Submitted:
11 February 2023
Posted:
13 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology of Cancer and BRCA
2.1. Pan-Cancer Overview
2.2. Ovarian Cancer
2.3. Breast Cancer
2.4. Pancreatic Cancer
2.5. Prostate Cancer
2.6. Mutations and the “Founder Effect”
3. Molecular Evolution of BRCA and Links to Human Cancers
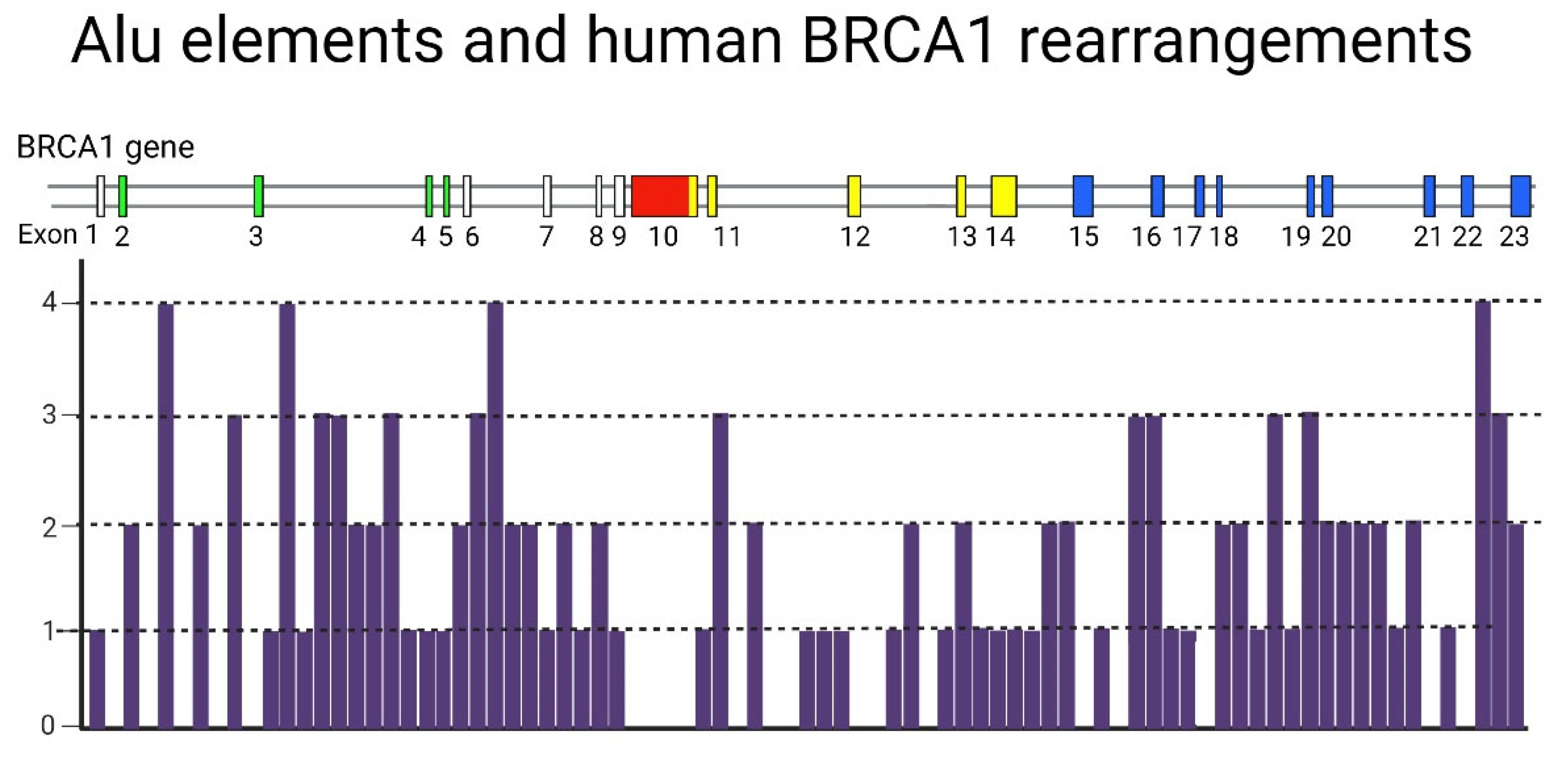
4. A Potential Mechanism for Enrichment of Mutations in the BRCA Genes
4.1. A Hypothetical Role of Transposable Elements (TEs) in BRCA-Associated Carcinogenesis
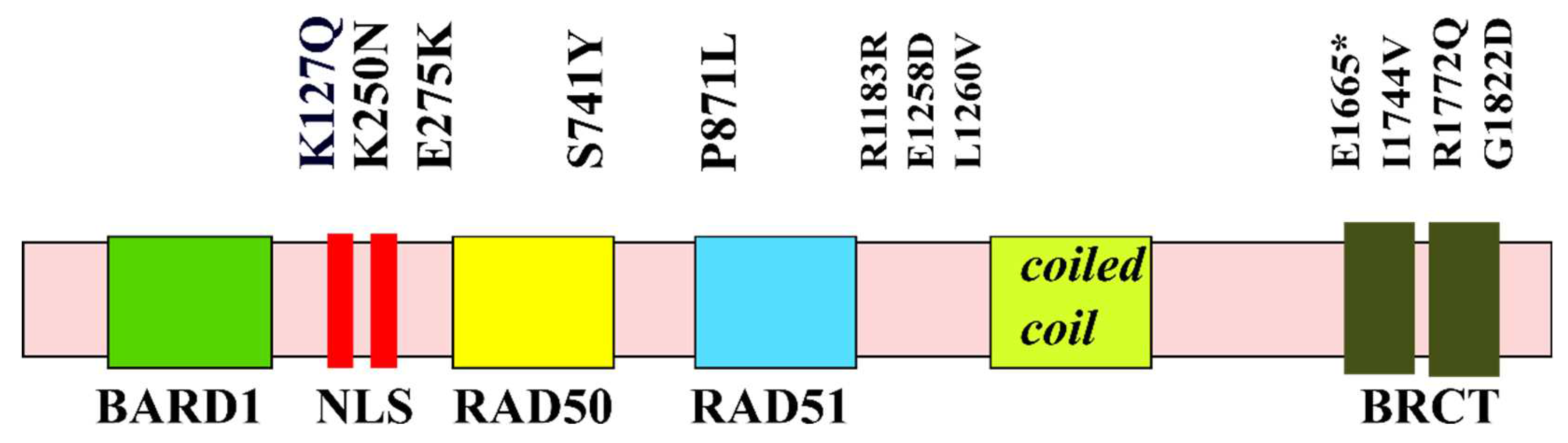
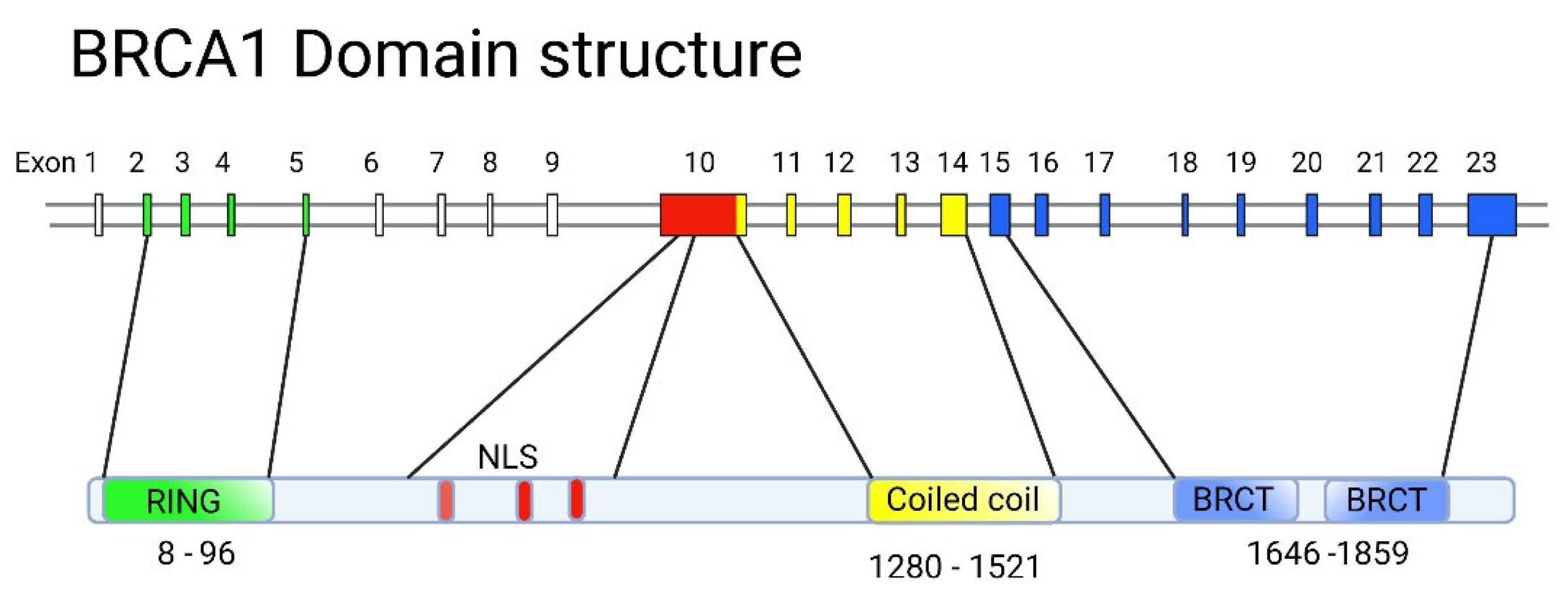
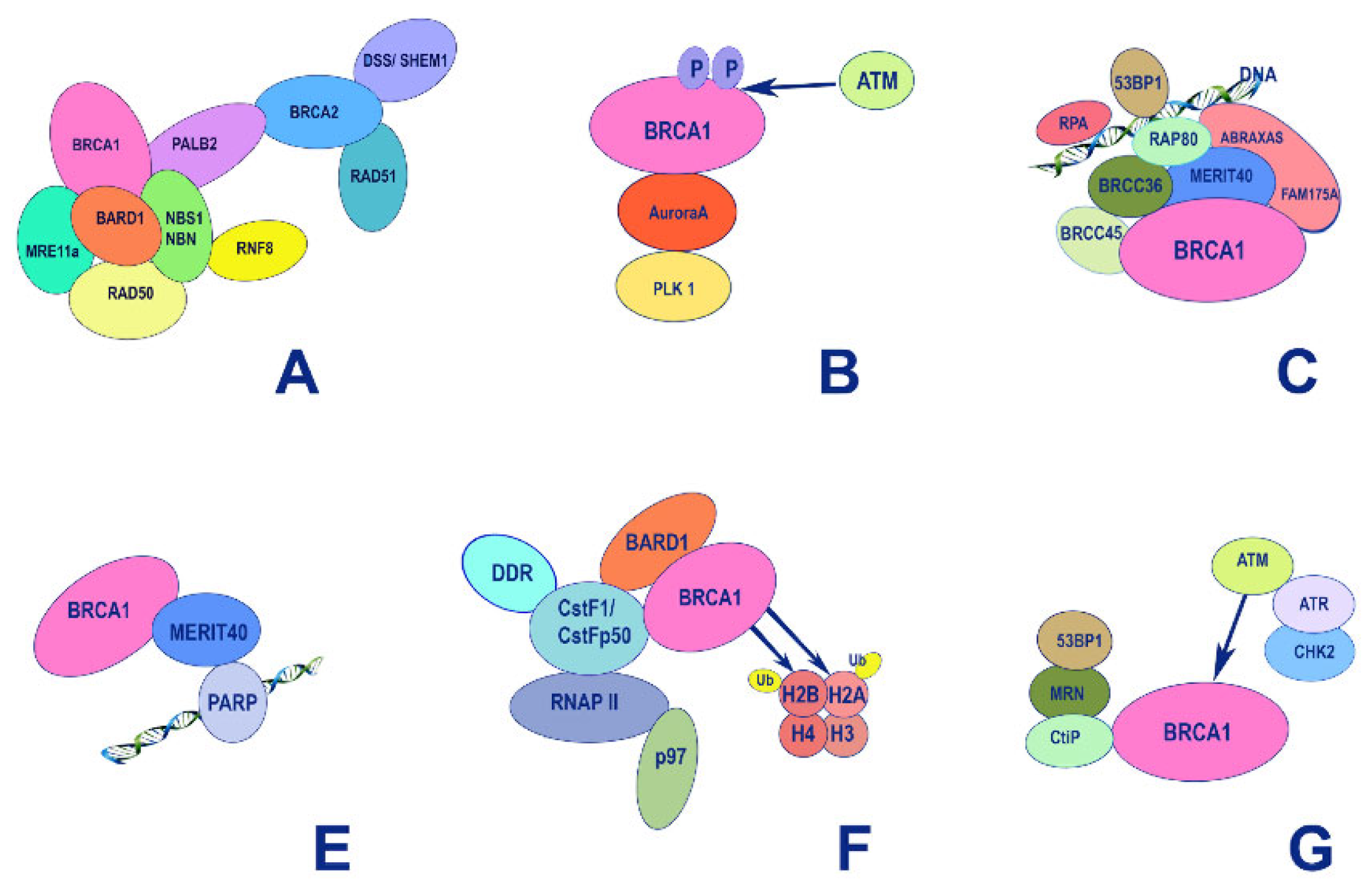
5. Structure-Function Analysis of Human BRCA1
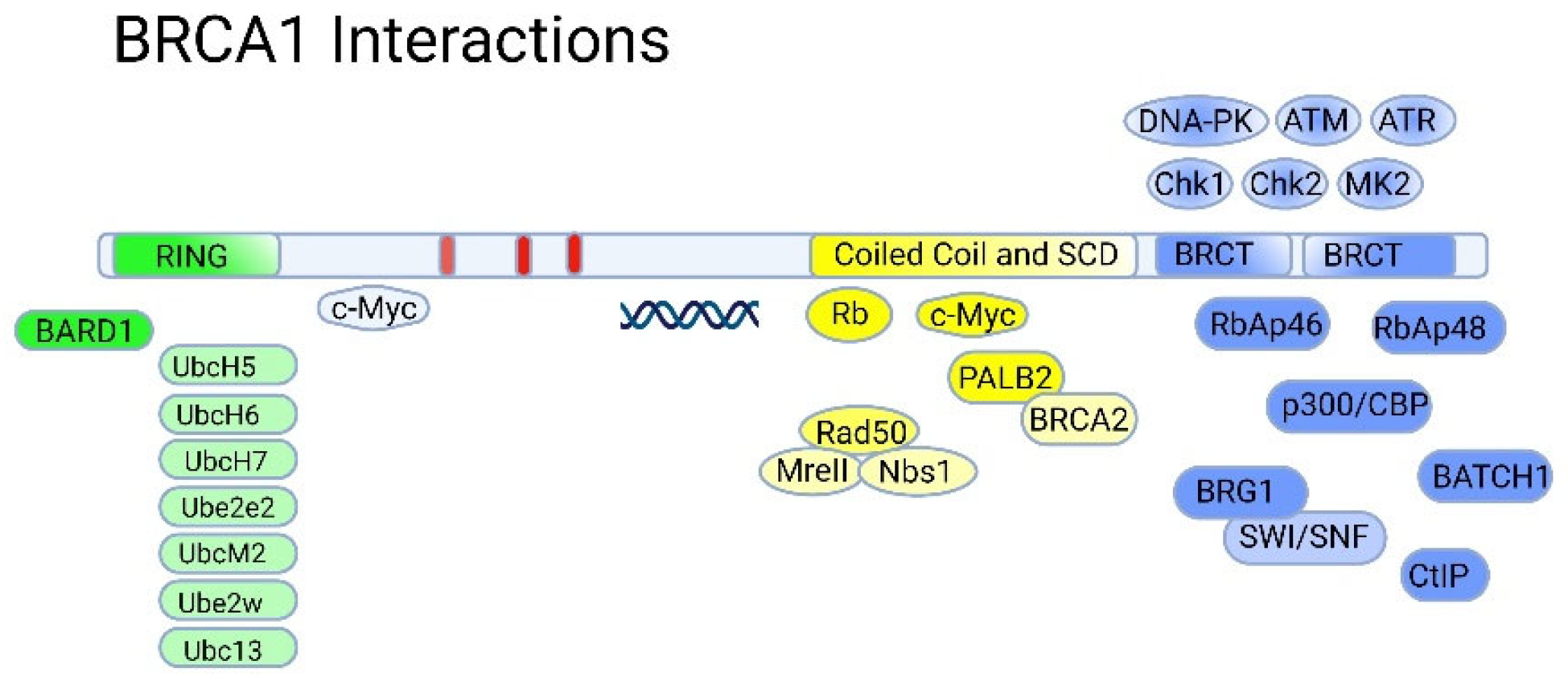
5.1. The RING Domain
5.2. The BRCT Domain
5.3. BRCA1 and p53
6. Survival of BRCA-Mutated Cancer Cells: Role of Tissue Microenvironment
6.1. Hypothesis: Role of Breast Adipocytes in Early Progression of BRCA1/2 Mutated Microtumors
7. Vulnerabilities of BRCA-Mutated Cancer Cells
7.1. Platinum Complexes
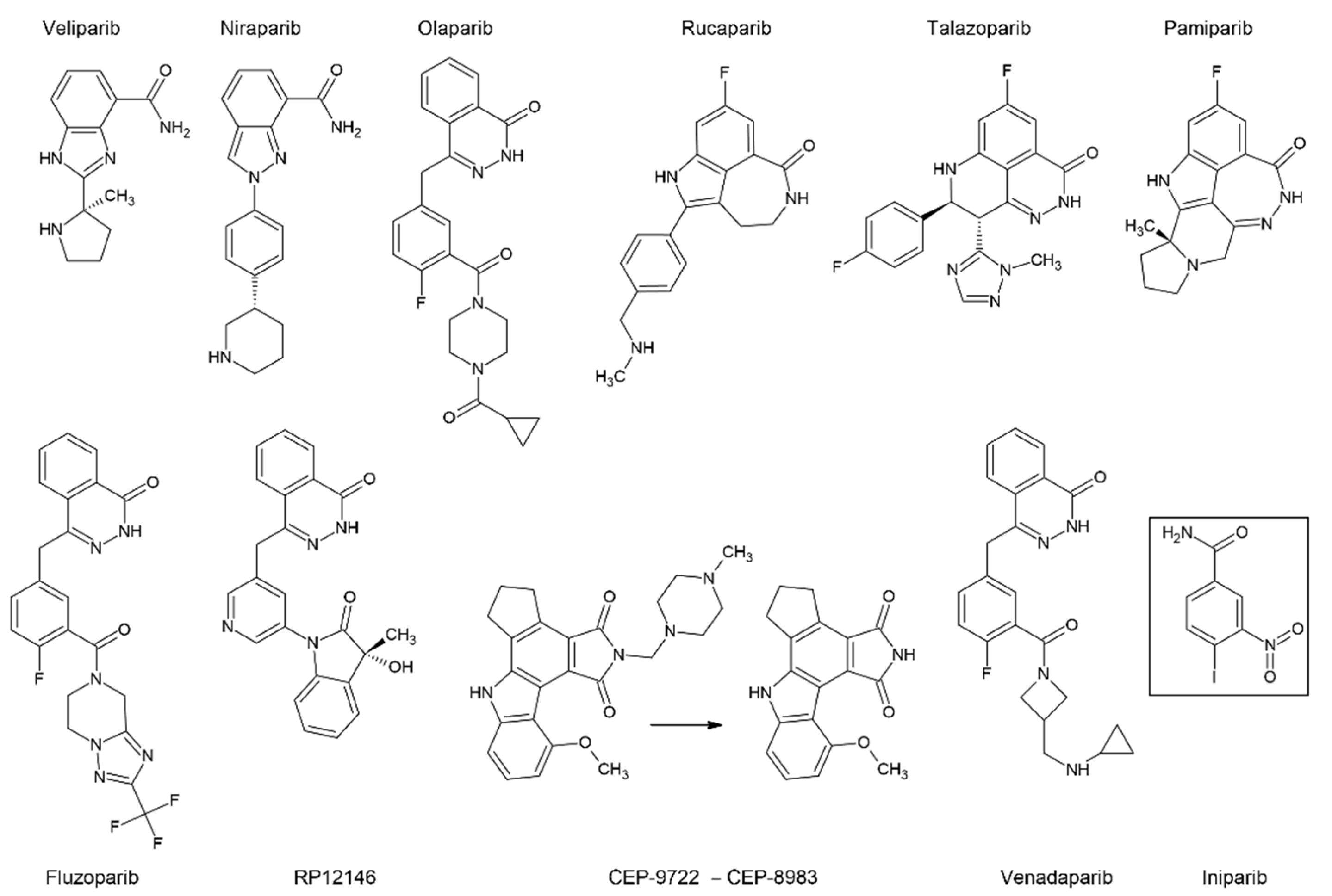
7.2. PARP Inhibitors
8. Future Perspectives and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, J.M.; Lee, M.K.; Newman, B.; Morrow, J.E.; Anderson, L.A.; Huey, B.; King, M.C. Linkage of Early-Onset Familial Breast Cancer to Chromosome 17q21. Science 1990, 250, 1684–1689. [Google Scholar] [CrossRef]
- Wooster, R.; Neuhausen, S.L.; Mangion, J.; Quirk, Y.; Ford, D.; Collins, N.; Nguyen, K.; Seal, S.; Tran, T.; Averill, D. Localization of a Breast Cancer Susceptibility Gene, BRCA2, to Chromosome 13q12-13. Science 1994, 265, 2088–2090. [Google Scholar] [CrossRef] [PubMed]
- King, T.A.; Li, W.; Brogi, E.; Yee, C.J.; Gemignani, M.L.; Olvera, N.; Levine, D.A.; Norton, L.; Robson, M.E.; Offit, K.; et al. Heterogenic Loss of the Wild-Type BRCA Allele in Human Breast Tumorigenesis. Ann Surg Oncol 2007, 14, 2510–2518. [Google Scholar] [CrossRef]
- Martins, F.C.; De, S.; Almendro, V.; Gönen, M.; Park, S.Y.; Blum, J.L.; Herlihy, W.; Ethington, G.; Schnitt, S.J.; Tung, N.; et al. Evolutionary Pathways in BRCA1-Associated Breast Tumors. Cancer Discov 2012, 2, 503–511. [Google Scholar] [CrossRef]
- Kotoula, V.; Fostira, F.; Papadopoulou, K.; Apostolou, P.; Tsolaki, E.; Lazaridis, G.; Manoussou, K.; Zagouri, F.; Pectasides, D.; Vlachos, I.; et al. The Fate of BRCA1-Related Germline Mutations in Triple-Negative Breast Tumors. Am J Cancer Res 2017, 7, 98–114. [Google Scholar]
- Yang, Q.; Yoshimura, G.; Nakamura, M.; Nakamura, Y.; Suzuma, T.; Umemura, T.; Mori, I.; Sakurai, T.; Kakudo, K. BRCA1 in Non-Inherited Breast Carcinomas (Review). Oncol. Rep. 2002, 9, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Neff, R.T.; Senter, L.; Salani, R. BRCA Mutation in Ovarian Cancer: Testing, Implications and Treatment Considerations. Ther Adv Med Oncol 2017, 9, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Lee, Y.-L.; Li, C.-Y. BRCA Genes and Related Cancers: A Meta-Analysis from Epidemiological Cohort Studies. Medicina (Kaunas) 2021, 57, 905. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lee, Y.-C.; Li, C.-Y.; Lee, Y.-L.; Chen, B.-L. BRCA1 and BRCA2 Gene Mutations and Lung Cancer Sisk: A Meta-Analysis. Medicina (Kaunas) 2020, 56, 212. [Google Scholar] [CrossRef]
- Gilks, C.B.; Prat, J. Ovarian Carcinoma Pathology and Genetics: Recent Advances. Hum Pathol 2009, 40, 1213–1223. [Google Scholar] [CrossRef]
