1. Introduction
In an era of medical innovation and technology, an estimated 20% of the global population are affected by nasal congestion and breathing disorders with many individuals undiagnosed or inadequately tested for nasal function and breathing. Nasal function affects overall health and contributes to pathophysiology of the respiratory system and sleep disorders that extend into somatic dysfunction and comorbid, pathological conditions. Access to interactive educational platforms as tools and guides for providers to improve their understanding of treatment options for such respiratory and sleep phenomena and the role of objective nasal measurements is not widely available and minimally applied [
1,
2].
Health information technology and digital health allows companies and providers to expand their reach in the healthcare market for analysis and personalization of patient care, specifically in the field of sleep disorders and airway measurement technology [
1]. Access to digital tools measuring airway function and patency permits providers to holistically view patient health and opportunities to improve outcomes while enhancing efficiency in the plan of care [
2]. Interactive computer programs with software functionalities and cloud-based informatics provide necessary access to research and develop algorithms guiding the healthcare provider (HCP) in understanding the particular data produced by a medical device. The data output and calculations within the software educate the HCP by guiding them through interpretations or analysis of a clinical test by providing certain types of limited clinical decision support [
3]. Growth in digital health required clarity and changes by the U.S. Food and Drug Administration (FDA) and U.S. Department of Health and Human Services (HHS) [
3]. They provided an amended legislative report on digital health in 2016 stating:
December 13, 2016 (Pub. L. 114-255), amended the Federal Food, Drug, and Cosmetic Act (FD&C Act) to exclude certain software functions from the definition of device under section 201(h) of the FD&C Act (21 U.S.C. 321(h)). These software functions are specified in section 520(o)(1) of the FD&C Act and the intended uses of such software functions can be summarize as follows: (1) administrative support of a health care facility; (2) maintaining or encouraging a healthy lifestyle and unrelated to the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition; (3) serving as electronic patient records when not intended to interpret or analyze patient records; (4) transferring, storing, converting formats, or displaying data; or (5) unless interpreting or analyzing a clinical test or other device data, providing certain types of limited clinical decision support to a healthcare provider [3]. The prevalence of nasal flow limitations and breathing disorders affects more than one billion people among the global population [
4]. A critical aspect of nasal airflow is the correct correlation the geometric measurements to the function of the nasal passages as the first port of entry into the respiratory system. The pathophysiological effect of dysfunctional breathing presents as a risk for obstructive sleep apnea and other disease comorbidities from birth through adulthood [
4]. Additional risk factors include obesity and an aging population. The World Health Organization (WHO) reported the number of people affected by obesity doubled over the past five years further exacerbating the diagnosis of breathing disorders resulting in obstructive sleep apnea (OSA) [
5].
Very few publications have proposed or defended the increasingly important need to measure nasal flow limitations in the clinical setting, yet more than two thousand publications have discussed nasal anatomy, nasal function, the nasal cycle, nasal resistance, and the objective measurement and testing options in research and clinical practice. Objective nasal measurements are an important part of clinical practice and could prove useful in early detection of disease pathology and progression before a sleep study [6, 7]. However, the learning curve understanding the technology data and what it means in application can take time and extensive training from clinical experts. Current interpretation training and education on the data output has been minimal, incongruent, or self-taught, at best.
The current FDA approved objective diagnostic tests for nasal airway measurements are rhinomanometry, peak nasal inspiratory flow (PNIF), and acoustic rhinometry, but interpretive software or educational websites with software functionalities are lacking. Unlike Europe, countries in South Asia, the United Kingdom, and Australia, the allergists and ENT specialists in the United States infrequently use acoustic rhinometry and rhinomanometry and employ mostly for research trials [
6]. Since 2017, and the upgrade in technology of the NR6 Rhinomanometer (GM Instruments, Ltd.) to 4-phase rhinomanometry, adoption of non-invasive, objective nasal measurements in the clinical workflow during pre, mid, and post treatment, is gaining recognition in dental and medical specialties. Because it functionally evaluates nasal aerodynamics and the transnasal pressure change as a biomarker, rhinomanometry may be preferable for evaluating nasal flow and function, while acoustic rhinometry is more helpful in locating the position and severity of nasal obstruction [
7]. PNIF, like rhinomanometry and acoustic rhinometry, is a simple, dependable, reproducible, cost effective, widely available objective tool in measuring nasal patency, but lacks a pressure sensor to measure breathing across a pressure gradient [
7].
