1. Introduction
Blood gas measurement is a monitoring or diagnostic test that is used to determine the levels of partial pressures of oxygen (pO2) and carbon dioxide (pCO2) circulating in the blood [
1]. These values can be used to calculate several biomedical parameters including bicarbonate levels and electrolytes [
2,
3]. In humans, pO2 and pCO2 can be measured either by invasive or non-invasive techniques. Invasive methods involve obtaining a sample of blood directly from an artery, such as the radial artery in the wrist, while non-invasive methods, primarily, use transcutaneous devices to measure the oxygen and carbon dioxide levels via skin [
4]. The arterial blood gas (ABG) requires specialized devices and expertise to analyze the results. In contrast, transcutaneous non-invasive methods do not involve withdrawal of blood sample and use sensors that are placed on the skin to measure the amount of oxygen and carbon dioxide in the blood. In addition, capnography is a non-invasive method that measures carbon dioxide in the exhaled air. The agreement between gas level in the exhaled air and ABG was high [
5]. Blood gas measurements can be used to assess the acid-base balance and to detect respiratory or metabolic disorders of the patient, especially, in emergency situations [
6]. The results of the test are used to determine the oxygenation status and to guide treatment decisions, such as the use of oxygen therapy, mechanical ventilation, or pharmacological interventions.
Transcutaneous devices employ sensors which are placed on the skin (e.g., arms, forehead, earlobe) to measure pO2 and pCO2 levels. Interestingly, the technology in the sensors for transcutaneous devices are fast evolving. For example, oxygen diffusion and pH-sensitive changes in voltage are used in transcutaneous devices [
7]. As oxygen diffuses through the membrane, it causes a change in the LED's light intensity which is then detected by the photodiode. The transcutaneous pO2 levels are measured using the principle of oxygen diffusion where the sensor is made up of a membrane that is permeable to oxygen and has a light-emitting diode (LED) on one side and a photodiode on the other side [
4,
8]. Similarly, transcutaneous pCO2 measurement uses the principle of pH changes caused by the presence of CO2. The sensor is composed of a pH-sensitive electrode which changes its voltage as per the skin pH and this voltage is then converted to pCO2 [
4]. These sensors subsequently utilize this information to calculate the amount of O2 or CO2 in the blood.
The use of algorithms to calculate different blood values from pO2 and pCO2 is a common approach in blood gas measurements. Mathematical equations are used to calculate and convert arterial or transcutaneous oxygen and carbon dioxide measurements into electrolyte and mineral levels. One example of an algorithm used for this purpose is the Stewart approach which includes pCO2, bicarbonate level and pH in the equation [
9]. Similarly, base excess (BE) algorithm derived from Siggaard-Andersen equation involves pCO2 and pH values to calculate the metabolic component of the acid-base balance in the blood and detect metabolic acidosis or alkalosis [
10,
11]. In Fick’s principle is employed to derive oxygen content in the blood using pO2 and hemoglobin values [
12]. The accuracy of these algorithms may vary depending on the patient physiology, the device used, and the environment.[
13]
The accuracy of the transcutaneous non-invasive measurements can be affected by several factors including skin perfusion, skin condition, patient movement, temperature, environmental factors, and potentially other health conditions of the patient [
6,
14,
15]. Since these devices rely on the diffusion of oxygen and carbon dioxide through the skin, poor skin perfusion will not allow accurate measurements [
16]. Similarly, factors such as dry or oily skin, eczema, and psoriasis can affect the accuracy of transcutaneous measurements because they can alter the ability of the skin to diffuse oxygen and carbon dioxide. Environmental factors such as temperature and humidity can affect the accuracy of the measurement [
17,
18]. Patients with certain conditions such as obesity or peripheral edema may have difficulty in placing the device properly, this can affect the accuracy of the measurement [
19]. In spite of the limitations of transcutaneous measurements, this non-invasive method is attractive and amenable to daily use in sports and healthcare setup.
In emergency settings, blood draws are performed to assess critical values and optimize care. There are several point-of-care testing (POCT) devices for measuring critical laboratory values, but real-time utility can be cumbersome considering need for blood draws, multiple cartridges, and potential for delays in obtaining results. Digital Blood Corporation (DBC; Fort Lauderdale, FL) developed and successfully patented [
20] a system for non - invasive real-time and continuous examination of a patient's blood environment parameters that includes having at least four user-input sensors operably configured to measure and hemoglobin content and partial pressure of O2 and CO2 in a patient’s, blood as well as the body temperature.