- McCluggage, W.G. Morphological Subtypes of Ovarian Carcinoma: A Review with Emphasis on New Developments and Pathogenesis. Pathology 2011, 43, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Lee, M.; Kim, H.S.; Chung, H.H.; Kim, J.-W.; Park, N.H.; Song, Y.-S. Effect of BRCA Mutational Status on Survival Outcome in Advanced-Stage High-Grade Serous Ovarian Cancer. J Ovarian Res 2019, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Risch, H.A.; McLaughlin, J.R.; Cole, D.E.; Rosen, B.; Bradley, L.; Kwan, E.; Jack, E.; Vesprini, D.J.; Kuperstein, G.; Abrahamson, J.L.; et al. Prevalence and Penetrance of Germline BRCA1 and BRCA2 Mutations in a Population Series of 649 Women with Ovarian Cancer. Am J Hum Genet 2001, 68, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Royer, R.; Li, S.; McLaughlin, J.R.; Rosen, B.; Risch, H.A.; Fan, I.; Bradley, L.; Shaw, P.A.; Narod, S.A. Frequencies of BRCA1 and BRCA2 Mutations among 1,342 Unselected Patients with Invasive Ovarian Cancer. Gynecol Oncol 2011, 121, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ramus, S.J.; Gayther, S.A. The Contribution of BRCA1 and BRCA2 to Ovarian Cancer. Mol Oncol 2009, 3, 138–150. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Soegaard, M.; Kjaer, S.K.; Cox, M.; Wozniak, E.; Høgdall, E.; Høgdall, C.; Blaakaer, J.; Jacobs, I.J.; Gayther, S.A.; Ramus, S.J. BRCA1 and BRCA2 Mutation Prevalence and Clinical Characteristics of a Population-Based Series of Ovarian Cancer Cases from Denmark. Clin Cancer Res 2008, 14, 3761–3767. [Google Scholar] [CrossRef] [PubMed]
- Alsop, K.; Fereday, S.; Meldrum, C.; deFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA Mutation Frequency and Patterns of Treatment Response in BRCA Mutation-Positive Women with Ovarian Cancer: A Report from the Australian Ovarian Cancer Study Group. J Clin Oncol 2012, 30, 2654–2663. [Google Scholar] [CrossRef]
- Norquist, B.M.; Brady, M.F.; Harrell, M.I.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Burger, R.A.; Tewari, K.S.; et al. Mutations in Homologous Recombination Genes and Outcomes in Ovarian Carcinoma Patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res 2018, 24, 777–783. [Google Scholar] [CrossRef]
- Tan, D.S.P.; Rothermundt, C.; Thomas, K.; Bancroft, E.; Eeles, R.; Shanley, S.; Ardern-Jones, A.; Norman, A.; Kaye, S.B.; Gore, M.E. “BRCAness” Syndrome in Ovarian Cancer: A Case-Control Study Describing the Clinical Features and Outcome of Patients with Epithelial Ovarian Cancer Associated with BRCA1 and BRCA2 Mutations. J Clin Oncol 2008, 26, 5530–5536. [Google Scholar] [CrossRef]
- Eoh, K.J.; Kim, H.M.; Lee, J.-Y.; Kim, S.; Kim, S.W.; Kim, Y.T.; Nam, E.J. Mutation Landscape of Germline and Somatic BRCA1/2 in Patients with High-Grade Serous Ovarian Cancer. BMC Cancer 2020, 20, 204. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.J.; Timms, K.M.; Carey, M.S.; Gutin, A.; Meyer, L.A.; Flake, D.D.; Abkevich, V.; Potter, J.; Pruss, D.; Glenn, P.; et al. Somatic Mutations in BRCA1 and BRCA2 Could Expand the Number of Patients That Benefit from Poly (ADP Ribose) Polymerase Inhibitors in Ovarian Cancer. J Clin Oncol 2010, 28, 3570–3576. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N Engl J Med 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and Somatic Mutations in Homologous Recombination Genes Predict Platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clin Cancer Res 2014, 20, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, M.; George, A.; Kaye, S.B.; Banerjee, S. BRCA Somatic Mutations and Epigenetic BRCA Modifications in Serous Ovarian Cancer. Ann Oncol 2016, 27, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [CrossRef] [PubMed]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Patients with Platinum-Sensitive Relapsed Serous Ovarian Cancer: A Preplanned Retrospective Analysis of Outcomes by BRCA Status in a Randomised Phase 2 Trial. Lancet Oncol 2014, 15, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Mehrgou, A.; Akouchekian, M. The Importance of BRCA1 and BRCA2 Genes Mutations in Breast Cancer Development. Med J Islam Repub Iran 2016, 30, 369. [Google Scholar]
- Aysola, K.; Desai, A.; Welch, C.; Xu, J.; Qin, Y.; Reddy, V.; Matthews, R.; Owens, C.; Okoli, J.; Beech, D.J.; et al. Triple Negative Breast Cancer—An Overview. Hered. Genet 2013, 2013, 001. [Google Scholar] [CrossRef]
- Kotsopoulos, J. BRCA Mutations and Breast Cancer Prevention. Cancers (Basel) 2018, 10, 524. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Brusco, L.; Daniels, M.; Wathoo, C.; Bailey, A.M.; Strong, L.; Shaw, K.; Lu, K.; Qi, Y.; Zhao, H.; et al. Incidental Germline Variants in 1000 Advanced Cancers on a Prospective Somatic Genomic Profiling Protocol. Ann Oncol 2016, 27, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. Carboplatin in BRCA1/2-Mutated and Triple-Negative Breast Cancer BRCAness Subgroups: The TNT Trial. Nat Med 2018, 24, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Nilsson, M.P.; Olsson, E.; George, A.M.; Chen, Y.; Kvist, A.; Törngren, T.; Vallon-Christersson, J.; Hegardt, C.; Häkkinen, J.; et al. Targeted Sequencing of BRCA1 and BRCA2 across a Large Unselected Breast Cancer Cohort Suggests That One-Third of Mutations Are Somatic. Ann Oncol 2016, 27, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- den Brok, W.D.; Schrader, K.A.; Sun, S.; Tinker, A.V.; Zhao, E.Y.; Aparicio, S.; Gelmon, K.A. Homologous Recombination Deficiency in Breast Cancer: A Clinical Review. JCO Precis Oncol 2017, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bodily, W.R.; Shirts, B.H.; Walsh, T.; Gulsuner, S.; King, M.-C.; Parker, A.; Roosan, M.; Piccolo, S.R. Effects of Germline and Somatic Events in Candidate BRCA-like Genes on Breast-Tumor Signatures. PLoS ONE 2020, 15, e0239197. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Tutt, A.; Ashworth, A. Hallmarks of “BRCAness” in Sporadic Cancers. Nat Rev Cancer 2004, 4, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Rechsteiner, M.; Dedes, K.; Fink, D.; Pestalozzi, B.; Sobottka, B.; Moch, H.; Wild, P.; Varga, Z. Somatic BRCA1 Mutations in Clinically Sporadic Breast Cancer with Medullary Histological Features. J Cancer Res Clin Oncol 2018, 144, 865–874. [Google Scholar] [CrossRef]
- Greer, J.B.; Whitcomb, D.C. Role of BRCA1 and BRCA2 Mutations in Pancreatic Cancer. Gut 2007, 56, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, A.; Szylberg, Ł.; Saganek, M.; Napiontek, W.; Antosik, P.; Grzanka, D. Emerging Strategies in BRCA-Positive Pancreatic Cancer. J Cancer Res Clin Oncol 2018, 144, 1503–1507. [Google Scholar] [CrossRef]
- Luo, G.; Lu, Y.; Jin, K.; Cheng, H.; Guo, M.; Liu, Z.; Long, J.; Liu, C.; Ni, Q.; Yu, X. Pancreatic Cancer: BRCA Mutation and Personalized Treatment. Expert Rev Anticancer Ther 2015, 15, 1223–1231. [Google Scholar] [CrossRef]