Lacking a methodological approach to understanding airway measurement interpretation, DAFNE Scoring System was created. By identifying the problem, the DAFNE Scoring System was written as an algorithm from published research, computed device output, and formulas to calculate, guide, and train the HCP in interpretation parameters of nasal aerodynamic values for better breathing, collaboration, and patient outcomes. Because there is no established algorithm nor clinical approach to interpreting airway measurements, a systematic literature review was required to form, identify, and describe the parameters of the three nasal measurement technologies incorporated into a software algorithm.
Once the algorithm was created, it was necessary to validate the performance and ability to score the severity of nasal breathing and the correct recommendations from evidenced based medicine from a literature review. The purpose of validating the performance of DAFNE was to assure that this algorithm will work correctly and independently of the issues concerning the programming language. Once the validity of the tool was recognized it was published on the internet and website interaction was implemented.
2. Materials and Methods
Details of the proprietary algorithm are complex and have been filed under pending patent #63/347,519 and 63/381,556, and Registration Number TXu-2-311-590 with a publication date of 2021. The DAFNE Score platform can be accessed at
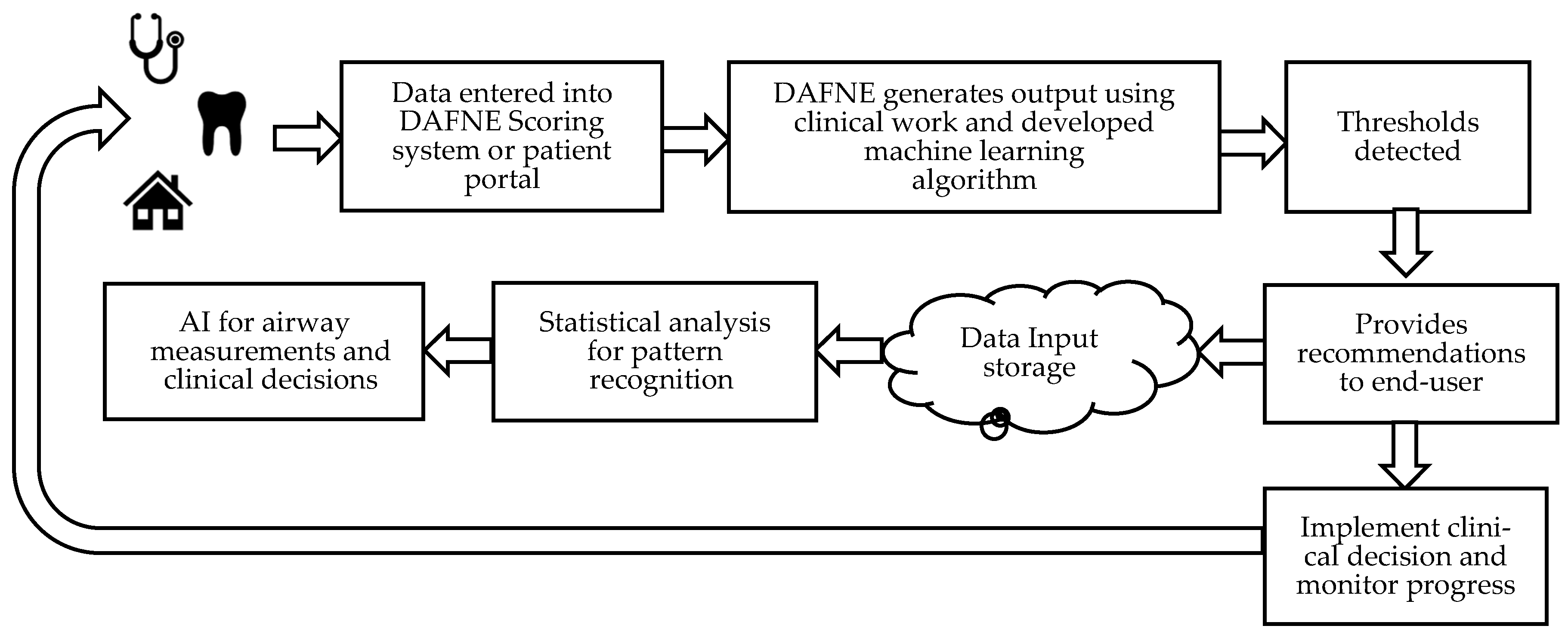
https://www.dafnescore.com/ . The performance and applicability of using DAFNE nasal measurement software algorithm was incorporated into an accessible framework model made of the components in
Figure 1. To provide a user-friendly access, the DAFNE platform consists of the following steps to guide a novel user or the experienced clinician:
- (i)
obtain clinical diagnostics and nasal airway measurements
- (ii)
the user enters the patient demographic information and test results
- (iii)
DAFNE generates the output based on the nasal geometry, nasal flow, or transnasal pressure change which determines the severity of nasal airway disease progression measured as RAW = where
- (iv)
testing thresholds are detected
- (v)
recommendations are produced and viewed
- (vi)
suggestions for collaborative care with other HCPs
- (viii)
nominal data is stored in the cloud for analysis of pattern recognition
- (ix)
AI in airway measurements for clinical decisions
- (x)
treatment is monitored, or annual measurements are obtained
The DAFNE Patient Portal
https://www.dafnescore.com/patient was designed as a proprietary tool to increase treatment compliance, hold patients accountable for their healthcare and plan of treatment, keep track of their airway measurements using the PNIF, educate the patient about their disease state and progression/improvement, and allow providers a new way to measure and monitor nasal airway health from a distance with telemedicine. The patient portal is the basic framework model consisting of steps 1-4 of the practitioner platform: the patient enters their breathing measurements, DAFNE calculates the average, and then determines if the measurement is normal or abnormal based on patient characteristics. The patient reports the measurement to their provider.
