Submitted:
11 March 2023
Posted:
13 March 2023
You are already at the latest version
Abstract
Keywords:
I. INTRODUCTION
- Materials selection like metals, polymers, ceramics, carbon-based materials, composites, biomaterials, and a combination of the above-mentioned.
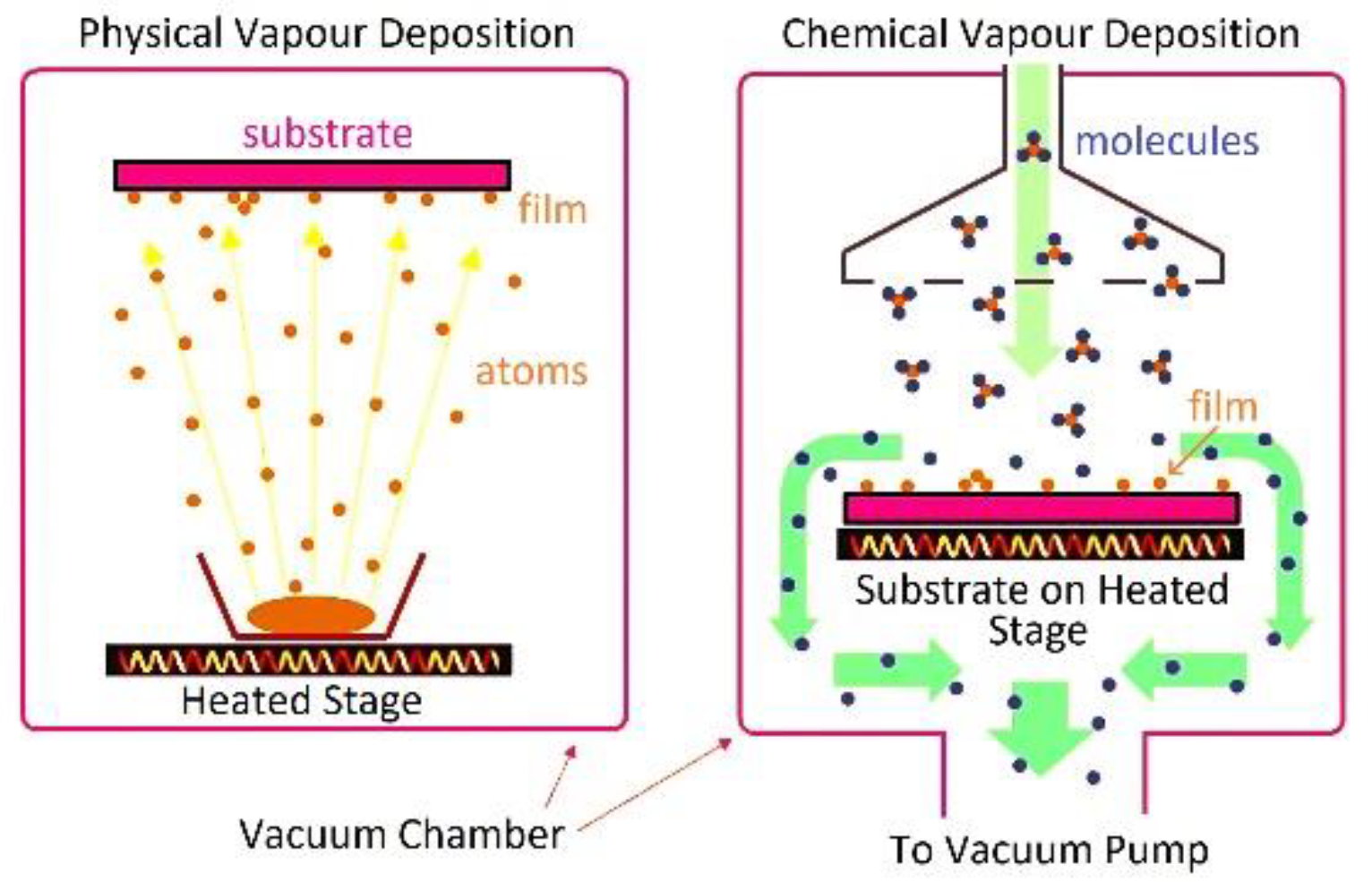
- Preparation techniques like laser micromachining, sol-gel, CVD, PVD, electrodeposition, electrospinning, and microfabrication.
- Electronic circuit designs like amplifiers and preamplifiers, filters, multiplexers, ADCs and DACs, power management, data transmission, control and feedback, and wireless technology.
- Implantation techniques, like targeted infusion, chronic implantation, and stereotaxic surgery [15].
- Exceptional Quality Neural Signal Recording with Implantable Microelectrodes.
- Long-Term Reliability in Chronic Implantation
- Accurate Brain Signal Decoding through High-Speed, High-Density Recording
- Multimodal Recording and Stimulation for Versatile Applications and Enhancing Implantable Microelectrodes through Integration with Other Technologies
- Standardizing the Manufacturing Process for Improved Implantable Microelectrodes Quality and Reliability
II. EXCEPTIONAL QUALITY NEURAL SIGNAL RECORDING WITH IMPLANTABLE MICROELECTRODES
- Filtering: removing noise and other unwanted signals from the brain signals. Examples include Kalman and Weiner filters. Kalman filter is a mathematical algorithm that uses a series of measurements and predictions to estimate the state of a system. It is commonly used in applications where real-time data is used to make predictions or control decisions. It improves SNR by combining sensor measurements, system dynamics, and knowledge of the noise characteristics to produce more accurate estimates of the true signal. Weiner filter is a type of filter that is used to remove noise from a signal or to improve the SNR by estimating the signal and noise in a statistical sense and then subtracting the noise from the original signal [23].
- Artifact reduction: removing artifacts, such as eye movements and muscle activity, can interfere with brain signals. Temporal filtering involves removing high-frequency noise and low-frequency drifts from the EEG signals. Spatial filtering: This involves removing noise from specific electrodes, for example, by using a common average reference. Independent Component Analysis (ICA) is a statistical technique used to separate independent sources of EEG signals. Artifact Subspace Reconstruction (ASR) involves removing artifacts from the EEG signals by projecting them into a subspace of artifact patterns. Removing muscle or eye movement artifacts involves identifying and removing artifacts generated by eye or muscle movements, which can produce large and sudden changes in the EEG signals [24].
- Feature extraction: identifying and extracting relevant features, such as frequency bands, event-related potentials, or power changes, from the brain signals. The most common features in feature extraction are time domain features, frequency domain features, event-related potentials, power spectral density, spatial features, and temporal features [25].
- Equalization: adjusting the balance of different frequency bands to optimize the signal quality. Equalization is a signal processing technique that can improve the SNR in electronic and biomedical devices. Equalization involves adjusting the amplitude or phase of different frequency components of a signal to achieve a desired response. There are various techniques, such as linear equalization, adaptive equalization, and non-linear equalization, which are used to improve the SNR. Linear equalization involves adjusting the amplitude or phase of different frequency components of a signal using a fixed filter. Linear equalization can be used to improve the SNR by reducing the level of background noise and enhancing the desired signal. Adaptive equalization is a type of linear equalization that uses an algorithm to adjust the filter coefficients based on the characteristics of the signal and noise. This can help to improve the SNR by adapting the filter to the specific conditions of the signal and noise. Non-linear equalization involves using non-linear processing techniques to adjust the amplitude or phase of different frequency components of a signal. Non-linear equalization can improve the SNR by reducing the level of background noise and enhancing the desired signal in a way that is not possible with linear equalization.