- Castro, E.; Eeles, R. The Role of BRCA1 and BRCA2 in Prostate Cancer. Asian J Androl 2012, 14, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Cattrini, C.; Soldato, D.; Vallome, G.; Caffo, O.; Castro, E.; Olmos, D.; Boccardo, F.; Zanardi, E. BRCA Mutations in Prostate Cancer: Prognostic and Predictive Implications. J Oncol 2020, 2020, 4986365. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A.; Neuhausen, S.; Vichodez, G.; Armel, S.; Lynch, H.T.; Ghadirian, P.; Cummings, S.; Olopade, O.; Stoppa-Lyonnet, D.; Couch, F.; et al. Rapid Progression of Prostate Cancer in Men with a BRCA2 Mutation. Br J Cancer 2008, 99, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Pal, T.; Vadaparampil, S.; Betts, J.; Miree, C.; Li, S.; Narod, S.A. BRCA1/2 in High-Risk African American Women with Breast Cancer: Providing Genetic Testing through Various Recruitment Strategies. Genet Test 2008, 12, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Ferla, R.; Calò, V.; Cascio, S.; Rinaldi, G.; Badalamenti, G.; Carreca, I.; Surmacz, E.; Colucci, G.; Bazan, V.; Russo, A. Founder Mutations in BRCA1 and BRCA2 Genes. Ann Oncol 2007, 18 (Suppl. 6), vi93–vi98. [Google Scholar] [CrossRef]
- Roa, B.B.; Boyd, A.A.; Volcik, K.; Richards, C.S. Ashkenazi Jewish Population Frequencies for Common Mutations in BRCA1 and BRCA2. Nat Genet 1996, 14, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Janavičius, R. Founder BRCA1/2 Mutations in the Europe: Implications for Hereditary Breast-Ovarian Cancer Prevention and Control. EPMA J 2010, 1, 397–412. [Google Scholar] [CrossRef]
- Sokolenko, A.P.; Sokolova, T.N.; Ni, V.I.; Preobrazhenskaya, E.V.; Iyevleva, A.G.; Aleksakhina, S.N.; Romanko, A.A.; Bessonov, A.A.; Gorodnova, T.V.; Anisimova, E.I.; et al. Frequency and Spectrum of Founder and Non-Founder BRCA1 and BRCA2 Mutations in a Large Series of Russian Breast Cancer and Ovarian Cancer Patients. Breast Cancer Res Treat 2020, 184, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Suspitsin, E.N.; Sherina, N.Y.; Ponomariova, D.N.; Sokolenko, A.P.; Iyevleva, A.G.; Gorodnova, T.V.; Zaitseva, O.A.; Yatsuk, O.S.; Togo, A.V.; Tkachenko, N.N.; et al. High Frequency of BRCA1, but Not CHEK2 or NBS1 (NBN), Founder Mutations in Russian Ovarian Cancer Patients. Hered Cancer Clin Pract. 2009, 7, 5. [Google Scholar] [CrossRef]
- Fabian, D.; Flatt, T. The Evolution of Aging; Nature Education Knowledge, 2011; Volume 3. [Google Scholar]
- Ben-Aharon, I.; Levi, M.; Margel, D.; Yerushalmi, R.; Rizel, S.; Perry, S.; Sharon, E.; Hasky, N.; Abir, R.; Fisch, B.; et al. Premature Ovarian Aging in BRCA Carriers: A Prototype of Systemic Precocious Aging? Oncotarget 2018, 9, 15931–15941. [Google Scholar] [CrossRef] [PubMed]
- Kępczyński, Ł.; Połatyńska, K.; Nykel, A.; Sałamunia, J.; Kałużewski, T.; Kużawczyk, A.; Gach, A. Age of Natural Menopause Onset in BRCA1/2 Carriers—Systematic Review and Meta-Analysis. Prz Menopauzalny 2020, 19, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Drechsel Katja, C.E.; van Tilborg Theodora, C.; Eijkemans Marinus, J.C.; Lentjes Eef, G.W.M.; Irene, H.; Mariette, G.; van Golde Ron, J.T.; Willem, V.; Lichtenbelt Klaske, D.; Broekmans Frank, J.M.; et al. The Impact of BRCA1- and BRCA2 Mutations on Ovarian Reserve Status. Reprod Sci 2022. [Google Scholar] [CrossRef]
- Semmler, L.; Reiter-Brennan, C.; Klein, A. BRCA1 and Breast Cancer: A Review of the Underlying Mechanisms Resulting in the Tissue-Specific Tumorigenesis in Mutation Carriers. J Breast Cancer 2019, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different Roles in a Common Pathway of Genome Protection. Nat Rev Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.H.; Serrano, L. Cell Type-Specific Properties and Environment Shape Tissue Specificity of Cancer Genes. Sci Rep 2016, 6, 20707. [Google Scholar] [CrossRef] [PubMed]