We also evaluated the proposal of using portions of DAFNE as a tool that can be correlated to sleep study parameters that determine the severity of sleep apnea such as apnea/hypopnea index (AHI), oxygen desaturation index (ODI), and respiratory disturbance index (RDI) based on the physics oriented numerical simulation from researched parameters. By properly identifying the severity of flow limitations found in sleep apnea from the data output, the results can be quickly cross referenced and categorized into 1 of 5 categories of disease progression found in DAFNE. The benefits of using this technique are: 1) quickly identifying patients predisposed to sleep apnea and nasal breathing dysfunction based on the test result entered into DAFNE and the level of disease progression, 2) provide a more accurate picture of nasal air flow limitation over time versus what a sleep study does in 1-3 nights, 3) provides a more accurate depiction of oxygenation versus pulse oximetry which can be affected by skin pigmentation and melanin, 4) adopt an approach that what Cone Beam Computed Tomography (CBCT) does without higher costs and radiation exposure, 5) reduce complex hospital visits and additional costs to the healthcare system, and 6) incorporate DAFNE Patient Portal into the plan of care for monitoring in a telemedicine platform.
2.1. Data Search
In order to create the DAFNE algorithm, a search was developed to identify validated measurement parameters published for the three technologies used in DAFNE. Electronic searches of PubMed, MEDLINE, EMBASE, Cochrane Library, and Scopus were performed to identify publications between 1988-2022 validating nasal function and structure measurement parameters to develop an algorithm for the DAFNE Scoring System [
8]. A checklist for this review with search terms included and related to “nasal function parameters,” “validation,” “nasal measurement software,” “nasal resistance,” “rhinomanometry,” “acoustic rhinometry,” “peak nasal inspiratory flow meter,” “practice patterns,” and “objective outcome measures.” Synonyms and related terms were connected with the Boolean operator “OR” and combined with “AND.” Medline (OVID) and EMBASE were searched for medical literature.
2.2. Data Extraction Data Extraction Process and Quality Assessment
Both authors independently reviewed the titles and abstracts that met the inclusion criteria and qualified the relevant measurement data for rhinomanometry, PNIF, and acoustic rhinometer for data extraction.
2.3. Approach Algorithm Development
The authors used criteria by selecting titles and abstracts that discussed the outcome variables nasal resistance measured in Pa/cm
3/s, nasal airflow measured in L/min, percentage of facial growth and development, and cross-sectional nasal volume measured in cm
2. From a literature review of 744 peer reviewed articles, twenty [9-30] were included in the algorithm (
Appendix A). Based on the literature search, we were able to identify seven variables necessary to make clinical decisions to improve nasal patency such as nasal flow limitations, patient characteristics, nasal resistance, nasal volume, and the number of respiratory cycles detected during a nasal function test.
The DAFNE algorithm was created using 73 measurement data sets of parameters from the research values dictating the therapeutic options and interprofessional collaboration necessary to solve complex airway and breathing problems. The platform language answered internal questions with mathematic and airway measurement input and logic that determined the output recommendations from the nominal entry. The combined algorithm model was able to analyze the measurements with a global health approach which will enhance the application of AI in airway measurement technology. After the algorithm is used over time, data collection will find correlations leading to improved care with identified normative values through coding instructions versus expert systems when creating prediction models determining what treatment protocols will work.