- Decoding: converting the extracted features into control signals that can be used to control the external device.
- Classification: using machine learning algorithms, such as support vector machines or neural networks, to categorize the brain signals into different classes, such as left/right-hand movement or object recognition.
- Feedback: providing visual, auditory, or haptic feedback to the user to inform them of the status of the BCI system and help them improve their control [26].

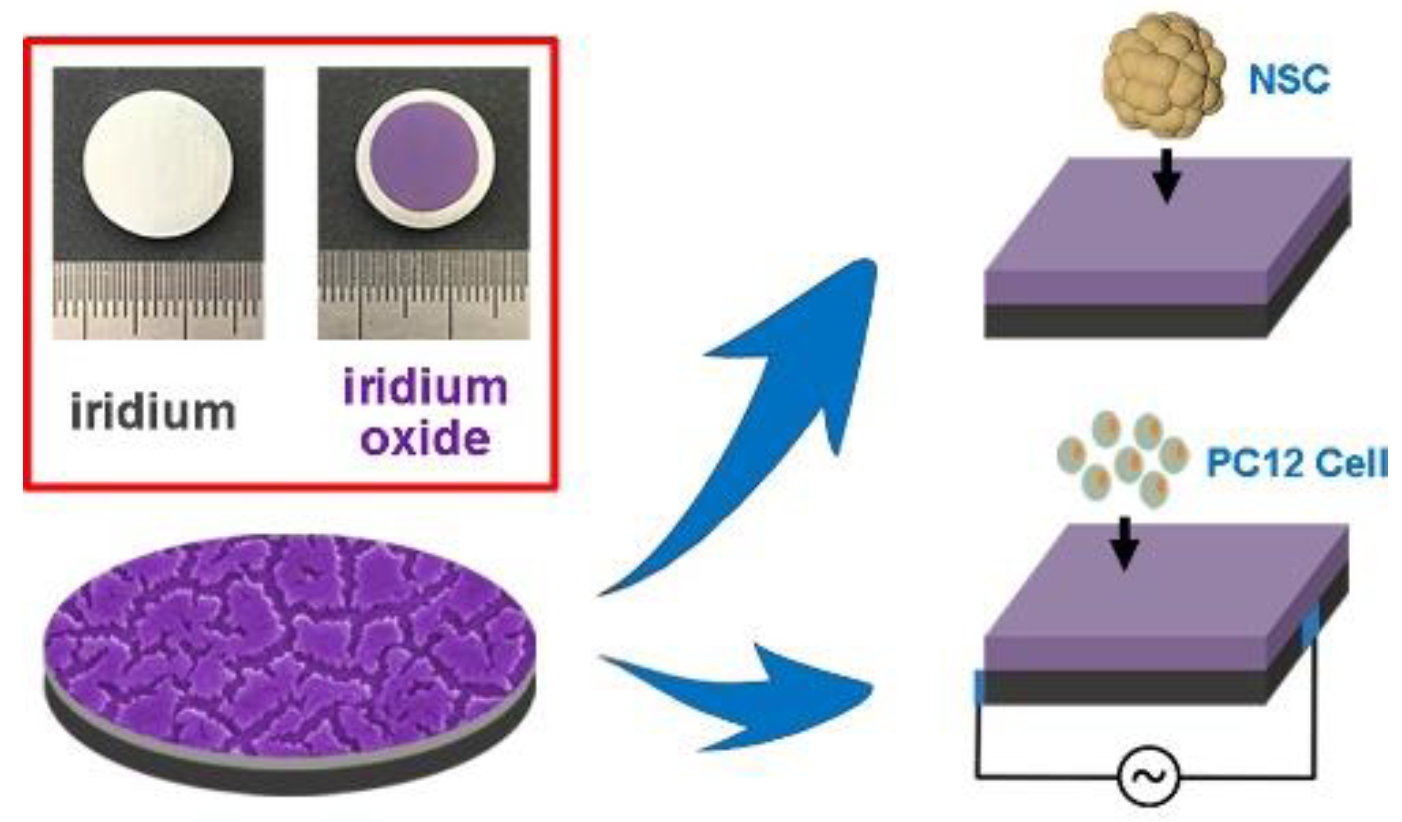
III. LONG-TERM RELIABILITY IN CHRONIC IMPLANTATION
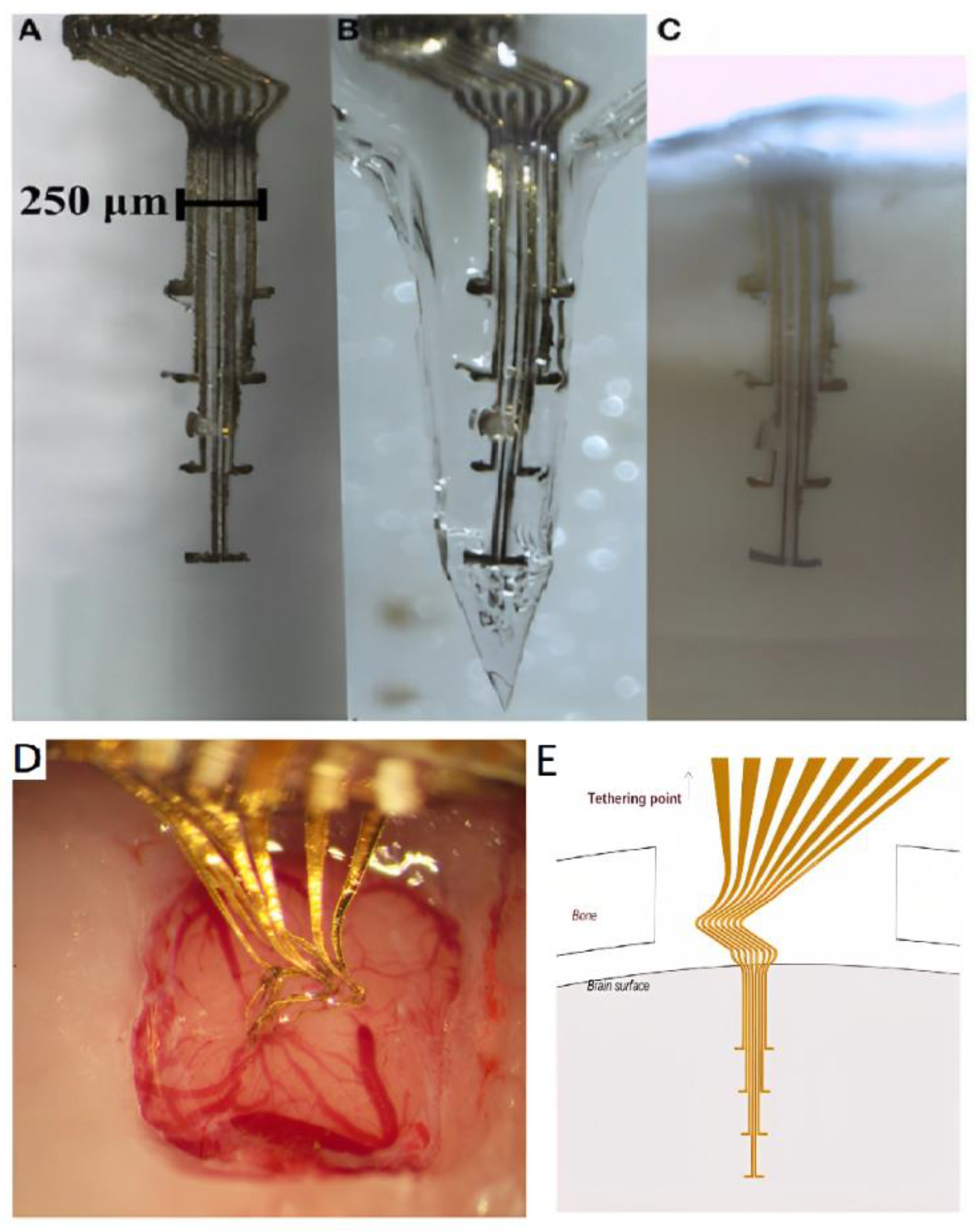
- Deep brain stimulation (DBS) for the treatment of movement disorders such as Parkinson's disease,
- Lesioning procedures for the treatment of psychiatric and neurological disorders,
- Placement of electrodes for recording neural activity
- Delivery of therapeutic agents such as gene therapy vectors or neurotrophins.
