Submitted:
28 February 2023
Posted:
01 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
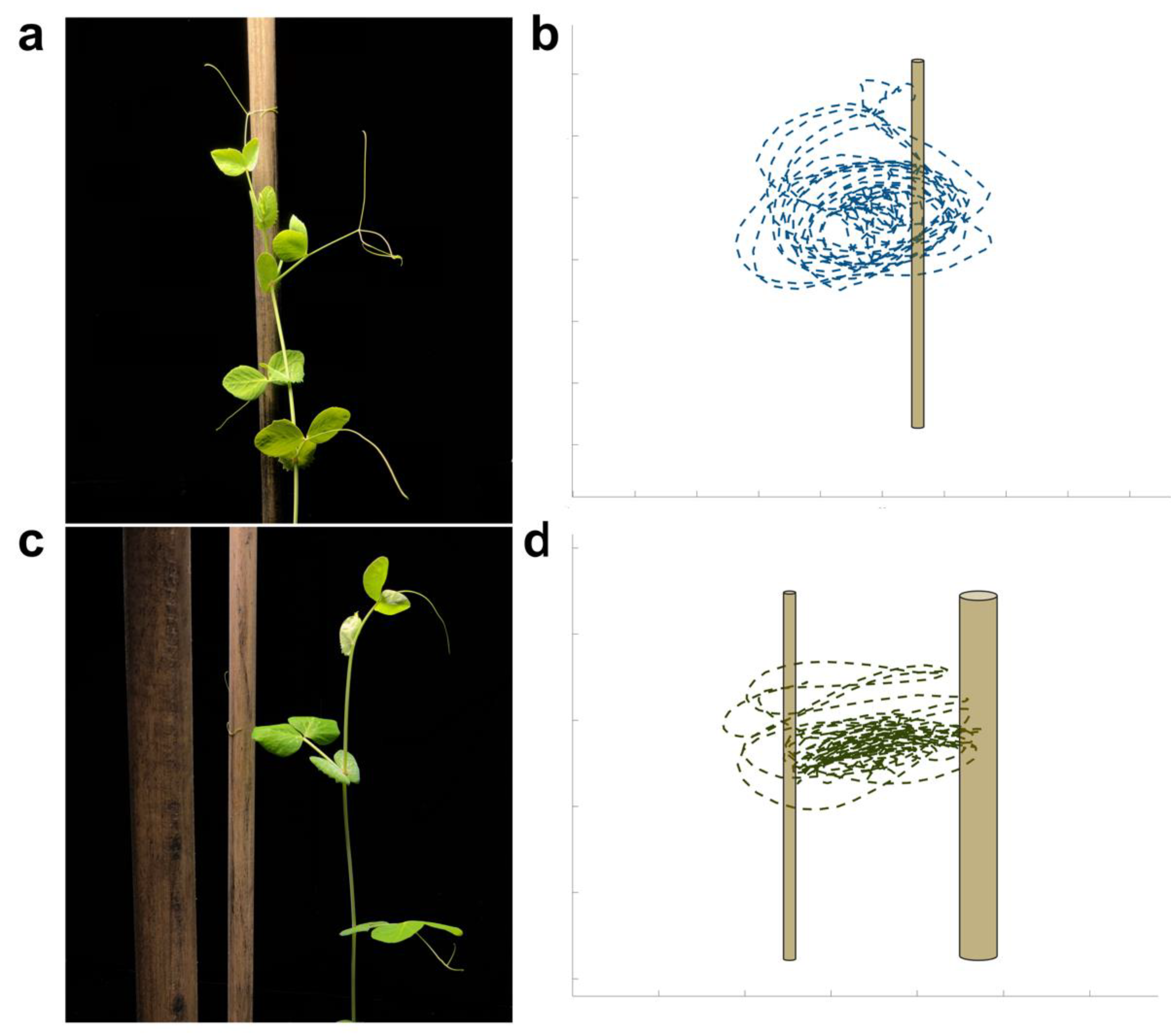
2.1. Qualitative results
2.2. Kinematic results
2.2.1. Number of circumnutation
2.2.2. Circunnutation duration