The objective of this study was to compare the values obtained using an established POCT device with the DBC’s non-invasive measurements in human subjects. For this purpose a set of eleven blood values was selected for calculation with DBC’s proprietary algorithm and software using four non-invasive core measurements (pO2, pCO2, Hb, T) and for a second data set using the pO2 and pCO2 values from the POC device to estimate the deviation and error resulting from transcutaneous measurements. A comfort assessment comparing the invasive and non-invasive methods was administered among the study participants.
3. Results and Discussion
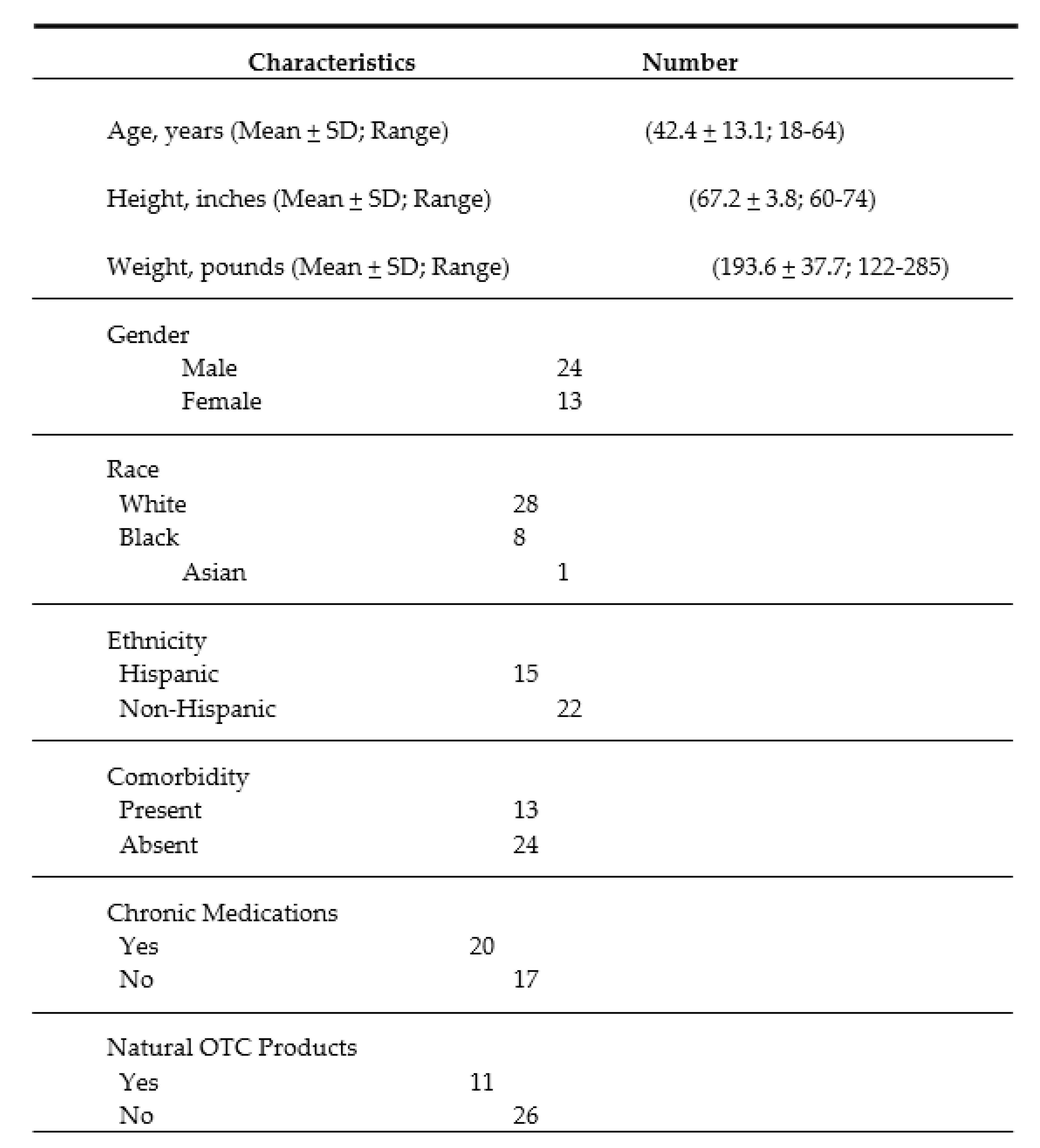
Data from 37 participants (mean age: 42.4 ± 13 years; range: 18-64 years) were included in the primary analysis. Participants were predominantly male (65%), and consisted of White (75%), Black (22%), and Asian (3%) (
Table 1). Forty percent of the participants identified themselves as Hispanic. The demographic data of the study participants have been summarized in
Table 1. Both methods were analyzed using Bland-Altman plots [
21]. For assessing the agreement between two quantitative methods of measurement, correlation coefficient and regression technique that studies the relationship between one variable and another, not the differences, are often inadequate and can be misleading when assessing agreement, because they evaluate only the linear association of two sets of observations [
22]. Bland-Altman plots represent an alternative analysis, based on the quantification of the agreement between two quantitative measurements by studying the mean difference and constructing limits of agreement. These statistical limits are calculated by using the mean and the standard deviation(s) of the differences between two measurements. The resulting graph the difference of the two paired measurements is plotted against the mean of the two measurements. Thus, the Bland Altman plot defines two important differences, the bias and 95% limits of agreement. The average bias, or the average of the differences, is computed as the value determined by one method minus the value determined by the other method. If we observe a value other than zero, the two methods are systematically producing different results, though you can’t tell only from the graph itself which method is better or worse. In our case we defined the iSTAT as the gold standard that we compare the DBC approach to. If on the contrary the average bias is close to zero, DBC’s approach is producing similar results as the standard POC device, although the average doesn’t tell the standard deviation (SD).