- Ade, C.; Roy-Engel, A.M.; Deininger, P.L. Alu Elements: An Intrinsic Source of Human Genome Instability. Curr Opin Virol 2013, 3, 639–645. [Google Scholar] [CrossRef]
- Welcsh, P.L.; King, M.C. BRCA1 and BRCA2 and the Genetics of Breast and Ovarian Cancer. Hum Mol Genet 2001, 10, 705–713. [Google Scholar] [CrossRef]
- Smith, T.M.; Lee, M.K.; Szabo, C.I.; Jerome, N.; McEuen, M.; Taylor, M.; Hood, L.; King, M.C. Complete Genomic Sequence and Analysis of 117 Kb of Human DNA Containing the Gene BRCA1. Genome Res 1996, 6, 1029–1049. [Google Scholar] [CrossRef]
- Montagna, M.; Santacatterina, M.; Torri, A.; Menin, C.; Zullato, D.; Chieco-Bianchi, L.; D’Andrea, E. Identification of a 3 Kb Alu-Mediated BRCA1 Gene Rearrangement in Two Breast/Ovarian Cancer Families. Oncogene 1999, 18, 4160–4165. [Google Scholar] [CrossRef]
- Girolimetti, G.; Perrone, A.M.; Santini, D.; Barbieri, E.; Guerra, F.; Ferrari, S.; Zamagni, C.; De Iaco, P.; Gasparre, G.; Turchetti, D. BRCA-Associated Ovarian Cancer: From Molecular Genetics to Risk Management. Biomed Res Int 2014, 2014, 787143. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.K.; Han, K.; Wang, J.; Lee, J.; Wang, H.; Callinan, P.A.; Dyer, M.; Cordaux, R.; Liang, P.; Batzer, M.A. Human Genomic Deletions Mediated by Recombination between Alu Elements. Am J Hum Genet 2006, 79, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Bozsik, A.; Pócza, T.; Papp, J.; Vaszkó, T.; Butz, H.; Patócs, A.; Oláh, E. Complex Characterization of Germline Large Genomic Rearrangements of the BRCA1 and BRCA2 Genes in High-Risk Breast Cancer Patients-Novel Variants from a Large National Center. Int J Mol Sci 2020, 21, 4650. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A.; Nathanson, K.L.; Calzone, K.; Antin-Ozerkis, D.; Shih, H.A.; Martin, A.M.; Lenoir, G.M.; Mazoyer, S.; Weber, B.L. Screening for Genomic Rearrangements in Families with Breast and Ovarian Cancer Identifies BRCA1 Mutations Previously Missed by Conformation-Sensitive Gel Electrophoresis or Sequencing. Am J Hum Genet 2000, 67, 841–850. [Google Scholar] [CrossRef]
- Nordling, M.; Karlsson, P.; Wahlström, J.; Engwall, Y.; Wallgren, A.; Martinsson, T. A Large Deletion Disrupts the Exon 3 Transcription Activation Domain of the BRCA2 Gene in a Breast/Ovarian Cancer Family. Cancer Res 1998, 58, 1372–1375. [Google Scholar] [PubMed]
- Ewald, I.P.; Ribeiro, P.L.I.; Palmero, E.I.; Cossio, S.L.; Giugliani, R.; Ashton-Prolla, P. Genomic Rearrangements in BRCA1 and BRCA2: A Literature Review. Genet Mol Biol 2009, 32, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-F.; Hu, Z.; Rao, N.-Y.; Song, C.-G.; Zhang, B.; Cao, M.-Z.; Su, F.-X.; Wang, Y.-S.; He, P.-Q.; Di, G.-H.; et al. The Prevalence of BRCA1 and BRCA2 Germline Mutations in High-Risk Breast Cancer Patients of Chinese Han Nationality: Two Recurrent Mutations Were Identified. Breast Cancer Res Treat 2008, 110, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Fachal, L.; Blanco, A.; Santamariña, M.; Carracedo, A.; Vega, A. Large Genomic Rearrangements of BRCA1 and BRCA2 among Patients Referred for Genetic Analysis in Galicia (NW Spain): Delimitation and Mechanism of Three Novel BRCA1 Rearrangements. PLoS ONE 2014, 9, e93306. [Google Scholar] [CrossRef]
- Lou, D.I.; McBee, R.M.; Le, U.Q.; Stone, A.C.; Wilkerson, G.K.; Demogines, A.M.; Sawyer, S.L. Rapid Evolution of BRCA1 and BRCA2 in Humans and Other Primates. BMC Evol Biol 2014, 14, 155. [Google Scholar] [CrossRef]