2.2.3. Distance from the circumnutation center to the origin
2.2.4. Length of the circumnutation major axis
2.2.5. Circumnutation length
2.2.6. Circumnutation area
2.2.7. Amplitude of maximum peak velocity
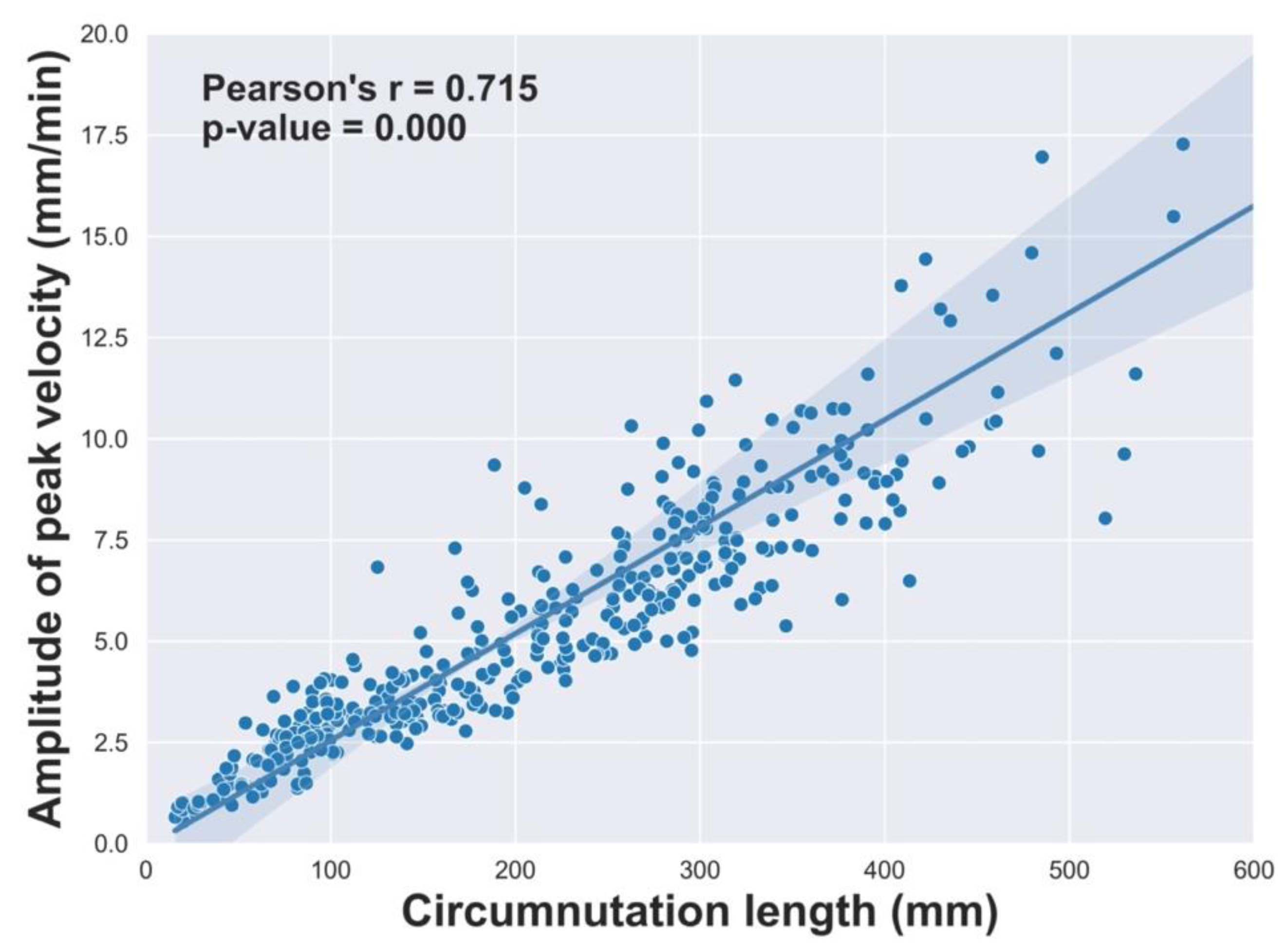
2.2.8. Correlational analyses