IV. ACCURATE BRAIN SIGNAL DECODING THROUGH HIGHSPEED, HIGH-DENSITY RECORDING
V. MULTIMODAL RECORDING AND STIMULATION FOR VERSATILE APPLICATIONS AND ENHANCING IMPLANTABLE MICROELECTRODES THROUGH INTEGRATION WITH OTHER TECHNOLOGIES
Some key areas of integration include
VI. STANDARDIZING THE MANUFACTURING PROCESS FOR IMPROVED IMPLANTABLE MICROELECTRODES QUALITY AND RELIABILITY
VII. CURRENTLY AVAILABLE SOLUTIONS
- 1.
-
NeuroPace RNS SystemThe NeuroPace RNS system is a type of implantable neural stimulation device developed by the company NeuroPace. It is a responsive neurostimulation (RNS) system designed to treat patients with epilepsy who have not responded to other treatments. The RNS system is designed to detect seizures as they are beginning to occur and then deliver a small electrical stimulus to the brain to stop the seizure from progressing.The RNS system consists of a small, implantable device (about the size of a pocket watch) that is placed under the skin of the skull and two thin wires (electrodes) that are surgically placed on the surface of the brain. These electrodes detect the abnormal electrical activity that occurs during a seizure and sends this information to the implantable device. The device then delivers a small electrical stimulus (a current-controlled and a charge-balanced biphasic electrical stimulation) to the brain to stop the seizure from progressing. The adjustability for frequency ranges from 1 to 333hz, and the intensity of current ranges from 1mA to 12mA [142]. It is considered a last resort treatment for patients who have not responded to other treatments and was FDA-approved in 2013. In 2017, NeuroPace marked its 1000th implant.
- 2.
-
NeuroNexusNeuroNexus Technologies is a medical device company that designs, manufactures, and sells advanced neurosensory and neurostimulation devices. The company was founded in 2000 and is based in Ann Arbor, Michigan, USA. NeuroNexus has many implantable and non-implantable neurotechnology products, including microelectrode arrays, flexible probes, cuff electrodes, wireless stimulators, and recording systems.The company's products are used in various applications, including basic neuroscience research, drug development, and treating neurological disorders such as Parkinson's, epilepsy, and chronic pain. NeuroNexus also provides custom design and manufacturing services for researchers and clinicians working in the field of neural engineering.One of the main focuses of the company is the development of flexible microelectrode arrays, which are thin, flexible probes that can be inserted into the brain or spinal cord to record neural activity or deliver electrical stimulation. These microelectrodes are made of thin film materials and are designed to conform to the contours of the brain, which helps to minimize the risk of tissue damage and improve the safety and efficacy of the stimulation. They offer up to 256 channel ECoG electrodes array [143].
- 3.
-
ImecImec is a research and development company based in Belgium specializing in nanoelectronics and digital technologies. They have a research program dedicated to developing advanced neural interfaces, which is focused on creating devices that can record and stimulate the activity of individual neurons in the brain with high spatial and temporal resolution. These devices are intended for use in both research and clinical applications, such as the treatment of neurological disorders and the restoration of sensory and motor function in people with disabilities. They are working with companies like Medtronic, Boston Scientific, and St. Jude Medical to develop implantable devices for applications like deep brain stimulation, spinal cord stimulation, and retinal prostheses.
- 4.
-
NeuropixelsNeuropixels electrodes are a type of neural recording electrode designed to record the activity of large numbers of neurons simultaneously. They are developed by the Neuropixels consortium, a collaboration between researchers at the Howard Hughes Medical Institute (HHMI), University College London (UCL), and the University of Cambridge.Neuropixels electrodes are made up of many individual recording sites, or "pixels," that are spaced closely on a flexible probe. Each pixel contains an electrode that can record the electrical activity of a single neuron. The pixels are arranged in a linear or 2D array on the probe, allowing the electrodes to be inserted into different regions of the brain or spinal cord.The Neuropixels electrodes are designed to have several key features that make them well-suited for large-scale neural recording. They have a high density of recording sites, which allows them to record the activity of many neurons at once. They are also designed to have low noise levels, so they can record the small electrical signals produced by neurons with high accuracy. Additionally, they are flexible and can be inserted through small openings in the skull, which allows them to record from deep brain regions with minimal invasiveness.Neuropixels electrodes are widely used in research studies to study brain function and neural circuits. They are particularly useful for studying the activity of large populations of neurons in different regions of the brain and for understanding how these neurons interact with each other to generate behavior.In summary, Neuropixels electrodes are a type of neural recording electrode designed to record the activity of large numbers of neurons simultaneously. They are developed by the Neuropixels consortium, a collaboration between researchers at the Howard Hughes Medical Institute (HHMI), University College London (UCL), and the University of Cambridge. They have a high density of recording sites, which allows them to record the activity of many neurons at once. They are also designed to have low noise levels, so they can record the small electrical signals produced by neurons with high accuracy. Additionally, they are flexible and can be inserted through small openings in the skull, which allows them to record from deep brain regions with minimal invasiveness. They are particularly useful for studying the activity of large populations of neurons in different regions of the brain and for understanding how these neurons interact with each other to generate behavior.
- 5.
-
ParadromicsParadromics is a neurotechnology company that develops implantable devices for recording and stimulating neural activity in the brain. The company was founded in 2014 by a group of researchers and engineers from Stanford University and is based in California, USA.One of the main focuses of Paradromics is the development of a high-bandwidth neural interface, which is a device that can be implanted in the brain to record and stimulate neural activity with high precision. The company's goal is to create a device that can record the activity of thousands of neurons simultaneously and stimulate these neurons with high temporal and spatial precision.The company's technology is based on the use of silicon-based electrodes that can be inserted into the brain. These electrodes are designed to be highly flexible and can be bent and folded to conform to the complex geometry of the brain. They are also designed to have a high density of recording sites, which allows them to record the activity of many neurons at once.Paradromics is also working on developing a high-bandwidth wireless link that can be used to transmit data from the implantable device to an external device. This wireless link will allow researchers to record and stimulate neural activity in real-time and to control the device remotely.Another focus of Paradromics is to make the devices more biocompatible. This way, the implantation of these devices in the human brain would be less invasive and have fewer side effects.In summary, Paradromics is a neurotechnology company that develops implantable devices for recording and stimulating neural activity in the brain. The company's main focus is the development of a high-bandwidth neural interface, which is a device that can be implanted in the brain to record and stimulate neural activity with high precision. The company's goal is to create a device that can record the activity of thousands of neurons simultaneously and stimulate these neurons with high temporal and spatial precision. They use silicon-based electrodes and a high-bandwidth wireless link and are also focused on developing more biocompatible devices; this way, the implantation of these devices in the human brain would be less invasive and have fewer side effects.