2.4. Technology Review Used in DAFNE
The three technologies used in creating DAFNE Scoring System were PNIF, acoustic rhinometry, and rhinomanometry (
Figure 2.). Rhinomanometry is a form of non-invasive mamometry measuring the transnasal pressure change as a biomarker indicating the amount of resistance in the nasal passages during active breathing. The functional measurement is a key indicator for sleep and and breathing disorders that takes 15 seconds to conduct [9-13]. Since 2015, the transition from the obsolete form of rhinomanometry to the current 4-phase rhinomanometry allows for greater detection in disease identification and progression found in the pathophysiology of nasal disease and breathing disorders [9-13]. Acoustic rhinometry is a dignostic structural measurement of the nasal passages using acoustic reflections produced through a sound tube that takes approximately 10 seconds with the A1 system (GM Instruments, Ltd.) and 30 seconds or more with other competitor systems [
14]. The PNIF is an inexpensive, rapid test to objectively measure nasal air flow during maximum inspiration [24-26].
Table 1 presents an overview and comparison of the nasal measurement technologies used in the algorithm.
2.5. Measurement Values of Rhinomanometry, Acoustic Rhinometry, and Peak Nasal Inspiratory Flow Meter (PNIF)
In the most basic form, DAFNE Scoring System evaluates the maximum mean testing score for each instrument and describes how to process the data into the treatment care plan. We found consistency among the measurement variables discussing the conclusive amount of decrease in nasal pressures, increase in cross-sectional measurements, increase in nasal volume, and increases in inspiration measured in liters per minute among gender differences, age, and height used in the context of the scoring platform [10,11,14-16,24-26,28,34]. The validated measurements and biomarkers guided the formulation of an algorithm for DAFNE Scoring System from the login page, to entering the measurement value, to understanding the data to suggestions for interprofessional collaborative care. Through a multicenter beta test, the validity and functionality of the scoring system was demonstrated and proven for future AI and machine learning for therapy predictability and outcomes.
The subsequent findings of the algorithm beta test showed how it may substitute the industry standard AHI in sleep studies by identifying patients at high risk with an abnormal score. Upper airway resistance (UARS) in the pathology of nasal disease is a precursor to OSA and correlates to the distribution of hypopneas and apneas measured in OSA [
18,
21]. Detection and scoring enhance early identification of airway disorders, especially in children [
23]. The abnormal score identifies disease progression with the classification flow limitations, Class 1-5, with < 60 ccm/s as the worst indicating severe sleep apnea/hypoventilation, or red on the y-axis of the rhinomanometry graph. The benefits of substituting the AHI with the DAFNE Score and classification of nasal flow limitation is a single measurement that is noninvasive, completed faster, more cost effective, and detects nasal function not detected by a sleep study [
18].
2.6. Beta-Testing
To ensure efficiency and performance quality of DAFNE, a series of steps were used in the two-phase project to determine the reliability of the calculations, organization, and functionality of the algorithmic platform as a useful tool in clinical practice and research. In Phase 1, a review of literature was necessary to obtain the normative values and ranges for the three techologies and treatment options used in DAFNE. Once measurement values and data were collected, the authors wrote the algorithm and framework. This is the first attempt in the medical industry to create a platform for machine learning and artificial intelligence with nasal airway measurement devices. The beta testing involved a group of five physicians who agreed to serve as reviewers of DAFNE, and how well they were able to follow the methods that drove therapuetic choices and disease improvement. After the testing, open ended comments and notes were reviewed for improvements. Collectively, we were able to establish the functionality and validation of the scoring system to leverage DAFNE and nasal airway technologies into clinical practice and research.
3. Results
3.1. Validation of Web-based DAFNE Scoring Software
Throughout the review of the literature, we were able to identify key parameters and values to incorporate into the algorithm of the DAFNE Scoring System that would define what is a normal or abnormal reading. Furthermore, we were able to design an educational tool based on the measuring technology with research findings of sensitivity and specificity as high as 96% [9-36]. The platform will assist health care providers from novel users to the most experienced around the globe on the designated values and parameters with decongestion, skeletal formation, nasal cycling, and suggestions for collaboration among other healthcare professionals [9-36].
3.2. Literature Data
To support the DAFNE scoring algorithm in clinical decision making, the literature showed a clear association between the measurement parameters and the distribution of apnea and hypopnea indices, nasal resistance, and symptoms at presentation among adults and children with sleep related disorders and breathing disorders, specifically in children with adenotonsillar hypertrophy [19, 23]. The association of skeletal growth and development were found to play a significant role in snoring and OSA compared to healthy children [
23]. The relevancy of these findings dictated the use of novel approaches used in the diagnosis of sleep and breathing disorders in adults and children and presented a [
20]. This translates the device testing and data output as clinically comparable and compatible to the outcome of a diagnosis from a sleep study or the development of a diagnosis OSA prior to the initiation of a sleep study [11, 12, 18, 20, 21, and 23]. The benefit of the device data proposes obtaining a baseline test of nasal resistance when a sleep study may not be an option or as an adjunct to the sleep study for comparative reasons pre, mid, and post treatment.