3. Discussion
4. Materials and Methods
4.1. Subjects
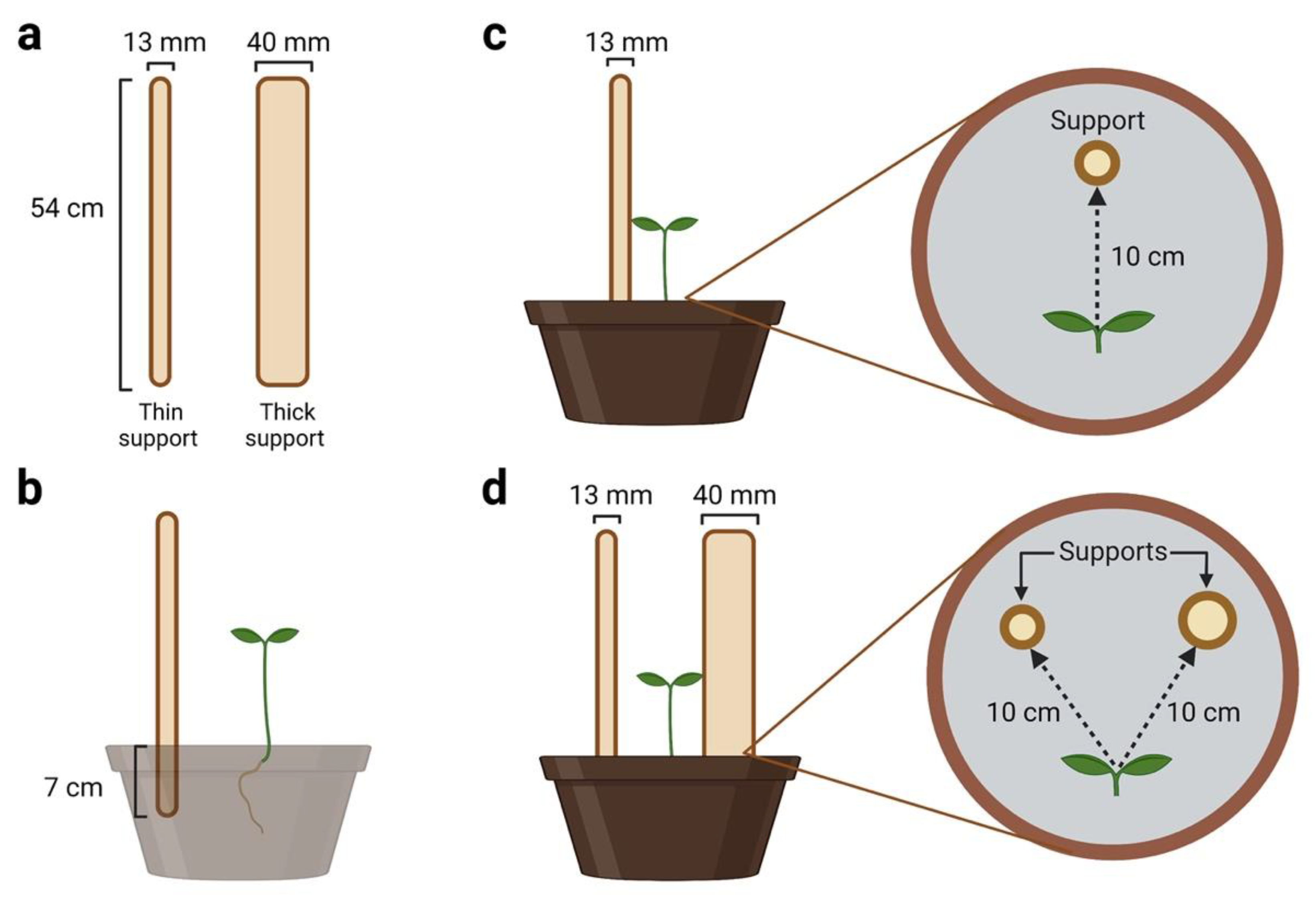
4.2. Type of support
4.3. Experimental conditions
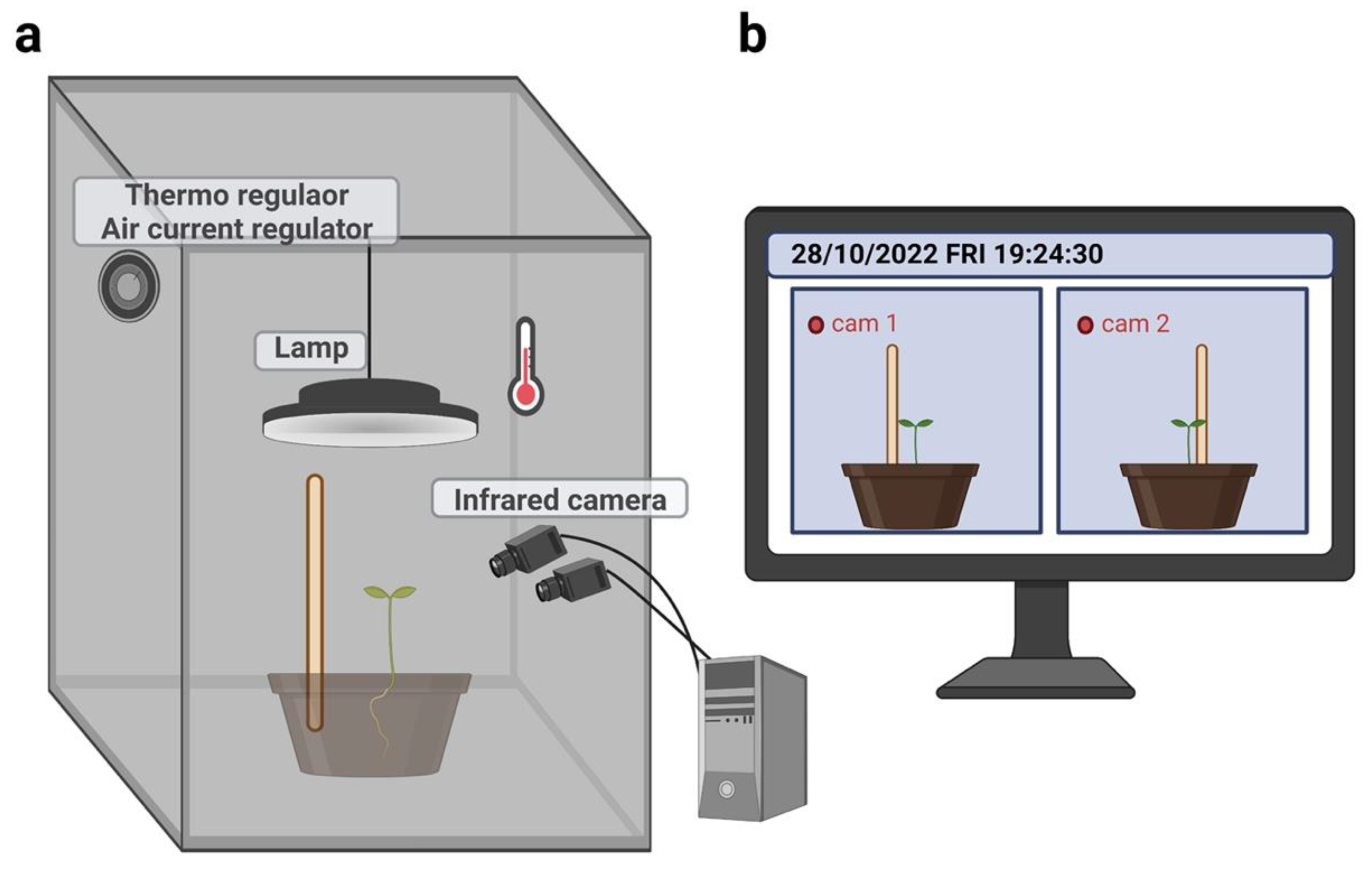
4.4. Experimental setup
4.5. Kinematic acquisition and data processing
4.6. Dependent measure
- (i)
- Number of circumnutations: the number of circumnutations performed by a plant from the time it was potted to the time it grasped the support.
- (ii)
- Circumnutation duration: the time taken by a plant to complete a single circumnutation.