- 6.
-
Ad-Tech Medical Instrument CorporationAd-Tech Medical Instrument Corporation is a medical device company that specializes in developing and manufacturing implantable neurostimulation devices and accessories. The company was founded in 1991 and is based in Racine, Wisconsin, USA.Ad-Tech's main product line includes deep brain stimulation (DBS) systems for treating movement disorders such as Parkinson's disease, dystonia, and essential tremor. They also produce spinal cord stimulation systems for the treatment of chronic pain and sacral nerve stimulation systems for the treatment of urinary and fecal incontinence.The company's DBS systems include neurostimulator devices and implantable leads, as well as a range of accessories such as extension cables, programming devices, and remote control devices. The spinal cord stimulation systems include neurostimulator devices and implantable leads, as well as a range of accessories such as extension cables, programming devices, and remote control devices.Ad-Tech's products are designed to be highly customizable, allowing physicians to tailor the stimulation parameters to the specific needs of each patient. The company's neurostimulator devices are also designed to be MRI-compatible, which allows patients to undergo MRI scans without removing the device.In addition to its products, the company provides a range of services to support physicians and patients, including technical support, clinical support, and patient education.In summary, Ad-Tech Medical Instrument Corporation is a medical device company that specializes in developing and manufacturing implantable neurostimulation devices and accessories. The company's main product line includes deep brain stimulation (DBS) systems for the treatment of movement disorders such as Parkinson's disease, dystonia, and essential tremor, spinal cord stimulation systems for the treatment of chronic pain, and sacral nerve stimulation systems for the treatment of urinary and fecal incontinence. They offer highly customizable products and services to support physicians and patients.
CONCLUSION
References
- He, G.; Dong, X.; Qi, M. From the perspective of material science: a review of flexible electrodes for brain-computer interface. Mater. Res. Express 2020, 7, 102001. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu Rev Biomed Eng 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Popović, D.B. Advances in functional electrical stimulation (FES). Journal of Electromyography and Kinesiology 2014, 24, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Experimental Neurology 2005, 195, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Restoration of grasp following paralysis through brain-controlled stimulation of muscles | Nature. Available online: https://www.nature.com/articles/nature10987.
- Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah Slanted Electrode Arrays (USEAs) implanted in residual peripheral arm nerves | Journal of NeuroEngineering and Rehabilitation | Full Text. Available online: https://jneuroengrehab.biomedcentral.com/articles/10.1186/s12984-017-0320-4.
- Wu, A.D.; Fregni, F.; Simon, D.K.; Deblieck, C.; Pascual-Leone, A. Noninvasive Brain Stimulation for Parkinson’s Disease and Dystonia. Neurotherapeutics 2008, 5, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H.S. Deep brain stimulation for treatment-resistant depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Frontiers | Vagus Nerve Stimulation (VNS) and Other Augmentation Strategies for Therapy-Resistant Depression (TRD): Review of the Evidence and Clinical Advice for Use. Available online: https://www.frontiersin.org/articles/10.3389/fnins.2018.00239/full.
- Osorio, I. Automated seizure abatement in humans using electrical stimulation. Annals of Neurology 2005, 57, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G. A Randomized Trial of Deep-Brain Stimulation for Parkinson’s Disease. New England Journal of Medicine 2006, 355, 896–908. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Annals of Indian Academy of Neurology 2008, 11, 13. [Google Scholar] [CrossRef]
- Gomez-Inhiesto, E. Direct Cost of Parkinson’s Disease: A Real-World Data Study of Second-Line Therapies. Parkinson’s Disease 2020, 2020, e9106026. [Google Scholar] [CrossRef]
- Reddy, K.V.M.K.; Reddy, B.M.; Reddy, K.C.S.; Kartheek, P.; Adarsh, T.S. Comparative investigation of electronic fuel injection in two-wheeler applications: A Review. IOP Conf. Ser.: Mater. Sci. Eng. 2021, 1116, 012073. [Google Scholar] [CrossRef]
- Grill, W.M.; Norman, S.E.; Bellamkonda, R.V. Implanted Neural Interfaces: Biochallenges and Engineered Solutions. Annual Review of Biomedical Engineering 2009, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat Rev Neurosci 2012, 13, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Maynard, E.M.; Fernandez, E.; Normann, R.A. A technique to prevent dural adhesions to chronically implanted microelectrode arrays. Journal of Neuroscience Methods 2000, 97, 93–101. [Google Scholar] [CrossRef]
- High-Amplitude Positive Spikes Recorded Extracellularly in Cat Visual Cortex | Journal of Neurophysiology. Available online: https://journals.physiology.org/doi/full/10.1152/jn.91365.2008.
- Hill, S.; Tononi, G. Modeling Sleep and Wakefulness in the Thalamocortical System. Journal of Neurophysiology 2005, 93, 1671–1698. [Google Scholar] [CrossRef] [PubMed]
- Lempka, S.F. Theoretical analysis of intracortical microelectrode recordings. J. Neural Eng. 2011, 8, 045006. [Google Scholar] [CrossRef] [PubMed]
- Camuñas-Mesa, L.A.; Quiroga, R.Q. A Detailed and Fast Model of Extracellular Recordings. Neural Computation 2013, 25, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Rousche, P.J. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Transactions on Biomedical Engineering 2001, 48, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Manju, B.R.; Sneha, M.R. ECG Denoising Using Wiener Filter and Kalman Filter. Procedia Computer Science 2020, 171, 273–281. [Google Scholar]
- Winkler, I.; Debener, S.; Müller, K.-R.; Tangermann, M. On the influence of high-pass filtering on ICA-based artifact reduction in EEG-ERP. in 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2015, 4101–4105. [Google Scholar] [CrossRef]
- Krishnan, S.; Athavale, Y. Trends in biomedical signal feature extraction. Biomedical Signal Processing and Control 2018, 43, 41–63. [Google Scholar] [CrossRef]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A comprehensive review of EEG-based brain–computer interface paradigms. J. Neural Eng. 2019, 16, 011001. [Google Scholar] [CrossRef]
- The effects of electrode impedance on data quality and statistical significance in ERP recordings - Kappenman - 2010 - Psychophysiology - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8986.2010.01009.x.
- Sharafkhani, N. Neural tissue-microelectrode interaction: Brain micromotion, electrical impedance, and flexible microelectrode insertion. Journal of Neuroscience Methods 2022, 365, 109388. [Google Scholar] [CrossRef]
- Lempka, S.F. Theoretical analysis of intracortical microelectrode recordings. J. Neural Eng. 2011, 8, 045006. [Google Scholar] [CrossRef] [PubMed]
- Surface patterning using templates: concept, properties and device applications - Chemical Society Reviews (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2011/CS/B924854.