These findings may catapult the redirection of biometrics used in diagnosing OSA and other breathing disorders, although the pattern of recognized behavior in the sleep testing industry over the past fifty years will not be bi-directional in acceptance among medical societies. This calls for additional research to replace the AHI and set the benchmark for new values considered in the progression of airway disease.
3.3. Ethnicity, Gender, and Age
Gender and age are two important patient characteristics used in DAFNE as part of treatment selection and AI in the pediatric and adult populations. Jones et al. (1994) found cephalometric development in kids and rhinomanometric measurements to be statistically significant specifically when measuring mandibular prognathism that also correlated to intranasal pressures and mean resistance [33, 34]. Juliet et al. concluded the lower age limit for testing rhinomanometry at five years old with the reference pressures with the most appropriate pressures at 75 and 100 Pascals [
35]. Nasal volume and mean cross-sectional area at the three measurement points in a congested or non-congested state are also incorporate into the DAFNE algorithm [11, 14, 18, 24, 26].
3.4. Indications, Treatment Modalities, and Outcomes
DAFNE Scoring System is able to guide the provider through proper patient identification and stratification by the cut off measurement for optimal CPAP titration, specifically in the mean resistance that indicates patient selection and tolerability of CPAP resulting in treatment non-compliance and failure, and the threshold for oral appliance therapy measuring the transnasal pressure at the proper pressure gradient [12, 18, 20, 21, and 28].
The DAFNE algorithm incorporated various measurement thresholds surgical intervention and the anatomical expansion that alter the nasal floor, nasal airflow velocity, and the downstream reduction of the negative pressure in the pharyngeal area, which proportionately affects the collapsibility in obstructive sleep apnea [21, 22, and 30]. It was also discovered that the AHI correlates well with nasal flow and velocity reductions [22, 36].
4. Discussion
As objective nasal measurements make their way into the clinical workspace, measurement data interpretation and collaboration remain one of the biggest challenges. In a time of technological advancements such as AI, software and clinical algorithms guide healthcare providers through automation in the decision process using objective data and measurements as a diagnostic tool for breathing disorders as a result of nasal obstruction. The limited amount of existing healthcare services related to nasal measurements are either not widely available or developed for collaborative care allowing the patient to choose a treatment path.
Healthcare decision makers in search of reliable information that compares health interventions increasingly turn to a logical structure and clear definition of a diagnostic approach or treatment plan for the best summary of the outcome evidence. Recognizing reviews for writing algorithms and AI allow systems to identify, select, assess, and interpret the pattern recognition, and can help clarify what is known and not known about the potential benefits and harms of drugs, devices, and other healthcare services.
The purpose of this contribution is to describe a way to improve the evaluation of normative values tested from various airway measurement devices through an algorithmic scoring system that evolves into AI. The scoring system meets an unmet need in an algorithm software publication for education and machine learning capabilities for airway measurements from previous validated output data. Due to the lack of an algorithm publication and an increased use of the airway measurement technology, the scoring system was created in order to improve implementation with the data output. Validation of any web-based software platform must answer the simple question of “Are we building the right technology at the right time for the right audience to better manage patient care?” Will this give them a better clinical understanding of a particular technology, the value of the data output, and how to incorporate it into their workflow?
This first of its kind web-based companion software is composed of statistically significant outcomes in research measured to decrease nasal resistance and increase nasal airflow, both with and without nasal decongestant, surgeries, and expansion. Ren et al [
9] explains how the reference values are useful in choosing and assessing a therapeutic intervention for nasal congestion or OSA, and Merkel et al. [
13] explained the normative values and parameters in nasal disease improvement. The previous research reviewed and tested the effects of maxillary expansion and the role it plays in nasal function measurements, too.
The tested and retested parameters are the foundation of the algorithm for DAFNE
https://www.dafnescore.com/ which further validate the existence of the platform for clinical care and education. The scoring system was specifically developed to accommodate the demands of telemedicine, telehealth, digital, electronic healthcare platforms, and educational needs for objective airway measurements requested at an international level. HCPs continuously use data-enabled infrastructures to support policy and planning, public health, and personalization of care.