- (iii)
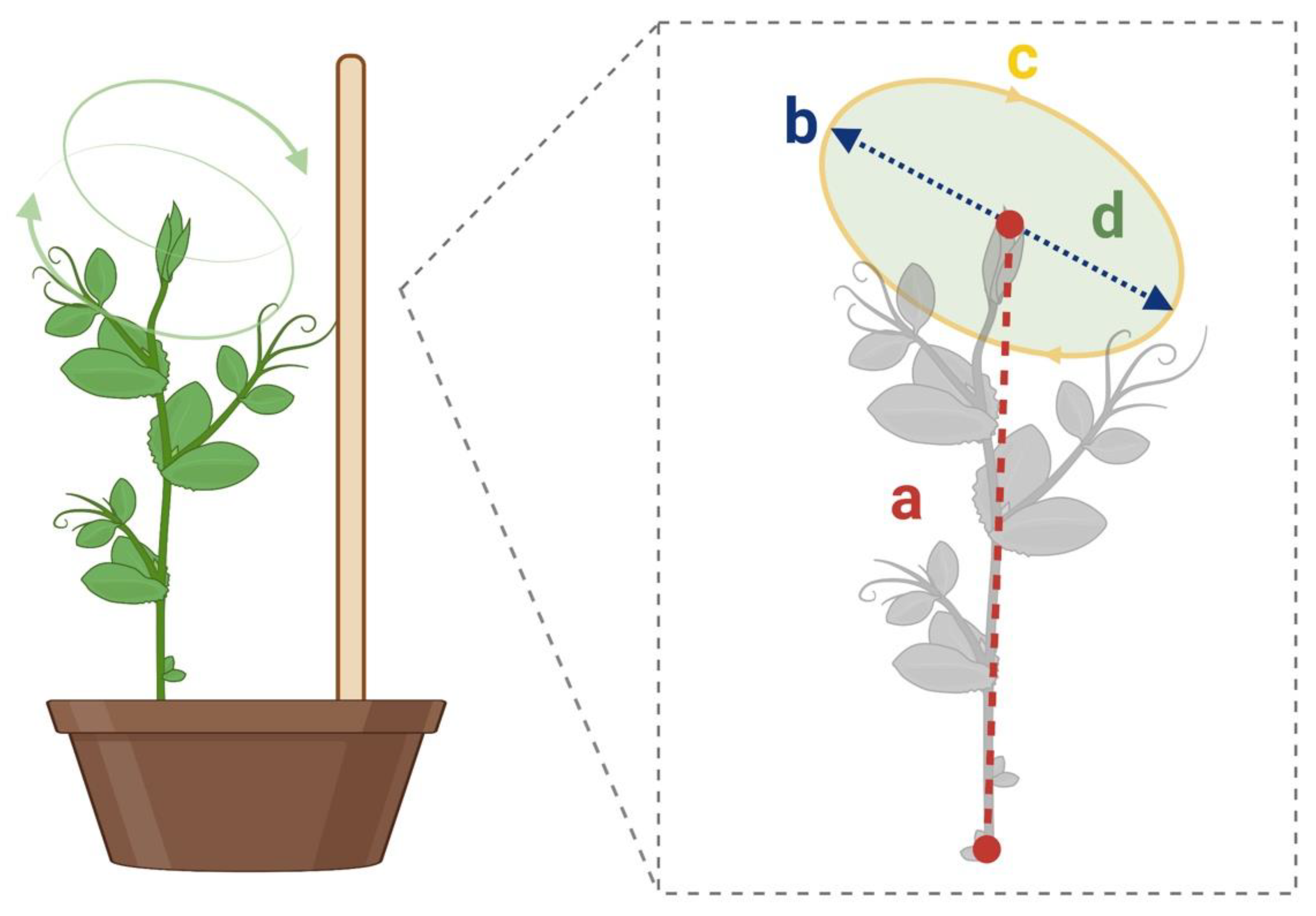
- Distance from the center of circumnutation to the origin (Figure 5. segment a): The distance between the circumnutation center and the plant origin.
- (iv)
- Length of the circumnutation major axis (Figure 5. segment b): the maximum distance between two points of the circumnutation trajectory.