- O’Donovan, P.J.; Livingston, D.M. BRCA1 and BRCA2: Breast/Ovarian Cancer Susceptibility Gene Products and Participants in DNA Double-Strand Break Repair. Carcinogenesis 2010, 31, 961–967. [Google Scholar] [CrossRef]
- Hemel, D.; Domchek, S.M. Breast Cancer Predisposition Syndromes. Hematol Oncol Clin North Am 2010, 24, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Preisler-Adams, S.; Schönbuchner, I.; Fiebig, B.; Welling, B.; Dworniczak, B.; Weber, B.H.F. Gross Rearrangements in BRCA1 but Not BRCA2 Play a Notable Role in Predisposition to Breast and Ovarian Cancer in High-Risk Families of German Origin. Cancer Genet Cytogenet 2006, 168, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rohlfs, E.M.; Puget, N.; Graham, M.L.; Weber, B.L.; Garber, J.E.; Skrzynia, C.; Halperin, J.L.; Lenoir, G.M.; Silverman, L.M.; Mazoyer, S. An Alu-Mediated 7.1 Kb Deletion of BRCA1 Exons 8 and 9 in Breast and Ovarian Cancer Families That Results in Alternative Splicing of Exon 10. Genes Chromosomes Cancer 2000, 28, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Agata, S.; Dalla Palma, M.; Callegaro, M.; Scaini, M.C.; Menin, C.; Ghiotto, C.; Nicoletto, O.; Zavagno, G.; Chieco-Bianchi, L.; D’Andrea, E.; et al. Large Genomic Deletions Inactivate the BRCA2 Gene in Breast Cancer Families. J Med Genet 2005, 42, e64. [Google Scholar] [CrossRef] [PubMed]
- Karhu, R.; Laurila, E.; Kallioniemi, A.; Syrjäkoski, K. Large Genomic BRCA2 Rearrangements and Male Breast Cancer. Cancer Detect Prev 2006, 30, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.M.; Davis, T.A.; Silva, A.G.S.; Kirk, J.A.; Leary, J.A.; kConFab Investigators. Large Genomic Rearrangements of Both BRCA2 and BRCA1 Are a Feature of the Inherited Breast/Ovarian Cancer Phenotype in Selected Families. J Med Genet 2005, 42, e31. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.; Santos, C.; Rocha, P.; Pinto, P.; Bizarro, S.; Teixeira, M.R. Molecular Diagnosis of the Portuguese Founder Mutation BRCA2 c.156_157insAlu. Breast Cancer Res Treat 2009, 117, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.A.; Reeves, J.R.; Cooke, T.G. BRCA1 and BRCA2 Proteins: Roles in Health and Disease. Mol Pathol 1998, 51, 237–247. [Google Scholar] [CrossRef]
- Brzovic, P.S.; Rajagopal, P.; Hoyt, D.W.; King, M.-C.; Klevit, R.E. Structure of a BRCA1–BARD1 Heterodimeric RING–RING Complex. Nat Struct Mol Biol 2001, 8, 833–837. [Google Scholar] [CrossRef]
- Wang, Y.; Bernhardy, A.J.; Johnson, N. Abstract A23: BRCA1 Mutations in the BRCT Domain Can Be Removed through Alternative Splicing and Induce PARP Inhibitor Resistance. Mol. Cancer Res. 2017, 15, A23. [Google Scholar] [CrossRef]
- Wu, W.; Koike, A.; Takeshita, T.; Ohta, T. The Ubiquitin E3 Ligase Activity of BRCA1 and Its Biological Functions. Cell Div 2008, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, R.; Fukuda, M.; Maeda, I.; Nishikawa, H.; Oyake, D.; Yabuki, Y.; Ogata, H.; Ohta, T. The RING Heterodimer BRCA1-BARD1 Is a Ubiquitin Ligase Inactivated by a Breast Cancer-Derived Mutation. J Biol Chem 2001, 276, 14537–14540. [Google Scholar] [CrossRef] [PubMed]