Efficiency of the physiology and pathophysiology of respiratory and nasal function has become a topic of importance in the medical and dental communities. The quantifiable, objective measurement data has been delivered to the forefront of the conversation through clinical practice, research, and societal panel discussions. An identifiable need for training and guidance to better understand nasal function data output has been uncovered yet absent in the marketplace. Feeling intimidated by the technology and data output, many practitioners do not perform the non-invasive objective tests. The data output has been evaluated and shown which medical modalities are approached at different measurement levels. Within the new era of AI and machine learning capabilities of airway measurements, the DAFNE Scoring System answers the unmet need.
The ease of use and the multiple access portals from a computer, notepad, or cell phone, allow HCPs to enter testing data after accepting the terms and conditions of the platform. The interface will direct the user to login with a username and password. They will tap on the technology tab, enter the population category, enter their mean resistance values, flow measurements in L/min, or cross-sectional values. The calculation or algorithm, based on clinical research and studies, will lead them to understand what that value means and what the next steps would be in order to help the patient breathe better. As part of the user agreement, it is noted that unidentifiable individual patient data protected under HIPAA will be accessible for larger research projects. The DAFNE Scoring System demonstrated the capabilities as a large data portal for further research in technology data and patient outcomes to discuss results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5. Conclusions
DAFNE Scoring System and the developed algorithm is able to detect the severity of nasal breathing disorders and determine airway treatment options in the clinical workflow. The validated web-based software companion platform is able to guide a healthcare practitioner who measures nasal obstruction and uses objective, non- invasive assessment tools in their approach to diagnosis airway and breathing disorders. By categorizing the airflow limitations based on disease progression, DAFNE is able to identify the severity of sleep apneas for improved screening prior to or as an adjunct to a sleep study potential replacing the AHI as the diagnostic value. DAFNE is available for global access and can be routinely used in clinical practice or research to further collaborate with other healthcare providers and improve patient outcomes with the proper treatment plans.
As clinical and research data improves, current measurement standards are refined, and new innovation becomes available, the algorithm measurement parameters in the software functions will be amended into a next version. The proprietary aspects of DAFNE are available to medical and companies for white labeling specific to their product(s) and market(s) for more detailed AI and ML.
6. Patents
All aspects of the DAFNE Score, DAFNE Patient Portal, and DAFNE Scoring System reported in this manuscript are proprietary, copyrighted, and patent pending works in the original form and licensed white labeling under U.S. Code: Title 17.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. This study protocol did not require a review by an Institutional Review Board (IRB) because the study did not involve human subjects. The unit of analyses were data from published articles and did not meet the definition of human subjects.
Informed Consent Statement
This study protocol did not require a review by an Institutional Review Board (IRB) because the study did not involve human subjects. The unit of analyses were articles from databases.
Data Availability Statement
The databases generated during or analyzed during the study are available from the corresponding author upon reasonable request.
Acknowledgments
Thank you to Justin Collins at Levitate Creative for his tireless support, efforts, and talent in the web-based software development and calculation methods of DAFNE Scoring System.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Literature Review of Parametric Measurement Data
| Publication |
Year |
Population |
Conclusions |
| Ren et al.9
|
2018 |
704 |
Reference values may be useful in evaluating nasal function and choosing and assessing efficacy of therapy for nasal congestion |
| Gammert et al.10
|
1988 |
46 |
Rhinomanometry is repeatable. Designated values for upper and lower values with decongestion |
| Janosević et al. 11
|
2009 |
108 |
Information on total nasal resistance normal values in healthy adult population important for computerized rhinomanometry |
| Inoue et al.12
|
2019 |
711 |
Nasal disease and nasal parameters are key factors for early CPAP therapy |
| Merkle et al.13
|
2014 |
38 |
Normative values for adults |
| Krzych-Fałta et al.14 |
2022 |
583 |
Acoustic rhinometry parameters depending on age and sex-component of standardization in nasal provocation test |
| Laine-Alava et al.15
|
2018 |
|
Guideline Values for Minimum Nasal Cross-Sectional Area in Children |
| Strasszek et al.16 |
2008 |
256 |
Presented material will facilitate the interpretation and evaluation of future and present epidemiologic studies based on AR in children. |
| Calvo-Henriques et al.17 |
2020 |
257 |
Impact of maxillary expansion on nasal breathing and resistance in adults |
| Hueto et al18. |
2016 |
38 |
Relationship between nasal resistance and continuous positive airway pressure |
| Laine-Alava et al.19
|
2016 |
115 |
Upper airway resistance during growth in children |
| Calvo-Henriques et al.20
|
2022 |
291 |
Recumbent position affects nasal resistance |
| Hoel et al.21
|
2020 |
126 |
Impact of nasal resistance and the distribution of apneas and hypopneas in OSA |
| Yoon et al.22
|
2018 |
20 |
Expansion of the nasal floor is associated with reducing air flow velocity and OSA |
| Rizzi et al.23
|
2002 |
73 |
Nasal resistance is useful in identifying OSA in children |
| Ottaviano et al.24
|
2012 |
109 |
Peak nasal values in adults |
| Ottaviano wt al.25 |
2016 |
- |
Review of nasal measurements |
| Prescott et al.26 |
1995 |
102 |
Peak nasal values in children infant to 8 years old |
| Calvo-Henriquez et al.27
|
2020 |
301 |
The role of pediatric maxillary expansion on nasal breathing in children |
| Hsu et al.28 |
2020 |
43 |
Rhinomanometry predicting CPAP failure |
References
- The Lancet Digital Health. Digital technologies: A new determinant of health. Lancet Digit Health 2021, 3, e684. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Anderson, M.; Albala, S.; et al. Health information technology and digital innovation for national learning health and care systems. Lancet Digit Health 2021, 3, e383–e396. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Center for Devices and Radiological Health. (2020, November). Report On Risks And Benefits To Health of Non-Device Software Functions. November 2020. Available online: https://www.fda.gov/media/143795/download (accessed on 13 July 2022).