- (v)
- Circumnutation length (Figure 5. segment c): the length of the overall path computed as the sum of all the Euclidean distances between subsequent points during a single circumnutation.
- (vi)
- Circumnutation area (Figure 5. segment d): the sum of pixels with a value equal to 1 obtained from the binarization of the circumnutation trajectory.
- (vii)
- Amplitude of peak velocity: values for the average of maximum velocity.
4.7. Statistical analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niklas, K.J. Climbing plants: attachment and the ascent for light. Current Biology 2011, 21, R199–R201. [Google Scholar] [CrossRef]
- Gianoli, E. The behavioural ecology of climbing plants. AoB Plants 2015, 7. [Google Scholar] [CrossRef]
- Gianoli, E.; Gonzalez-Teuber, M. Effect of support availability, mother plant genotype and maternal support environment on the twining vine Ipomoea purpurea. Plant Ecology 2005, 179, 231–235. [Google Scholar] [CrossRef]
- Darwin, C. The movements and habits of climbing plants; John Murray: London, UK, 1875. [Google Scholar]
- Ceccarini, F.; Guerra, S.; Peressotti, A.; Peressotti, F.; Bulgheroni, M.; Baccinelli, W.; Bonato, B.; Castiello, U. Speed–accuracy trade-off in plants. Psychonomic Bulletin & Review 2020, 27, 966–973. [Google Scholar]
- Ceccarini, F.; Guerra, S.; Peressotti, A.; Peressotti, F.; Bulgheroni, M.; Baccinelli, W.; Bonato, B.; Castiello, U. On-line control of movement in plants. Biochemical and Biophysical Research Communications 2020. [CrossRef]
- Guerra, S.; Peressotti, A.; Peressotti, F.; Bulgheroni, M.; Baccinelli, W.; D’Amico, E.; Gomez, A.; Massaccesi, S.; Ceccarini, F.; Castiello, U. Flexible control of movement in plants. Sci Rep 2019, 9, 16570. [Google Scholar] [CrossRef]
- Guerra, S.; Bonato, B.; Wang, Q.; Peressotti, A.; Peressotti, F.; Baccinelli, W.; Bulgheroni, M.; Castiello, U. Kinematic Evidence of Root-to-Shoot Signaling for the Coding of Support Thickness in Pea Plants. Biology 2022, 11, 405. [Google Scholar] [CrossRef]
- Castiello, U. (Re) claiming plants in comparative psychology. Journal of Comparative Psychology 2020, 135, 127–141. [Google Scholar] [CrossRef]
- Carrasco-Urra, F.; Gianoli, E. Abundance of climbing plants in a southern temperate rain forest: host tree characteristics or light availability? Journal of Vegetation Science 2009, 20, 1155–1162. [Google Scholar] [CrossRef]
- Goriely, A.; Neukirch, S. Mechanics of climbing and attachment in twining plants. Physical Review Letters 2006, 97, 184302. [Google Scholar] [CrossRef]
- Saito, K. A study on diameter-dependent support selection of the tendrils of Cayratia japonica. Sci Rep 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Putz, F.E.; Holbrook, N.M. Biomechanical studies of vines. The Biology of Vines 1992, 73–98. [Google Scholar]
- Rowe, N.P.; Isnard, S.; Gallenmüller, F.; Speck, T. 2 Diversity of Mechanical Architectures in Climbing Plants: An Ecological Perspective. Ecology and Biomechanics: A Mechanical Approach to the Ecology of Animals and Plants 2006, 35.
- Sousa-Baena, M.S.; Hernandes-Lopes, J.; Van Sluys, M.-A. Reaching the top through a tortuous path: helical growth in climbing plants. Current Opinion in Plant Biology 2021, 59, 101982. [Google Scholar] [CrossRef]
- Viviani, P.; McCollum, G. The relation between linear extent and velocity in drawing movements. Neuroscience 1983, 10, 211–218. [Google Scholar] [CrossRef]
- Cohen, I.; Huang, Y.; Chen, J.; Benesty, J.; Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson correlation coefficient. Noise Reduction in Speech Processing 2009, 1–4. [Google Scholar]
- Van Rossum, G.; Drake Jr, F.L. Python tutorial; Centrum voor Wiskunde en Informatica Amsterdam, The Netherlands: 1995; Volume 620.