The standard deviation of the differences between the two methods (labeled as the SD of bias) is used to calculate the 95% limits of agreement, computed as the bias plus or minus 1.96 times its SD. 95% of the differences between the two methods would be expected to be within the range of values described by the 95% limits of agreement. The limits of agreement include both systematic (bias) and random error (precision), and provide a useful measure for comparing the likely differences between individual results measured by two methods. For interpreting the Bland-Altman results the following questions are addressed:
1) How big is the average discrepancy between the POC and DBC’s approach (the bias)? This is interpreted clinically, not statistically, and addresses if the discrepancy is large enough to be important clinically.
2) How wide are the limits of agreement between the POC and DBC’s approach? Wide limits as defined clinically, indicate ambiguous results while narrow limits and a tiny bias indicate that the two methods are essentially equivalent.
3) Can a trend be observed in the data? Does the difference between the two methods tend to get larger or smaller as the average increases?
4) Is the variability consistent across the graph or does the scatter around the bias line get larger or smaller as the average gets higher?
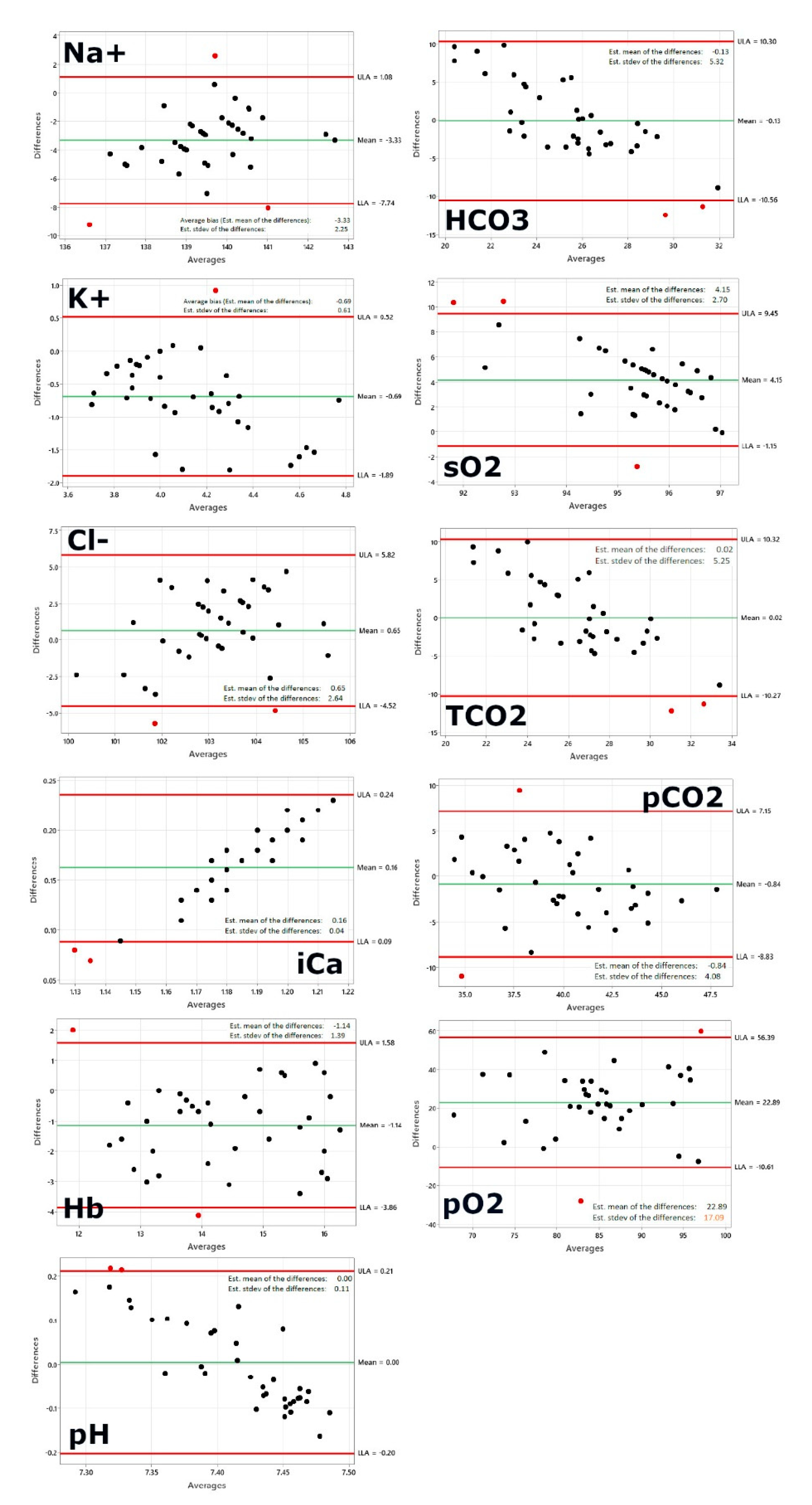
Figure 1, first panel shows the Bland-Altman plot for sodium. The following plotted points are beyond the limits of agreement: 1, 6, 10. As 95% of the data points should be in the confidence interval, 2-3 data point outliers outside the interval would be acceptable given our sample size of 37. The average bias, or the average of the differences, is computed as the value determined by one method minus the value determined by the other method. If one method is sometimes higher, and sometimes the other method is higher, the average of the differences will be close to zero. If it is not close to zero, this indicates that the two assay methods are systematically producing different results. This seems to be the case for sodium on a first glance, however a closer look allows to re-evaluate. A systematic shift by ~3 is not that drastically given the fact that a normal blood sodium level is between 135 and 145 milliequivalents per liter (mEq/L) and severe hyponatremia occurs when levels drop below 125 mEq/L [
23]