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690–702. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. 9 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 July 2022).
- Krouse, J.; Lund, V.; Fokkens, W.; Meltzer, E.O. Diagnostic strategies in nasal congestion. Int. J. Gen. Med. 2010, 3, 59–67. [Google Scholar] [CrossRef]
- Ta, N.H.; Gao, J.; Philpott, C. A systematic review to examine the relationship between objective and patient-reported outcome measures in sinonasal disorders: Recommendations for use in research and clinical practice. Int. Forum Allergy Rhinol. 2021, 11, 910–923. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, L.; Duan, S.; Zhang, W.; Zhang, Y. Nasal airflow resistance measured by rhinomanometry in a healthy population of China. Int. Forum Allergy Rhinol. 2018, 8, 1308–1314. [Google Scholar] [CrossRef]
- Gammert, C.; Hampl, K.; Herrmann, P. Beitrag zu den Normwerten in der Rhinomanometrie [Normal values in rhinomanometry]. HNO. 1988, 36, 399–405. [Google Scholar]
- Janosević, L.; Dotlić, J.; Janosević, S.; Dudvarski, Z.; Milovanović, A.; Pendjer, I. Computerized rhinomanometry: A study of total nasal resistance normal values. Acta Chir. Iugosl. 2009, 56, 51–54. [Google Scholar] [CrossRef]
- Inoue, A.; Chiba, S.; Matsuura, K.; Osafune, H.; Capasso, R.; Wada, K. Nasal function and CPAP compliance. Auris Nasus Larynx 2019, 46, 548–558. [Google Scholar] [CrossRef]
- Merkle, J.; Kohlhas, L.; Zadoyan, G.; Mösges, R.; Hellmich, M. Rhinomanometric reference intervals for normal total nasal airflow resistance. Rhinology 2014, 52, 292–299. [Google Scholar] [CrossRef]
- Krzych-Fałta, E.; Szczęsnowicz-Dąbrowska, P.; Samoliński, B.; Grzanka, A.; Wojas, O. The normal ranges of selected acoustic rhinometry parameters depending on age and sex-component of standarization in nasal provocation test. Adv. Dermatol. Allergol. 2022; 39, 171–181. [Google Scholar]
- Laine-Alava, M.T.; Murtolahti, S.; Crouse, U.K.; Warren, D.W. Guideline Values for Minimum Nasal Cross-Sectional Area in Children. Cleft Palate Craniofac. J. 2018, 55, 1043–1050. [Google Scholar] [CrossRef]
- Straszek, S.P.; Moeller, A.; Hall, G.L.; Zhang, G.; Stick, S.M.; Franklin, P.J. Reference values for acoustic rhinometry in children from 4 to 13 years old. Am. J. Rhinol. 2008, 22, 285–291. [Google Scholar] [CrossRef]
- Calvo-Henriquez, C.; Capasso, R.; Chiesa-Estomba, C.; et al. The role of pediatric maxillary expansion on nasal breathing. A systematic review and metanalysis. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110139. [Google Scholar] [CrossRef]
- Hueto, J.; Santaolalla, F.; Sanchez-Del-Rey, A.; Martinez-Ibargüen, A. Usefulness of rhinomanometry in the identification and treatment of patients with obstructive sleep apnoea: An algorithm for predicting the relationship between nasal resistance and continuous positive airway pressure. a retrospective study. Clin. Otolaryngol. 2016, 41, 750–757. [Google Scholar] [CrossRef]
- Laine-Alava, M.T.; Murtolahti, S.; Crouse, U.K.; Warren, D.W. Upper airway resistance during growth: A longitudinal study of children from 8 to 17 years of age. Angle Orthod. 2016, 86, 610–616. [Google Scholar] [CrossRef]
- Calvo-Henríquez, C.; Chiesa-Estomba, C.; Lechien, J.R.; et al. The Recumbent Position Affects Nasal Resistance: A Systematic Review and Meta-Analysis. Laryngoscope 2022, 132, 6–16. [Google Scholar] [CrossRef]