- Putz, F.E. The natural history of lianas on Barro Colorado Island, Panama. Ecology 1984, 65, 1713–1724. [Google Scholar] [CrossRef]
- Karban, R. Plant behaviour and communication. Ecology Letters 2008, 11, 727–739. [Google Scholar] [CrossRef]
- Bowling, A.J.; Vaughn, K.C. Gelatinous fibers are widespread in coiling tendrils and twining vines. American Journal of Botany 2009, 96, 719–727. [Google Scholar] [CrossRef]
- Guerra, S.; Bonato, B.; Wang, Q.; Ceccarini, F.; Peressotti, A.; Peressotti, F.; Baccinelli, W.; Bulgheroni, M.; Castiello, U. The coding of object thickness in plants: When roots matter. Journal of Comparative Psychology 2021. [CrossRef]
- Sartori, L.; Camperio-Ciani, A.; Bulgheroni, M.; Castiello, U. Reach-to-grasp movements in Macaca fascicularis monkeys: the Isochrony Principle at work. Frontiers in Psychology 2013, 4, 114. [Google Scholar] [CrossRef]
- Svenson, O. Process descriptions of decision making. Organizational Behavior and Human Performance 1979, 23, 86–112. [Google Scholar] [CrossRef]
- Lunenburg, F.C. The decision making process. In Proceedings of the National Forum of Educational Administration & Supervision Journal; 2010. [Google Scholar]
- Eisenfuhr, F. Decision making. Academy of Management Review 2011, 19, 312–330. [Google Scholar]
- Kacelnik, A.; El Mouden, C. Triumphs and trials of the risk paradigm. Animal Behaviour 2013, 86, 1117–1129. [Google Scholar] [CrossRef]
- Dener, E.; Kacelnik, A.; Shemesh, H. Pea plants show risk sensitivity. Current Biology 2016, 26, 1763–1767. [Google Scholar] [CrossRef]
- Gruntman, M.; Groß, D.; Májeková, M.; Tielbörger, K. Decision-making in plants under competition. Nature Communications 2017, 8, 2235. [Google Scholar] [CrossRef]
- McNamara, J.M.; Houston, A.I. Risk-sensitive foraging: a review of the theory. Bulletin of Mathematical Biology 1992, 54, 355–378. [Google Scholar] [CrossRef]
- Schmid, B. Decision-making: are plants more rational than animals? Current Biology 2016, 26, R675–R678. [Google Scholar] [CrossRef]
- Calvo, P. The philosophy of plant neurobiology: a manifesto. Synthese 2016, 193, 1323–1343. [Google Scholar] [CrossRef]
- Souza, G.M.; Toledo, G.R.; Saraiva, G.F. Towards systemic view for plant learning: ecophysiological perspective. Memory and Learning in Plants 2018, 163–189. [Google Scholar]
- Chen, C.; Xiao, Y.-G.; Li, X.; Ni, M. Light-regulated stomatal aperture in Arabidopsis. Molecular Plant 2012, 5, 566–572. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Raschke, K. Separation and measurement of direct and indirect effects of light on stomata. Plant Physiology 1981, 68, 33–40. [Google Scholar] [CrossRef]
- Souza, G.M.; Ferreira, A.S.; Saraiva, G.F.; Toledo, G.R. Plant “electrome” can be pushed toward a self-organized critical state by external cues: Evidences from a study with soybean seedlings subject to different environmental conditions. Plant Signaling & Behavior 2017, 12, e1290040. [Google Scholar]
- Debono, M.W.; Souza, G.M. Plants as electromic plastic interfaces: A mesological approach. Progress in Biophysics and Molecular Biology 2019, 146, 123–133. [Google Scholar] [CrossRef]
- Saraiva, G.; Ferreira, A.; Souza, G. Osmotic stress decreases complexity underlying the electrophysiological dynamic in soybean. Plant Biology 2017, 19, 702–708. [Google Scholar] [CrossRef]
- Bak, P.; Tang, C.; Wiesenfeld, K. Self-organized criticality: An explanation of the 1/f noise. Physical Review Letters 1987, 59, 381. [Google Scholar] [CrossRef]
- Simonetti, V.; Bulgheroni, M.; Guerra, S.; Peressotti, A.; Peressotti, F.; Baccinelli, W.; Ceccarini, F.; Bonato, B.; Wang, Q.; Castiello, U. Can Plants Move Like Animals? A Three-Dimensional Stereovision Analysis of Movement in Plants. Animals 2021, 11, 1854. [Google Scholar] [CrossRef]
- JASP Team, JASP (Version 0.17) [Computer software], 2023.