The second panel shows potassium and only one data point is outside the limits of agreement. Given our sample size of 37 the one data point outside the interval is perfectly acceptable as >95% of the data points are in the confidence interval. Normally, the blood potassium level is 3.6 to 5.2 millimoles per liter (mmol/L). A very low potassium level (less than 2.5 mmol/L) can be life-threatening and requires urgent medical attention [
24]. The DBC data range fells well into that interval while the Istat shows an average of 3.79 mmol/L, so at the lower limit, indicating a slight underestimation as one would expect most of the values to be in the interval of 4 to 5 millimoles per liter.
Figure 1, third panel shows the Bland-Altman plot for chloride. Two data points are outside the limits of agreement: 6, 24. Given our sample size of 37 the two data points outside the interval are acceptable as >95% of the data points are in the confidence interval. A typical normal range is 96 to 106 milliequivalents per liter (mEq/L) or 96 to 106 millimoles per liter (mmol/L) [
25]. Normal value ranges may vary slightly among different laboratories. All patient data in the study are in the normal range (except one value of 107 measured by the POC device, this patient showed a value of 102.3 mEq/L by DBC) and the range of acceptance equals the normal range interval.
Figure 1, fourth panel shows the BA plot for calcium. The data points are outside the limits of agreement: 1, 9. Given our sample size of 37 and the fact that 95% of the data points should be in the confidence interval, two data points outside the interval would be acceptable. Calcium concentration, both total and free, is characterized by a high physiological variation, depending on age, sex, physiological state (eg, pregnancy), and even season, owing to the seasonal variation of vitamin D. Therefore, separate reference intervals have been established according to the age and sex of the individual being tested [
26]. Total calcium reference ranges for Adults 2.25-2.62 mmol/L and values tend to be reduced in elderly persons. Possible critical values for total calcium are < 1.5 mmol/L or >3.25 mmol/L. Both methods applied in this study do measure ionized calcium that is the amount of free calcium in the blood (not attached to proteins) whereas serum calcium is the total amount of calcium present in the blood. Measurement of serum free (ionized) calcium (Ca++) reflects true calcium status of the body in health and disease. In healthy individuals, plasma ionized calcium concentration is maintained between approximately 1.15 and 1.30 mmol/L. Hypercalcemia (increased amount of calcium in blood), diagnosed if ionized calcium is >1.30 mmol/L, is more common than hypocalcemia (reduced amount of calcium in blood).