- Chapman, C.A.R. Nanoporous Gold Biointerfaces: Modifying Nanostructure to Control Neural Cell Coverage and Enhance Electrophysiological Recording Performance. Advanced Functional Materials 2017, 27, 1604631. [Google Scholar] [CrossRef]
- Woo, H. Au Hierarchical Nanostructure-Based Surface Modification of Microelectrodes for Improved Neural Signal Recording. Anal. Chem. 2021, 93, 11765–11774. [Google Scholar] [CrossRef] [PubMed]
- Nanostructured platinum grass enables superior impedance reduction for neural microelectrodes - ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0142961215006183?via%3Dihub.
- Valle E della Welle, E.J.; Chestek, C.A.; Weiland, J.D. Compositional and morphological properties of platinum-iridium electrodeposited on carbon fiber microelectrodes. J. Neural Eng. 2021, 18, 054001. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H. Recent Progress on Microelectrodes in Neural Interfaces. Materials 2018, 11, 1995. [Google Scholar] [CrossRef]
- Sait, R.A.; Cross, R.B.M. Synthesis and characterization of sputtered titanium nitride as a nucleation layer for novel neural electrode coatings. Applied Surface Science 2017, 424, 290–298. [Google Scholar] [CrossRef]
- Rodrigues, F. Fabrication and characterization of polyimide-based ‘smooth’ titanium nitride microelectrode arrays for neural stimulation and recording. J. Neural Eng. 2019, 17, 016010. [Google Scholar] [CrossRef]
- Kim, G.H. et al. CNT-Au nanocomposite deposition on gold microelectrodes for improved neural recordings.
- Wang, X.-D. et al. Synthesis of Carbon Nanotubes by Catalytic Chemical Vapor Deposition. Perspective of Carbon Nanotubes (IntechOpen, 2019). [CrossRef]
- Wang, J. A review of graphene synthesis at low temperatures by CVD methods. New Carbon Materials 2020, 35, 193–208. [Google Scholar] [CrossRef]
- Lu, Y. Anodically electrodeposited iridium oxide films microelectrodes for neural microstimulation and recording. Sensors and Actuators B: Chemical 2009, 137, 334–339. [Google Scholar] [CrossRef]
- Meyer, R.D. , Cogan, S.F., Nguyen, T.H. & Rauh, R.D. Electrodeposited iridium oxide for neural stimulation and recording electrodes. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2001, 9, 2–11. [Google Scholar] [PubMed]
- Ryu, M. et al. Enhancement of interface characteristics of neural probe based on graphene, ZnO nanowires, and conducting polymer PEDOT.
- Deng, M. Electrochemical deposition of polypyrrole/graphene oxide composite on microelectrodes towards tuning the electrochemical properties of neural probes. Sensors and Actuators B: Chemical 2011, 158, 176–184. [Google Scholar] [CrossRef]
- Pickup, P.G.; Osteryoung, R.A. Electrochemical polymerization of pyrrole and electrochemistry of polypyrrole films in ambient temperature molten salts. J. Am. Chem. Soc. 1984, 106, 2294–2299. [Google Scholar] [CrossRef]
- Conducting polymers for corrosion protection: a review | SpringerLink. Available online: https://link.springer.com/article/10.1007/s11998-014-9586-7.
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Chen, C.; Ruan, S.; Bai, X.; Lin, C.; Xie, C.; Lee, I.-S. Patterned iridium oxide film as neural electrode interface: Biocompatibility and improved neurite outgrowth with electrical stimulation. Materials Science and Engineering: C 2019, 103, 109865. [Google Scholar] [CrossRef]
- Sputtered iridium oxide films for neural stimulation electrodes - Cogan - 2009 - Journal of Biomedical Materials Research Part B: Applied Biomaterials - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/jbm.b.31223.
- Chen, C. Patterned iridium oxide film as neural electrode interface: Biocompatibility and improved neurite outgrowth with electrical stimulation. Materials Science and Engineering: C 2019, 103, 109865. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Mohammed, M.R.; Radhi, S.H.; Hadi, A.N. A Review on the Latest Developments of Conducting Polymer and Composite Coatings for Enhancing Biocompatibility and Corrosion Resistance of Metallic Biomedical Implants. European Journal of Engineering and Technology Research 2021, 6, 146–155. [Google Scholar] [CrossRef]
- Cui, X.; Wiler, J.; Dzaman, M.; Altschuler, R.A.; Martin, D.C. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials 2003, 24, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Negi, Y.S.; Adhyapak, P.V. Development in Polyaniline Conducting Polymers. Journal of Macromolecular Science, Part C 2002, 42, 35–53. [Google Scholar] [CrossRef]
- Chen, N. Nanotunnels within Poly(3,4-ethylenedioxythiophene)-Carbon Nanotube Composite for Highly Sensitive Neural Interfacing. ACS Nano 2020, 14, 8059–8073. [Google Scholar] [CrossRef] [PubMed]
- Topological supramolecular network enabled highly conductive and stretchable organic bioelectronics | bioRxiv. Available online: https://www.biorxiv.org/content/10.1101/2022.01.16.476423v1.
- Manivasagam, G. , Dhinasekaran, D. & Rajamanickam, A. Biomedical Implants: Corrosion and its Prevention - A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar]
- Implantable Device Fabrication and Packaging | SpringerLink. Available online: https://link.springer.com/referenceworkentry/10.1007/978-981-15-2848-4_102-1.
- Wellman, S.M. A Materials Roadmap to Functional Neural Interface Design. Advanced Functional Materials 2018, 28, 1701269. [Google Scholar] [CrossRef]
- Prasad, A. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J. Neural Eng. 2012, 9, 056015. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Composites Part A: Applied Science and Manufacturing 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Civantos, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. Nanomaterials. (BoD – Books on Demand, 2011).
- Frontiers | Low-Temperature Atomic Layer Deposited Oxide on Titanium Nitride Electrodes Enables Culture and Physiological Recording of Electrogenic Cells. https://www.frontiersin.org/articles/10.3389/fnins.2020.552876/full.
- Kim, B.J., Gutierrez, C.A. & Meng, E. Parylene-based electrochemical-MEMS force sensor for studies of intracortical probe insertion mechanics.