- R Development Core Team, R: A language and environment for statistical computing, R Foundation for Satistical Computing: 2010.
- van Doorn, J.; van den Bergh, D.; Böhm, U.; Dablander, F.; Derks, K.; Draws, T.; Etz, A.; Evans, N.J.; Gronau, Q.F.; Haaf, J.M. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychonomic Bulletin & Review 2021, 28, 813–826. [Google Scholar]
- Ly, A.; Verhagen, J.; Wagenmakers, E.-J. Harold Jeffreys’s default Bayes factor hypothesis tests: Explanation, extension, and application in psychology. Journal of Mathematical Psychology 2016, 72, 19–32. [Google Scholar] [CrossRef]
- Jeffreys, H. The theory of probability; OuP Oxford: New York, NY, USA, 1998. [Google Scholar]
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| Group | Mean | SD | SE | Coefficient of variation |
Lower | Upper | |
| Number of circumnutations | DS | 24.924 | 4.247 | 0.303 | 0.170 | 24.327 | 25.521 |
| SS | 26.553 | 6.156 | 0.439 | 0.232 | 25.688 | 27.418 | |
| Circumnutation duration (min) |
DS | 66.746 | 13.190 | 0.940 | 0.198 | 64.893 | 68.600 |
| SS | 69.000 | 14.451 | 1.030 | 0.209 | 66.969 | 71.031 | |
| Distance from the circumnutation center to the origin (cm) | DS | 16.004 | 10.145 | 0.723 | 0.634 | 14.579 | 17.430 |
| SS | 28.660 | 25.146 | 1.792 | 0.877 | 25.127 | 32.194 | |
| Length of the circumnutation major axis (mm) |
DS | 91.214 | 38.929 | 2.774 | 0.427 | 85.744 | 96.684 |
| SS | 72.908 | 43.538 | 3.102 | 0.597 | 66.791 | 79.026 | |
| Circumnutation length (mm) |
DS | 243.403 | 124.957 | 8.903 | 0.513 | 225.846 | 260.961 |
| SS | 188.148 | 115.972 | 8.263 | 0.616 | 171.853 | 204.443 | |
| Circumnutation area (mm2) |
DS | 4992.504 | 4634.422 | 330.189 | 0.928 | 4341.325 | 5643.684 |
| SS | 3217.099 | 3505.097 | 249.728 | 1.090 | 2724.601 | 3709.598 | |
| Amplitude of maximum peak velocity (mm/min) |
DS | 6.541 | 5.650 | 0.403 | 0.864 | 5.748 | 7.335 |
| SS | 4.660 | 2.840 | 0.202 | 0.610 | 4.260 | 5.059 | |
| Note. DS = double-support condition; SS = single-support condition; SD = standard deviation; SE = standard error; CI = credible interval | |||||||
| BF₁₀ | W | R-hat | |
|---|---|---|---|
| Number of circumnutation | 314.656 | 14220.000 | 1.008 |
| Circumnutation duration | 0.387 | 17083.000 | 1.000 |
| Distance from the circumnutation center to the origin | 43.665 | 15057.000 | 1.007 |
| Length of the circumnutation major axis | 734.705 | 24455.000 | 1.016 |
| Circumnutation length | 980.421 | 24433.000 | 1.015 |
| Circumnutation area | 1267.886 | 24611.500 | 1.008 |
| Amplitude of maximum peak velocity | 4137.588 | 25438.000 | 1.014 |
| Note. Result based on data augmentation algorithm with 5 chains of 1000 iterations. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





