.Y.; Chia, J.S. Enrichment of Human CCR6(+) Regulatory T Cells with Superior Suppressive Activity in Oral Cancer. J Immunol 2017, 199, 467–476. [Google Scholar] [CrossRef]
- Tanaka, T.; Li, G.; Asano, T.; Saito, M.; Kaneko, M.K.; Suzuki, H.; Kato, Y. Development of a Novel Anti-Mouse CCR2 Monoclonal Antibody (C(2)Mab-6) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 80–86. [Google Scholar] [CrossRef]
- Asano, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Tanaka, T.; Sano, M.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 3 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 107–112. [Google Scholar] [CrossRef]
- Saito, M.; Harigae, Y.; Li, G.; Asano, T.; Tanaka, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. C(3)Mab-2: An Anti-Mouse CCR3 Monoclonal Antibody for Immunocytochemistry. Monoclon Antib Immunodiagn Immunother 2022, 41, 45–49. [Google Scholar] [CrossRef]
- Asano, T.; Suzuki, H.; Tanaka, T.; Saito, M.; Li, G.; Goto, N.; Nanamiya, R.; Kaneko, M.K.; Kato, Y. C(3)Mab-3: A Monoclonal Antibody for Mouse CC Chemokine Receptor 3 for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2022, 41, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Suzuki, H.; Goto, N.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Establishment of Novel Anti-Mouse CCR3 Monoclonal Antibodies (C(3)Mab-6 and C(3)Mab-7) by N-terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Takei, J.; Suzuki, H.; Asano, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-Mouse CCR4 Monoclonal Antibody (C(4)Mab-1) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 87–93. [Google Scholar] [CrossRef]
- Asano, T.; Tanaka, T.; Suzuki, H.; Li, G.; Nanamiya, R.; Tateyama, N.; Isoda, Y.; Okada, Y.; Kobayashi, H.; Yoshikawa, T.; et al. Development of a Novel Anti-Mouse CCR6 Monoclonal Antibody (C(6)Mab-13) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 343–349. [Google Scholar] [CrossRef]
- Tanaka, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Sano, M.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 8 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 65–70. [Google Scholar] [CrossRef]
- Kobayashi, H.; Asano, T.; Suzuki, H.; Tanaka, T.; Yoshikawa, T.; Kaneko, M.K.; Kato, Y. Establishment of a Sensitive Monoclonal Antibody Against Mouse CCR9 (C(9)Mab-24) for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2022. [CrossRef]
- Kitamura, K.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Cx(6)Mab-1: A Novel Anti-Mouse CXCR6 Monoclonal Antibody Established by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Li, G.; Saito, M.; Suzuki, H.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of an Anti-human CCR2 Monoclonal Antibody (C(2)Mab-9) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 188–193. [Google Scholar] [CrossRef]
- Nanamiya, R.; Takei, J.; Asano, T.; Tanaka, T.; Sano, M.; Nakamura, T.; Yanaka, M.; Hosono, H.; Kaneko, M.K.; Kato, Y. Development of Anti-Human CC Chemokine Receptor 9 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Suzuki, H.; Isoda, Y.; Asano, T.; Nakamura, T.; Yanaka, M.; Handa, S.; Takahashi, N.; Okuno, S.; Yoshikawa, T.; et al. Development of a Sensitive Anti-Human CCR9 Monoclonal Antibody (C(9)Mab-11) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 303–310. [Google Scholar] [CrossRef]
- Kobayashi, H.; Asano, T.; Tanaka, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Determination of the Binding Epitope of an Anti-Mouse CCR9 Monoclonal Antibody (C(9)Mab-24) Using the 1× Alanine and 2× Alanine-Substitution Method. Antibodies (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Tateyama, N.; Asano, T.; Suzuki, H.; Li, G.; Yoshikawa, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Epitope Mapping of Anti-Mouse CCR3 Monoclonal Antibodies Using Flow Cytometry. Antibodies (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Isoda, Y.; Tanaka, T.; Suzuki, H.; Asano, T.; Yoshikawa, T.; Kitamura, K.; Kudo, Y.; Ejima, R.; Ozawa, K.; Kaneko, M.K.; et al. Epitope Mapping Using the Cell-Based 2 × Alanine Substitution Method About the Anti-mouse CXCR6 Monoclonal Antibody, Cx(6)Mab-1. Monoclon Antib Immunodiagn Immunother 2023, 42, 22–26. [Google Scholar] [CrossRef]