All DBC values are very close to the DBC average of 1.1 mmol/L which seems a bit too low for healthy patients, with a max deviation of +-0.01 mmol/L only. The fact that the DBC value is practically constant is leading to the shift observed in the Bland-Altman plot that shows clearly a x-axis dependency of the value, while the POC device values vary with a standard deviation of 0.04 mmol/L and a range from 1.17-1.31 mmol/L. The DBC results do not seem to reflect the same range. The linearity of the dots in the plot would predict that values from patients with higher or lower than the normal range concentrations would be outside the confidence interval and not captured correctly. However, only an actual experiment/study with patients suffering from reduced calcium in blood could tell.
Figure 1, fifth panel shows the BA plot for hemoglobin. The following datapoints are outside the limits of agreement: 11, 25. Given our sample size the two data points outside the interval are acceptable. The healthy range for hemoglobin is for men, 13.2 to 16.6 g/dL and for women, 11.6 to 15 g/dL [
27]. The range of acceptance equals the normal range interval.
Figure 1, sixth panel shows the Bland Altman plot for the pH. Two data points are beyond the limits of agreement: 15, 27. Given our sample size two data points outside the interval are acceptable. The pH of blood in the arteries should be between 7.35 and 7.45 for the body’s metabolic processes and other systems to work well [
28,
29]. These processes produce acids, much of the acid made in the body is carbonic acid, so the body has a complex system of feedback and regulation to maintain healthy pH levels. Acidosis occurs when the blood is too acidic, with a pH below 7.35. Alkalosis occurs when the blood is not acidic enough, with a pH above 7.45. While all data measured with POC device is in the range 7.35-7.45 the DBC data range from 7.21-7.51 with more than half of the values outside the expected range for healthy patients thus predicting either acidosis or alkalosis which however is in no case confirmed or supported by the i-STAT. Given the small normal range of 0.1 the standard deviation of +-0.1 of the DBC measurement is too large to allow the correct prediction of the disease state.
Figure 1, seventh panel shows the Bland-Altman plot for serum bicarbonate. Two data points are outside the limits of agreement: 5, 7. Given our sample size two data points outside the interval are acceptable. An acceptable normal range of serum bicarbonate (HCO3) is 22-26 meq/L [
30]. Serum bicarbonate is used for systematic arterial blood gas interpretation. Interpretation leads to an understanding of the degree or severity of abnormalities, whether the abnormalities are acute or chronic, and if the primary disorder is metabolic or respiratory in origin. The Romanski method of analysis is most simplistic for all levels of providers [
31,
32]. This method helps determine the presence of an acid-base disorder, its primary cause, and whether compensation is present. The first step is to look at the pH and assess for the presence of acidemia (pH < 7.35) or alkalemia (pH > 7.45). If the pH is in the normal range (7.35-7.45), use a pH of 7.40 as a cutoff point. In other words, a pH of 7.37 would be categorized as acidosis, and a pH of 7.42 would be categorized as alkalemia. Next, evaluate the respiratory and metabolic components of the ABG results, the PaCO2 and HCO3, respectively. The PaCO2 indicates whether the acidosis or alkalemia is primarily from a respiratory or metabolic acidosis/alkalosis. PaCO2 > 40 with a pH < 7.4 indicates a respiratory acidosis, while PaCO2 < 40 and pH > 7.4 indicates a respiratory alkalosis (but is often from hyperventilation from anxiety or compensation for a metabolic acidosis). Next, assess for evidence of compensation for the primary acidosis or alkalosis by looking for the value (PaCO2 or HCO3) that is not consistent with the pH. Lastly, assess the PaO2 for any abnormalities in oxygenation.
As an example, in respiratory acidosis the expected physiologic response is an increased HCO3. The increase in concentration of bicarbonate ions (HCO3) in plasma (P HCO3) is tiny in patients with acute respiratory acidosis, but is much larger in patients with chronic respiratory acidosis. As it is important to be able to distinguish between normal, slightly elevated and elevated levels of HCO3, the interval of confidence of more than 10 is not acceptable and doesn’t allow to use this value in correctly predicting the disease state at this point.
Figure 1, eighth panel shows the Bland-Altman plot for Oxygen saturation. Three data points are outside the limits of agreement: 5, 19,27. Given our sample size three data point outside the interval are still acceptable. Oxygen saturation (sO2) refers to the percentage amount of oxygen in the blood. Among other things, it provides information about the functional capacity of the lungs and the effectiveness of oxygen transport in the blood. Normal values 95-99% (SaO2) resp. venous oxygen saturation 73% (SvO2) [
28,
29]. The brain gets affected when the SpO2 level falls below 80-85%. Cyanosis develops when the SpO2 level drops below 67%. The normal oxygen levels in a pulse oximeter usually range from 95% to 100%. While the range of agreement is acceptable and within the clinically relevant interval for healthy patients, DBC seems to slightly underestimate the oxygen levels. As this seems to be a systematic error a correction factor might resolve this issue easily.