- Seymour, J.P. , Elkasabi, Y.M., Chen, H.-Y., Lahann, J. & Kipke, D.R. The insulation performance of reactive parylene films in implantable electronic devices. Biomaterials 2009, 30, 6158–6167. [Google Scholar] [PubMed]
- Patrick, E.; Orazem, M.E.; Sanchez, J.C.; Nishida, T. Corrosion of tungsten microelectrodes used in neural recording applications. Journal of Neuroscience Methods 2011, 198, 158–171. [Google Scholar] [CrossRef] [PubMed]
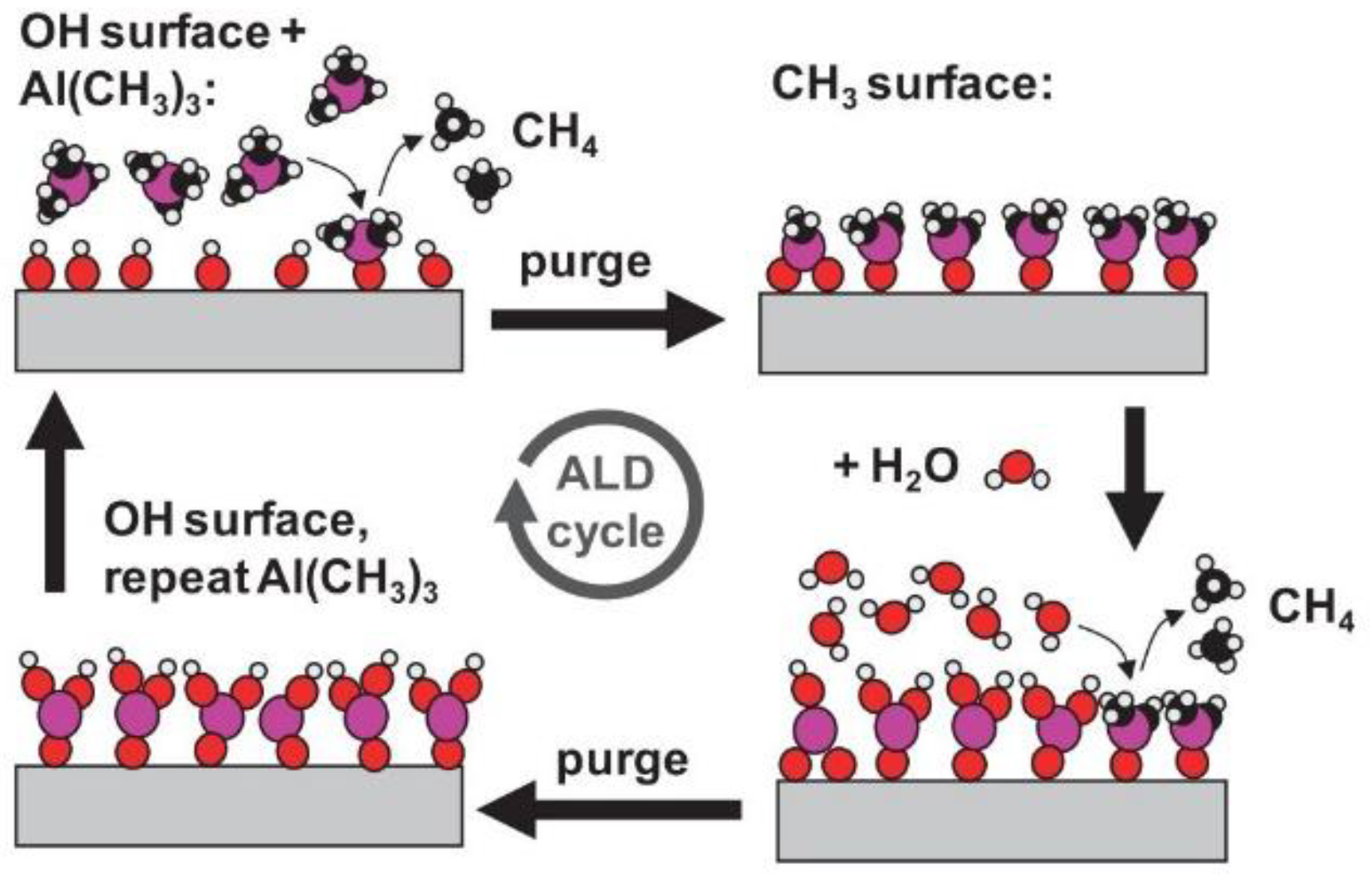
- “Basics of ALD. Parsons Research Group, Nov. 16, 2015. Available online: https://thinfilm.wordpress.ncsu.edu/research/basics-of-ald/ (accessed on 11 March 2023).
- Implantable neurotechnologies: a review of micro- and nanoelectrodes for neural recording | SpringerLink. Available online: https://link.springer.com/article/10.1007/s11517-015-1430-4.
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A Review of Organic and Inorganic Biomaterials for Neural Interfaces. Advanced Materials 2014, 26, 1846–1885. [Google Scholar] [CrossRef] [PubMed]
- Thelin, J. Implant Size and Fixation Mode Strongly Influence Tissue Reactions in the CNS. PLOS ONE 2011, 6, e16267. [Google Scholar] [CrossRef] [PubMed]
- Szarowski, D.H. Brain responses to micro-machined silicon devices. Brain Research 2003, 983, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Edell, D.J.; Toi, V.V.; McNeil, V.M.; Clark, L.D. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Transactions on Biomedical Engineering 1992, 39, 635–643. [Google Scholar] [CrossRef]
- Turner, J.N. Cerebral Astrocyte Response to Micromachined Silicon Implants. Experimental Neurology 1999, 156, 33–49. [Google Scholar] [CrossRef]
- Glial responses to implanted electrodes in the brain | Nature Biomedical Engineering. Available online: https://www.nature.com/articles/s41551-017-0154-1.
- Frontiers | Biomedical and Tissue Engineering Strategies to Control Foreign Body Reaction to Invasive Neural Electrodes. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2021.659033/full.
- Kozai, T.D.Y.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Brain Tissue Responses to Neural Implants Impact Signal Sensitivity and Intervention Strategies. ACS Chem. Neurosci. 2015, 6, 48–67. [Google Scholar]
- Carbon fiber on polyimide ultra-microelectrodes - IOPscience. Available online: https://iopscience.iop.org/article/10.1088/1741-2552/aa8c88.
- Chronic interfacing with the autonomic nervous system using carbon nanotube (CNT) yarn electrodes | Scientific Reports. Available online: https://www.nature.com/articles/s41598-017-10639-w.
- Subbaroyan, J.; Martin, D.C.; Kipke, D.R. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex.
- The density difference between tissue and neural probes is a key factor for glial scarring | Scientific Reports. Available online: https://www.nature.com/articles/srep02942.
- Kil, D. Dextran as a Resorbable Coating Material for Flexible Neural Probes. Micromachines 2019, 10, 61. [Google Scholar] [CrossRef]
- Wang, X.C. et al. A Parylene Neural Probe Array for Multi-Region Deep Brain Recordings.
- Stieglitz, T.; Beutel, H.; Schuettler, M.; Meyer, J.-U. Micromachined, Polyimide-Based Devices for Flexible Neural Interfaces. Biomedical Microdevices 2000, 2, 283–294. [Google Scholar] [CrossRef]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. Journal of Polymer Science Part B: Polymer Physics 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Altuna, A.; Berganzo, J.; Fernández, L.J. Polymer SU-8-Based Microprobes for Neural Recording and Drug Delivery. Frontiers in Materials 2015, 2. [Google Scholar] [CrossRef]
- Xiang, Z. Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle. J. Micromech. Microeng. 2014, 24, 065015. [Google Scholar] [CrossRef]
- Spencer, K.C. Characterization of Mechanically Matched Hydrogel Coatings to Improve the Biocompatibility of Neural Implants. Sci Rep 2017, 7, 1952. [Google Scholar] [CrossRef]
- Stice, P.; Gilletti, A.; Panitch, A.; Muthuswamy, J. Thin microelectrodes reduce GFAP expression in the implant site in rodent somatosensory cortex. J. Neural Eng. 2007, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Silk-Backed Structural Optimization of High-Density Flexible Intracortical Neural Probes. Journal of Microelectromechanical Systems 2015, 24, 62–69. [Google Scholar] [CrossRef]
- Lecomte, A. Silk and PEG as means to stiffen a parylene probe for insertion in the brain: toward a double time-scale tool for local drug delivery. J. Micromech. Microeng. 2015, 25, 125003. [Google Scholar] [CrossRef]
- Nguyen, T.P. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Kipke, D.R. Insertion shuttle with carboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain. Journal of Neuroscience Methods 2009, 184, 199–205. [Google Scholar] [CrossRef]
- Insertion Of Flexible Neural Probes Using Rigid Stiffeners Attached With Biodissolvable Adhesive - Video. Available online: https://www.jove.com/t/50609/insertion-of-flexible-neural-probes-using-rigid-stiffeners-attached-with-biodissolvable-adhesive.