- Hoel, H.C.; Kvinnesland, K.; Berg, S. Impact of nasal resistance on the distribution of apneas and hypopneas in obstructive sleep apnea. Sleep Med. 2020, 71, 83–88. [Google Scholar] [CrossRef]
- Yoon, A.; Guilleminault, C.; Zaghi, S.; Liu, S.Y. Distraction Osteogenesis Maxillary Expansion (DOME) for adult obstructive sleep apnea patients with narrow maxilla and nasal floor. Sleep Med. 2020, 65, 172–176. [Google Scholar] [CrossRef]
- Rizzi, M.; Onorato, J.; Andreoli, A.; et al. Nasal resistances are useful in identifying children with severe obstructive sleep apnea before polysomnography. Int. J. Pediatr. Otorhinolaryngol. 2002, 65, 7–13. [Google Scholar] [CrossRef]
- Ottaviano, G.; Scadding, G.K.; Scarpa, B.; Accordi, D.; Staffieri, A.; Lund, V.J. Unilateral peak nasal inspiratory flow, normal values in adult population. Rhinology 2012, 50, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, G.; Fokkens, W.J. Measurements of nasal airflow and patency: A critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy 2016, 71, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.A.J.; Prescott, K.E. Peak Nasal Inspiratory flow measurement: An investigation in children. Int. J. Pediatr. Otorhinolaryngol. 1995, 32, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Henriquez, C.; Capasso, R.; Chiesa-Estomba, C.; et al. The role of pediatric maxillary expansion on nasal breathing. A systematic review and metanalysis. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110139. [Google Scholar] [CrossRef]
- Hsu, Y.B.; Liu, S.Y.; Lan, M.Y.; Huang, Y.C.; Tzeng, I.S.; Lan, M.C. Role of rhinomanometry in the prediction of therapeutic positive airway pressure for obstructive sleep apnea. Respir. Res. 2020, 21, 115. [Google Scholar] [CrossRef]
- Vogt, K.; Jalowayski, A.A.; Althaus, W.; et al. 4-Phase-Rhinomanometry (4PR)--basics and practice 2010. Rhinol. Suppl. 2010, 21, 1–50. [Google Scholar]
- Vogt, K.; Wernecke, K.D.; Behrbohm, H. Four-phase rhinomanometry: A multicentric retrospective analysis of 36,563 clinical measurements. Eur. Arch. Otorhinolaryngol. 2016, 273, 1185–1198. [Google Scholar] [CrossRef]
- Huang, Y.S.; Guilleminault, C. Pediatric obstructive sleep apnea and the critical role of oral-facial growth: Evidences. Front. Neurol. 2013, 3, 184. [Google Scholar] [CrossRef]
- Harvold, E.P.; Tomer, B.S.; Vargervik, K.; Chierici, G. Primate experiments on oral respiration. Am. J. Orthod. 1981, 79, 359–372. [Google Scholar] [CrossRef]
- Gong, X.; Li, W.; Gao, X. Effects of Craniofacial Morphology on Nasal Respiratory Function and Upper Airway Morphology. J. Craniofacial Surg. 2018, 29, 1717–1722. [Google Scholar] [CrossRef]
- Jones, A.G.; Bhatia, S. A study of nasal respiratory resistance and craniofacial dimensions in white and West Indian black children. Am. J. Orthod. Dentofac. Orthop. 1994, 106, 34–39. [Google Scholar] [CrossRef]
- Juliá, J.C.; Burchés, M.E.; Martorell, A. Active anterior rhinomanometry in paediatrics. Normality criteria. Allergol. Immunopathol. (Madr). 2011, 39, 342–346. [Google Scholar] [CrossRef]
- Iwasaki, T.; Yoon, A.; Guilleminault, C.; Yamasaki, Y.; Liu, S.Y. How does distraction osteogenesis maxillary expansion (DOME) reduce severity of obstructive sleep apnea? Sleep Breath. 2020, 24, 287–296. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).