Figure 1, nineth panel shows the Bland-Altman plot for total carbon dioxide. Two data points are outside the limits of agreement: 5, 7. Given our sample size two data points outside the interval are well acceptable. The normal range for total carbon dioxide is 23 to 29 mEq/L or 23 to 29 mmol/L [
32]. While all data measured with the POC device is in the normal range the DBC data range 16-38 mmol/L with roughly one third of the values outside the expected range for healthy patients which however is in no case confirmed or supported by POC values. Given the small normal range of 6 mmol/L the standard deviation of +-5.25 mmol/L of the DBC measurement is too large to allow the correct prediction of the disease state.
Figure 1, tenth panel shows the Bland -Altman plot for partial pressure of carbon dioxide. Two data points are outside the limits of agreement: 1, 30. Given our sample size two data points outside the interval are well acceptable. The partial pressure of carbon dioxide (PCO2) is the measure of carbon dioxide within arterial or venous blood. It often serves as a marker of sufficient alveolar ventilation within the lungs. Generally, under normal physiologic conditions, the value of PCO2 ranges between 35 to 45 mmHg [
28,
29]. The standard deviation is higher than the range for healthy patients and as this is a direct measured value by the TINA instrument, the transcutaneous measurement as carried out in this study seems to have a too large variation and the values gained do not match or follow the same pattern as the values observed with iSTAT.
Figure 1, eleventh panel shows the Bland-Altman plot for partial pressure of oxygen. Two data points are outside the limits of agreement: 5, 30. Given our sample size two data points outside the interval are well acceptable. Most healthy adults have a PaO2 within the normal range of 75-100mmHg [
28,
29]. If a PaO2 level is lower than 75 mmHg, it means that a person is not getting enough oxygen. Hypoxemia is PaO2 < 50 mmHg.
Transcutaneous oxygen measurements are different (generally lower) and published normal transcutaneous oxygen partial pressures (PtcO2) for the chest and lower limb have defined tissue hypoxia as a value of < 40 mmHg (< 30 mmHg in some patients, < 50 mmHg in others). A study with thirty-two volunteers had transcutaneous oxygen measurements (TCOM) performed on the chest, upper and lower limbs breathing air, with leg then arm elevated [
33]. Results were: Room-air PtcO2 (mmHg, mean (95% confidence interval)) were: chest: 53.6 (48.7–58.5); upper arm: 60.0 (56.1−64.0); forearm: 52.3 (44.8–55.8); dorsum of hand: 50.2 (46.1–54.3); thenar eminence: 70.8 (67.7–73.8); hypothenar eminence: 77.9 (75.1–80.7); lateral leg: 50.2 (46.2–54.2); lateral malleolus: 50.5 (46.6–54.3); medial malleolus: 48.9 (45.6–52.1); dorsum, between first and second toe: 53.1 (49.2–57.0); dorsum, proximal to fifth toe: 58.5 (55.0–62.0); plantar, 1st MTP: 73.7 (70.3–77.1). Nineteen subjects had at least one room-air PtcO2 below 40 mmHg (nine upper limb, 13 lower limbs, four chest). Approximately 10% lower limb PtcO2 were < 100 mmHg on normobaric oxygen. Only one subject at one site had an upper limb PtcO2 < 100 mmHg breathing oxygen. The broad dispersion in PtcO2 in this healthy cohort reflects the inherent biologic variability in dermal perfusion and oxygen delivery, making it difficult to define narrow, rigid 'normal' values. Thus, the authors stated that they cannot recommend a single PtcO2 value as 'normal' for the upper or lower limb. A thorough patient assessment is essential to establish appropriateness for hyperbaric oxygen therapy, with TCOM used as an aid to guide this decision and not as an absolute. Our standard deviation of 17 is by far too high and makes the resulting values very unreliable, overriding even the systematic shift between arterial blood draw and transcutaneous oxygen measurements (which is about 22 mmHg, or 76% of the arterial blood draw). This hints on the one hand to a correction factor to account for the systematic error for the body part measured, and on the other hand to the need a more robust protocol or measurement to gain transcutaneous pO2 to get a smaller variation and values that after correction would match or follow the same pattern as the values observed with iSTAT.
In a next step, the whole dataset was recalculated using iSTAT pO2 and pCO2 values, while leaving the initial hemoglobin and temperature values. As the software is expecting transcutaneous pO2 values, pO2 values used have been corrected with a factor of 0.76 to match the systematic difference between transcutaneous measurements and an arterial blood draw observed in our study.