- Liu, J. Syringe-injectable electronics. Nature Nanotech 2015, 10, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Musk, E.; Neuralink. An Integrated Brain-Machine Interface Platform With Thousands of Channels. Journal of Medical Internet Research 2019, 21, e16194. [Google Scholar] [CrossRef]
- Paralikar, K.J.; Clement, R.S. Collagenase-Aided Intracortical Microelectrode Array Insertion: Effects on Insertion Force and Recording Performance. IEEE Transactions on Biomedical Engineering 2008, 55, 2258–2267. [Google Scholar] [CrossRef]
- Nguyen, J.K. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J. Neural Eng. 2014, 11, 056014. [Google Scholar] [CrossRef]
- Ware, T. Fabrication of Responsive, Softening Neural Interfaces. Advanced Functional Materials 2012, 22, 3470–3479. [Google Scholar] [CrossRef]
- Lee, S.E. A Flexible Depth Probe Using Liquid Crystal Polymer. IEEE Transactions on Biomedical Engineering 2012, 59, 2085–2094. [Google Scholar]
- Rezaei, S.; Xu, Y.; Pang, S.W. Control of neural probe shank flexibility by fluidic pressure in embedded microchannel using PDMS/PI hybrid substrate. PLOS ONE 2019, 14, e0220258. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, I.H.; Kording, K.P. How advances in neural recording affect data analysis. Nat Neurosci 2011, 14, 139–142. [Google Scholar] [CrossRef]
- Buzsáki, G. Large-scale recording of neuronal ensembles. Nat Neurosci 2004, 7, 446–451. [Google Scholar] [CrossRef]
- Abruña, H.D.; Kiya, Y.; Henderson, J.C. Batteries and electrochemical capacitors. Physics Today 2008, 61, 43–47. [Google Scholar] [CrossRef]
- Schiavone, G.; Kang, X.; Fallegger, F.; Gandar, J.; Courtine, G.; Lacour, S.P. Guidelines to Study and Develop Soft Electrode Systems for Neural Stimulation. Neuron 2020, 108, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Chang, Y.; Venton, B.J. Carbon microelectrodes with customized shapes for neurotransmitter detection: A review. Analytica Chimica Acta 2022, 1223, 340165. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, S.; Rajan, M. Chapter One - Biosynthesis of nanoparticles and their roles in numerous areas. In Comprehensive Analytical Chemistry; Verma, S.K., Das, A.K., Eds.; Elsevier, 2021; pp. 1–47. [Google Scholar] [CrossRef]
- Wei, D.; Wu, B.; Guo, Y.; Yu, G.; Liu, Y. Controllable Chemical Vapor Deposition Growth of Few Layer Graphene for Electronic Devices. Acc. Chem. Res. 2013, 46, 106–115. [Google Scholar] [CrossRef] [PubMed]
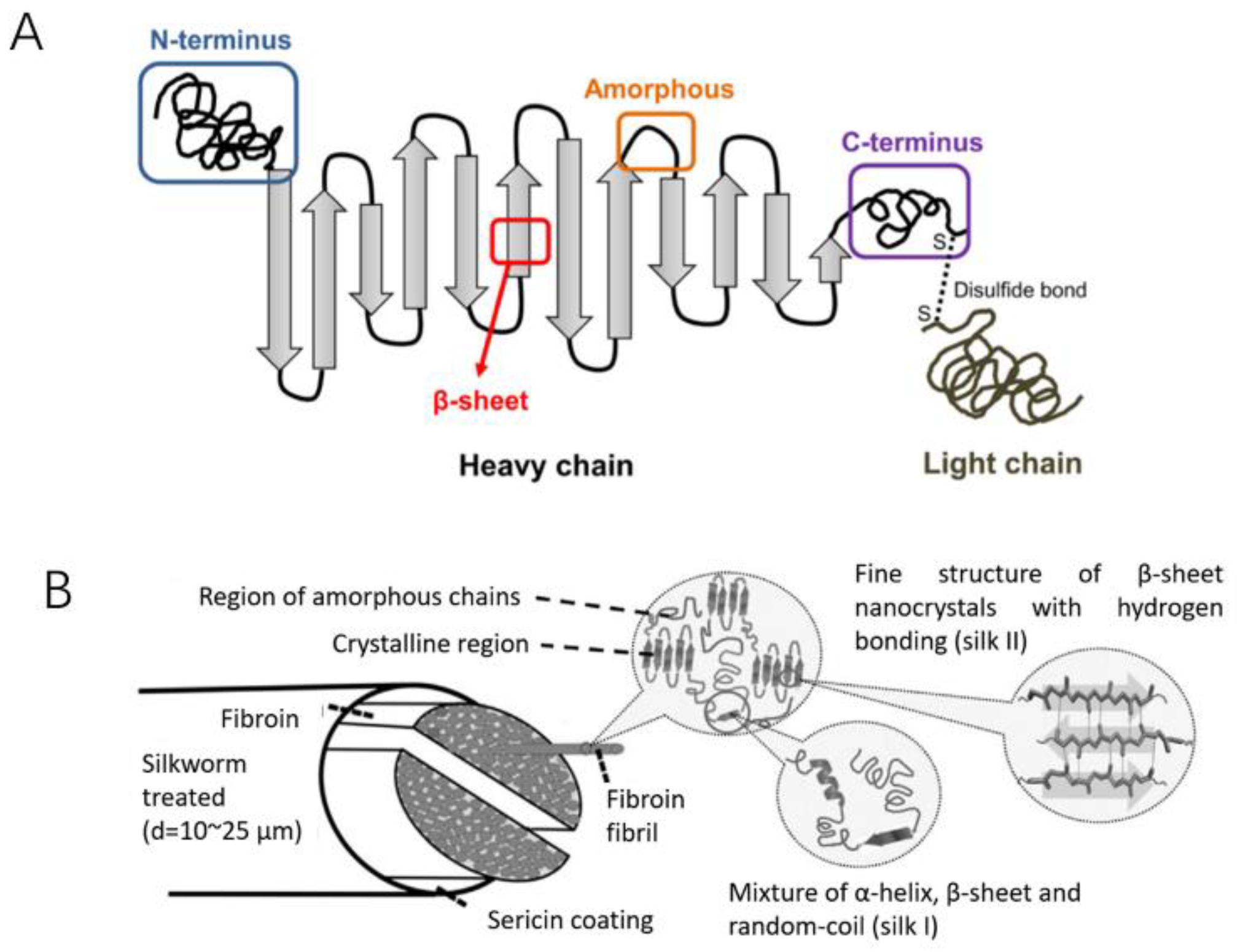
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk Materials – A Road to Sustainable High Technology. Advanced Materials 2012, 24, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Frontiers in Bioengineering and Biotechnology 2021, 9. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2021.622524 (accessed on 10 February 2023). [Online].