- Birrane, G.; Varma, A.K.; Soni, A.; Ladias, J.A.A. Crystal Structure of the BARD1 BRCT Domains. Biochemistry 2007, 46, 7706–7712. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, X.L.; Yang, M.C.; Wei, F.; Ayi, T.C.; Bowcock, A.M.; Baer, R. Cell Cycle-Dependent Colocalization of BARD1 and BRCA1 Proteins in Discrete Nuclear Domains. Proc Natl Acad Sci U S A 1997, 94, 12075–12080. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Matsuoka, S.; Ballif, B.A.; Zhang, D.; Smogorzewska, A.; Gygi, S.P.; Elledge, S.J. Abraxas and RAP80 Form a BRCA1 Protein Complex Required for the DNA Damage Response. Science 2007, 316, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Paull, T.T.; Cortez, D.; Bowers, B.; Elledge, S.J.; Gellert, M. Direct DNA Binding by Brca1. Proc Natl Acad Sci U S A 2001, 98, 6086–6091. [Google Scholar] [CrossRef]
- Starita, L.M.; Parvin, J.D. The Multiple Nuclear Functions of BRCA1: Transcription, Ubiquitination and DNA Repair. Curr Opin Cell Biol 2003, 15, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Pan, H.; Li, S.; Flesken-Nikitin, A.; Chen, P.L.; Boyer, T.G.; Lee, W.H. Sequence-Specific Transcriptional Corepressor Function for BRCA1 through a Novel Zinc Finger Protein, ZBRK1. Mol Cell 2000, 6, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.L.; Rodriguez, A.M.; Snyder, R.R.; Hankins, G.D.V.; Boehning, D. Structure-Function of the Tumor Suppressor BRCA1. Comput Struct Biotechnol J 2012, 1, e201204005. [Google Scholar] [CrossRef]
- Lee, M.S.; Green, R.; Marsillac, S.M.; Coquelle, N.; Williams, R.S.; Yeung, T.; Foo, D.; Hau, D.D.; Hui, B.; Monteiro, A.N.A.; et al. Comprehensive Analysis of Missense Variations in the BRCT Domain of BRCA1 by Structural and Functional Assays. Cancer Res 2010, 70, 4880–4890. [Google Scholar] [CrossRef]
- Williams, R.S.; Bernstein, N.; Lee, M.S.; Rakovszky, M.L.; Cui, D.; Green, R.; Weinfeld, M.; Glover, J.N.M. Structural Basis for Phosphorylation-Dependent Signaling in the DNA-Damage Response. Biochem Cell Biol 2005, 83, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Willers, H.; Feng, Z.; Ghosh, J.C.; Kim, S.; Weaver, D.T.; Chung, J.H.; Powell, S.N.; Xia, F. Chk2 Phosphorylation of BRCA1 Regulates DNA Double-Strand Break Repair. Mol Cell Biol 2004, 24, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R.; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR Substrate Analysis Reveals Extensive Protein Networks Responsive to DNA Damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, H.C.; Yaffe, M.B. Kinases That Control the Cell Cycle in Response to DNA Damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol 2009, 21, 245–255. [Google Scholar] [CrossRef] [PubMed]
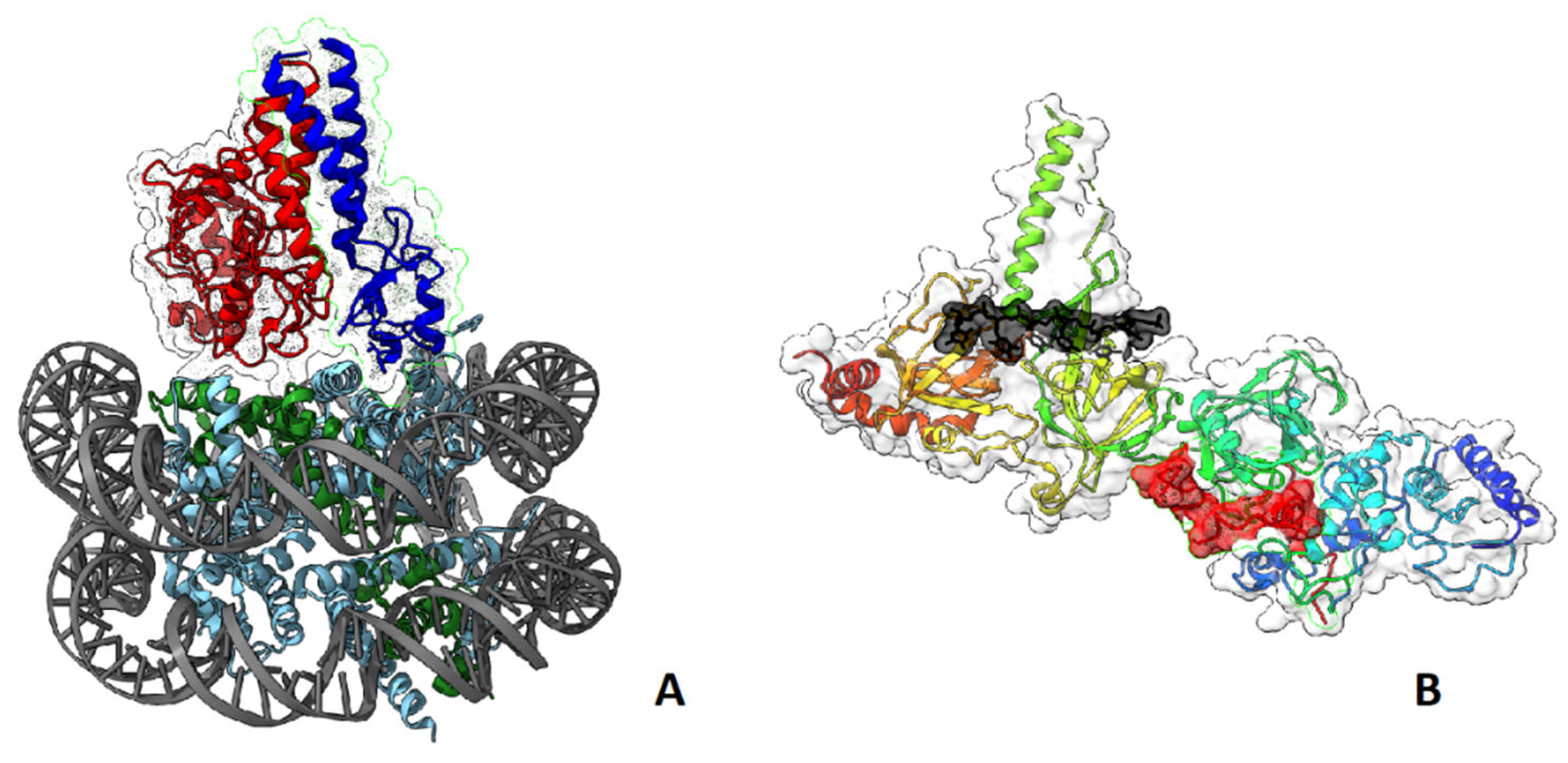
- Hu, Q.; Botuyan, M.V.; Zhao, D.; Cui, G.; Mer, E.; Mer, G. Mechanisms of BRCA1-BARD1 Nucleosome Recognition and Ubiquitylation. Nature 2021, 596, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.R.; Clifford, G.; Bonnet, C.; Groth, A.; Wilson, M.D.; Chapman, J.R. BARD1 Reads H2A Lysine 15 Ubiquitination to Direct Homologous Recombination. Nature 2021, 596, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Witus, S.R.; Burrell, A.L.; Farrell, D.P.; Kang, J.; Wang, M.; Hansen, J.M.; Pravat, A.; Tuttle, L.M.; Stewart, M.D.; Brzovic, P.S.; et al. BRCA1/BARD1 Site-Specific Ubiquitylation of Nucleosomal H2A Is Directed by BARD1. Nat Struct Mol Biol 2021, 28, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jeffrey, P.D.; Miller, J.; Kinnucan, E.; Sun, Y.; Thoma, N.H.; Zheng, N.; Chen, P.-L.; Lee, W.-H.; Pavletich, N.P. BRCA2 Function in DNA Binding and Recombination from a BRCA2-DSS1-SsDNA Structure. Science 2002, 297, 1837–1848. [Google Scholar] [CrossRef]