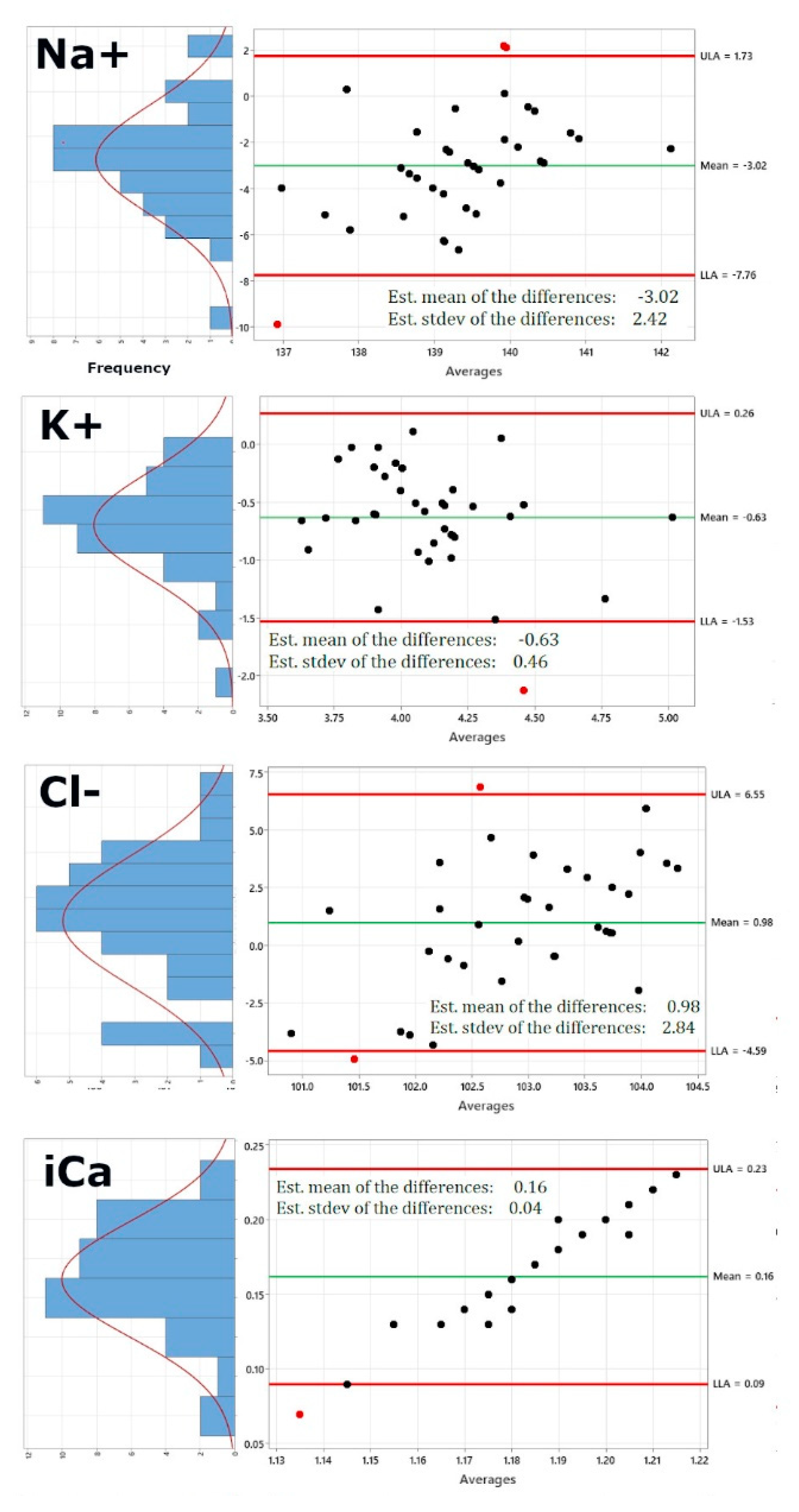
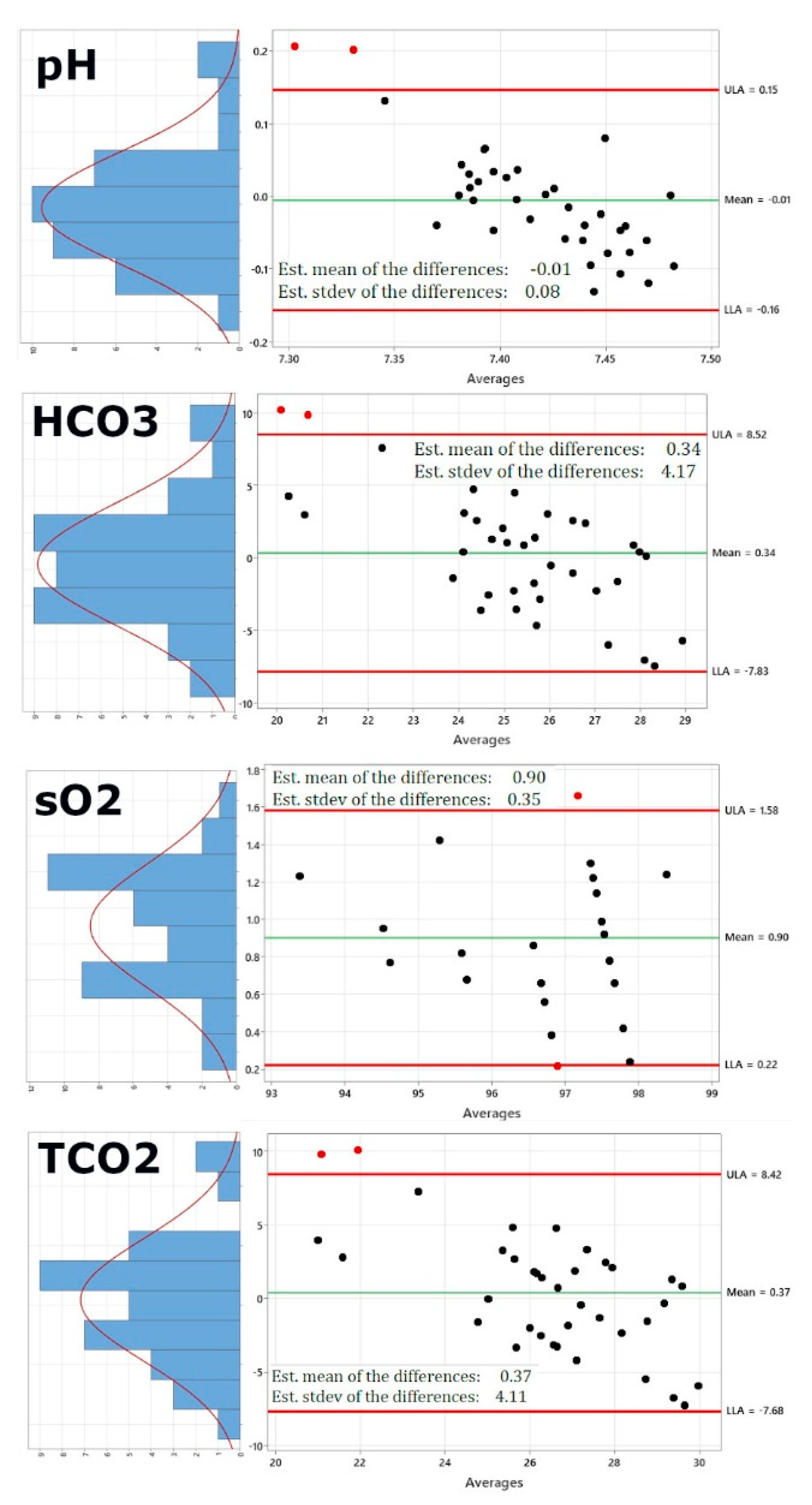
Figure 2 shows the re-calculated Bland-Altman plots using this new dataset. Of course, pO2 and PCO2 have been excluded now, as these would be the same values. In addition, the right panel also shows on the left of each Bland-Altman plot a distribution plot of differences between measurements by the two methods. The red curve represents Normal distribution.
Figure 2 shows that while sodium, potassium, chloride and ionized Calcium are not altered significantly by using the iSTAT values for pO2 and pCO2 (Panels 1-4), pH (panel 5) shows a lower standard deviation of 0.08 from 0.11, bicarbonate (panel 6) shows a lower standard deviation of 4.17 from 5.32, the standard deviation for oxygen saturation very significantly decreases from 2.7 to 0.35 and the same is true for the standard deviation of total carbon dioxide, which decreases from 5.25 to 4.11, generally bringing the limits of agreement within the acceptable and clinically relevant range.
Table 2 compares the comfort level of the study participants for both approaches. Participants filled out the questionnaire directly after their participation.
Author Contributions
Conceptualization, R.H.E. and J.C.; Methodology, J.C., S.D., T.H., S.A.; Software, J.P., J.C.; Formal Analysis, R.H.E., J.P., and J.C.; Investigation, J.C., S.D., T.H., S.A.; Data Curation, R.H.E, J.P., J.C.; Writing – Original Draft Preparation, R.H.E., S.D.; Writing – Review & Editing, R.H.E, S.D., J.P., J.C., T.H., S.A.; Project Administration, J.C. and S.D.; Funding Acquisition, R.H.E., J.C., and S.D.