- Buccino, P.; et al. How does the presence of neural probes affect extracellular potentials? J. Neural Eng. 2019, 16, 026030. [Google Scholar] [CrossRef] [PubMed]
- Rihani, R.T.; et al. Liquid Crystal Elastomer-Based Microelectrode Array for In Vitro Neuronal Recordings. Micromachines 2018, 9, 8. [Google Scholar] [CrossRef]
- Castagnola, E.; et al. Smaller, softer, lower-impedance electrodes for human neuroprosthesis: a pragmatic approach. Frontiers in Neuroengineering 2014, 7. Available online: https://www.frontiersin.org/articles/10.3389/fneng.2014.00008 (accessed on 10 February 2023). [Online.
- He, W.; Bellamkonda, R.V. Nanoscale neuro-integrative coatings for neural implants. Biomaterials 2005, 26, 2983–2990. [Google Scholar] [CrossRef]
- Rajkalyan, C.; Tewari, A.; Rao, S.; Avitsian, R. Anesthetic considerations for stereotactic electroencephalography implantation. J Anaesthesiol Clin Pharmacol 2019, 35, 434–440. [Google Scholar] [CrossRef]
- Draz, H.H. et al., Mechanical Analysis of Human DBS Electrodes. in 2020 32nd International Conference on Microelectronics (ICM), Aqaba, Jordan, 2020; 2020; pp. 1–6. [CrossRef]
- Alivisatos, P. The Brain Activity Map. Science 2013, 339, 1284–1285. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, P.; Chun, M.; Church, G.M.; Greenspan, R.J.; Roukes, M.L.; Yuste, R. The Brain Activity Map Project and the Challenge of Functional Connectomics. Neuron 2012, 74, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Marblestone* et al. Physical principles for scalable neural recording. Frontiers in Computational Neuroscience 2013, 7. Available online: https://www.frontiersin.org/articles/10.3389/fncom.2013.00137 (accessed on 11 February 2023). [Online].
- Shin, H. Multifunctional multi-shank neural probe for investigating and modulating long-range neural circuits in vivo. Nat Commun 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Pavuluri, S.K.; Cummins, G.; Desmulliez, M.P.Y. Wireless Power Transfer Techniques for Implantable Medical Devices: A Review. Sensors 2020, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y. Powering Implantable and Ingestible Electronics. Advanced Functional Materials 2021, 31, 2009289. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S. Lee, A. C. Molnar, and S. Cash. Injectable wireless microdevices: challenges and opportunities. Bioelectronic Medicine 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Moradi, F.; Heidari, H. Biointegrated and Wirelessly Powered Implantable Brain Devices: A Review. IEEE Transactions on Biomedical Circuits and Systems 2020, 14, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Arshad, J. Intelligent Control of Robotic Arm Using Brain Computer Interface and Artificial Intelligence. Applied Sciences 2022, 12, 21. [Google Scholar] [CrossRef]
- Mirza, K.B.; Golden, C.T.; Nikolic, K.; Toumazou, C. Closed-Loop Implantable Therapeutic Neuromodulation Systems Based on Neurochemical Monitoring. Frontiers in Neuroscience 2019, 13. (accessed on 11 February 2023). [Online]. [Google Scholar] [CrossRef]
- JNakai; Ohkura, M.; Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol 2001, 19, 2. [CrossRef] [PubMed]
- Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods, vol. 6, no. 12, Art. no. 12, Dec. 2009. [CrossRef] [PubMed]
- Xiao, G. Microelectrode Arrays Modified with Nanocomposites for Monitoring Dopamine and Spike Firings under Deep Brain Stimulation in Rat Models of Parkinson’s Disease. ACS Sens. 2019, 4, 1992–2000. [Google Scholar] [CrossRef]
- Kiyatkin, E.A. Brain temperature and its role in physiology and pathophysiology: Lessons from 20 years of thermorecording. Temperature 2019, 6, 271–333. [Google Scholar] [CrossRef] [PubMed]
- Rezaei-Mazinani, S. Monitoring fluorescent calcium signals in neural cells with organic photodetectors. J. Mater. Chem. C 2019, 7, 9049–9056. [Google Scholar] [CrossRef]
- Storace, A.; Braubach, O.R.; Jin, L.; Cohen, L.B.; Sung, U. Monitoring Brain Activity with Protein Voltage and Calcium Sensors. Sci Rep 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Borchers, S.; Himmelbach, M.; Logothetis, N.; Karnath, H.-O. Direct electrical stimulation of human cortex — the gold standard for mapping brain functions? . Nat Rev Neurosci 2012, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Son, Y.; Kim, J.; Lee, C.J.; Yoon, E.-S.; Cho, I.-J. A multichannel neural probe with embedded microfluidic channels for simultaneous in vivo neural recording and drug delivery. Lab Chip 2015, 15, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Deisseroth, K. Recent advances in optogenetics and pharmacogenetics. Brain Research 2013, 1511, 1–5. [Google Scholar] [CrossRef]
- Wu, F.; Stark, E.; Ku, P.-C.; Wise, K.D.; Buzsáki, G.; Yoon, E. Monolithically Integrated μLEDs on Silicon Neural Probes for High-Resolution Optogenetic Studies in Behaving Animals. Neuron 2015, 88, 1136–1148. [Google Scholar] [CrossRef]
- Agorelius, J.; Tsanakalis, F.; Friberg, A.; Thorbergsson, P.T.; Pettersson, L.; Schouenborg, J. An array of highly flexible electrodes with a tailored configuration locked by gelatin during implantation—initial evaluation in cortex cerebri of awake rats. Frontiers in Neuroscience 2015, 9. Available online: https://wwwfrontiersinorg/articles/103389/fnins201500331 (accessed on 11 March 2023). [Online]. [CrossRef] [PubMed]
- Sekiguchi, H. Adhesionable flexible GaN-based microLED array film to brain surface for in vivo optogenetic stimulation. Appl. Phys. Express 2022, 15, 046501. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yang, X.; Wu, X.; Wang, Y.; Pei, W. Analysis of Electromagnetic Interference and Shielding in the μLED Optrode Based on Finite Element Method. Frontiers in Nanotechnology 2021, 3. Available online: https://www.frontiersin.org/articles/10.3389/fnano.2021.758484 (accessed on 11 March 2023). [Online]. [CrossRef]
- Sodagar, M.; Wise, K.D.; Najafi, K. A Wireless Implantable Microsystem for Multichannel Neural Recording. IEEE Transactions on Microwave Theory and Techniques 2009, 57, 2565–2573. [Google Scholar] [CrossRef]
- Neely, R.M.; Piech, D.K.; Santacruz, S.R.; Maharbiz, M.M.; Carmena, J.M. Recent advances in neural dust: towards a neural interface platform. Current Opinion in Neurobiology 2018, 50, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.P.; Jobst, B.C. Critical review of the responsive neurostimulator system for epilepsy. MDER 2015, 8, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Jonak, R.; Lovelace, J.W.; Ethell, I.M.; Razak, K.A.; Binder, D.K. Reusable Multielectrode Array Technique for Electroencephalography in Awake Freely Moving Mice. Frontiers in Integrative Neuroscience 2018, 12. Available online: https://www.frontiersin.org/articles/10.3389/fnint.2018.00053 (accessed on 11 March 2023). [Online]. [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. International Journal of Molecular Sciences 2021, 22, 3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