- Tanaka, T.; Suzuki, H.; Asano, T.; Li, G.; Nanamiya, R.; Tateyama, N.; Isoda, Y.; Okada, Y.; Kobayashi, H.; Yoshikawa, T.; et al. Epitope Mapping of an Anti-Mouse CCR2 Monoclonal Antibody (C(2)Mab-6) Using Enzyme-Linked Immunosorbent Assay. Monoclon Antib Immunodiagn Immunother 2022, 41, 339–342. [Google Scholar] [CrossRef]
- Isoda, Y.; Tanaka, T.; Suzuki, H.; Asano, T.; Nakamura, T.; Yanaka, M.; Handa, S.; Komatsu, Y.; Okuno, S.; Takahashi, N.; et al. Epitope Mapping of an Anti-Mouse CXCR6 Monoclonal Antibody (Cx(6)Mab-1) Using the 2 × Alanine Scanning Method. Monoclon Antib Immunodiagn Immunother 2022, 41, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Li, G.; Asano, T.; Kaneko, M.K.; Suzuki, H.; Kato, Y. Epitope Mapping of the Anti-Human CCR2 Monoclonal Antibody C(2)Mab-9. Monoclon Antib Immunodiagn Immunother 2022, 41, 150–156. [Google Scholar] [CrossRef]
- Chamorro, S.; Vela, M.; Franco-Villanueva, A.; Carramolino, L.; Gutiérrez, J.; Gómez, L.; Lozano, M.; Salvador, B.; García-Gallo, M.; Martínez, A.C.; et al. Antitumor effects of a monoclonal antibody to human CCR9 in leukemia cell xenografts. MAbs 2014, 6, 1000–1012. [Google Scholar] [CrossRef]
- Chain, B.; Arnold, J.; Akthar, S.; Brandt, M.; Davis, D.; Noursadeghi, M.; Lapp, T.; Ji, C.; Sankuratri, S.; Zhang, Y.; et al. A Linear Epitope in the N-Terminal Domain of CCR5 and Its Interaction with Antibody. PLoS One 2015, 10, e0128381. [Google Scholar] [CrossRef]
- Gómez-Melero, S.; García-Maceira, F.I.; García-Maceira, T.; Luna-Guerrero, V.; Montero-Peñalvo, G.; Túnez-Fiñana, I.; Paz-Rojas, E. Amino terminal recognition by a CCR6 chemokine receptor antibody blocks CCL20 signaling and IL-17 expression via β-arrestin. BMC Biotechnol 2021, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Durán, G.; Romo-Mancillas, A. Behavior of Chemokine Receptor 6 (CXCR6) in Complex with CXCL16 Soluble form Chemokine by Molecular Dynamic Simulations: General Protein‒Ligand Interaction Model and 3D-QSAR Studies of Synthetic Antagonists. Life (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Wasilko, D.J.; Johnson, Z.L.; Ammirati, M.; Che, Y.; Griffor, M.C.; Han, S.; Wu, H. Structural basis for chemokine receptor CCR6 activation by the endogenous protein ligand CCL20. Nat Commun 2020, 11, 3031. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Orr, C.M.; Chan, H.T.C.; James, S.; Penfold, C.A.; Kim, J.; Inzhelevskaya, T.; Mockridge, C.I.; Cox, K.L.; Essex, J.W.; et al. Reducing affinity as a strategy to boost immunomodulatory antibody agonism. Nature 2023, 614, 539–547. [Google Scholar] [CrossRef]
- Asano, T.; Kaneko, M.K.; Kato, Y. RIEDL tag: A novel pentapeptide tagging system for transmembrane protein purification. Biochem Biophys Rep 2020, 23, 100780. [Google Scholar] [CrossRef]
- Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Epitope Mapping System: RIEDL Insertion for Epitope Mapping Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 162–167. [Google Scholar] [CrossRef]
- Rutihinda, C.; Haroun, R.; Saidi, N.E.; Ordoñez, J.P.; Naasri, S.; Lévesque, D.; Boisvert, F.M.; Fortier, P.H.; Belzile, M.; Fradet, L.; et al. Inhibition of the CCR6-CCL20 axis prevents regulatory T cell recruitment and sensitizes head and neck squamous cell carcinoma to radiation therapy. Cancer Immunol Immunother 2022. [CrossRef]
- Chen, K.J.; Lin, S.Z.; Zhou, L.; Xie, H.Y.; Zhou, W.H.; Taki-Eldin, A.; Zheng, S.S. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One 2011, 6, e24671. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Cao, L.; Zhu, Y.; Cao, J.; Li, X.; Zhou, J.; Liu, B.; Zhao, T. Enhance anti-lung tumor efficacy of chimeric antigen receptor-T cells by ectopic expression of C-C motif chemokine receptor 6. Sci Bull (Beijing) 2021, 66, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Chen, J. CAR-T cell engineering with CCR6 exhibits superior anti-solid tumor efficacy. Sci Bull (Beijing) 2021, 66, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Kang, M.; Jeon, M.; Chung, Y.; Kim, A.R.; Lee, Y.J.; Kim, E.S.; Nam, H.; Park, J.; Lee, J.Y.; et al. CEACAM1 marks highly suppressive intratumoral regulatory T cells for targeted depletion therapy. Clin Cancer Res 2023. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