- Trego, K.S.; Groesser, T.; Davalos, A.R.; Parplys, A.C.; Zhao, W.; Nelson, M.R.; Hlaing, A.; Shih, B.; Rydberg, B.; Pluth, J.M.; et al. Non-Catalytic Roles for XPG with BRCA1 and BRCA2 in Homologous Recombination and Genome Stability. Mol Cell 2016, 61, 535–546. [Google Scholar] [CrossRef]
- Scully, R.; Livingston, D.M. In Search of the Tumour-Suppressor Functions of BRCA1 and BRCA2. Nature 2000, 408, 429–432. [Google Scholar] [CrossRef]
- Deng, C.X.; Scott, F. Role of the Tumor Suppressor Gene Brca1 in Genetic Stability and Mammary Gland Tumor Formation. Oncogene 2000, 19, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Foray, N.; Marot, D.; Randrianarison, V.; Venezia, N.D.; Picard, D.; Perricaudet, M.; Favaudon, V.; Jeggo, P. Constitutive Association of BRCA1 and C-Abl and Its ATM-Dependent Disruption after Irradiation. Mol Cell Biol 2002, 22, 4020–4032. [Google Scholar] [CrossRef] [PubMed]
- Levav-Cohen, Y.; Goldberg, Z.; Zuckerman, V.; Grossman, T.; Haupt, S.; Haupt, Y. C-Abl as a Modulator of P53. Biochem Biophys Res Commun 2005, 331, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Hantschel, O.; Rix, U.; Schmidt, U.; Bürckstümmer, T.; Kneidinger, M.; Schütze, G.; Colinge, J.; Bennett, K.L.; Ellmeier, W.; Valent, P.; et al. The Btk Tyrosine Kinase Is a Major Target of the Bcr-Abl Inhibitor Dasatinib. Proc Natl Acad Sci U S A 2007, 104, 13283–13288. [Google Scholar] [CrossRef] [PubMed]
- Althubiti, M.; Rada, M.; Samuel, J.; Escorsa, J.M.; Najeeb, H.; Lee, K.-G.; Lam, K.-P.; Jones, G.D.D.; Barlev, N.A.; Macip, S. BTK Modulates P53 Activity to Enhance Apoptotic and Senescent Responses. Cancer Res 2016, 76, 5405–5414. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Barlev, N.; Macip, S. BTK: A Two-Faced Effector in Cancer and Tumour Suppression. Cell Death Dis 2018, 9, 1064. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, W.; Linke, S.P.; Cao, L.; Li, W.M.; Furth, P.A.; Harris, C.C.; Deng, C.X. Genetic Interactions between Tumor Suppressors Brca1 and P53 in Apoptosis, Cell Cycle and Tumorigenesis. Nat Genet 2001, 28, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Somasundaram, K.; Peng, Y.; Tian, H.; Zhang, H.; Bi, D.; Weber, B.L.; El-Deiry, W.S. BRCA1 Physically Associates with P53 and Stimulates Its Transcriptional Activity. Oncogene 1998, 16, 1713–1721. [Google Scholar] [CrossRef]
- Pietrasik, S.; Zajac, G.; Morawiec, J.; Soszynski, M.; Fila, M.; Blasiak, J. Interplay between BRCA1 and GADD45A and Its Potential for Nucleotide Excision Repair in Breast Cancer Pathogenesis. Int J Mol Sci 2020, 21, 870. [Google Scholar] [CrossRef]
- Clarke, C.L.; Sandle, J.; Jones, A.A.; Sofronis, A.; Patani, N.R.; Lakhani, S.R. Mapping Loss of Heterozygosity in Normal Human Breast Cells from BRCA1/2 Carriers. Br J Cancer 2006, 95, 515–519. [Google Scholar] [CrossRef]
- Armes, J.E.; Egan, A.J.M.; Southey, M.C.; Dite, G.S.; McCredie, M.R.E.; Giles, G.G.; Hopper, J.L.; Venter, D.J. The Histologic Phenotypes of Breast Carcinoma Occurring before Age 40 Years in Women with and without BRCA1 or BRCA2 Germline Mutations. Cancer 1998, 83, 2335–2345. [Google Scholar] [CrossRef]
- Mote, P.A.; Leary, J.A.; Avery, K.A.; Sandelin, K.; Chenevix-Trench, G.; Kirk, J.A.; Clarke, C.L. Germ-Line Mutations in BRCA1 or BRCA2 in the Normal Breast Are Associated with Altered Expression of Estrogen-Responsive Proteins and the Predominance of Progesterone Receptor A. Genes Chromosomes Cancer 2004, 39, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Ingthorsson, S.; Traustadottir, G.A.; Gudjonsson, T. Cellular Plasticity and Heterotypic Interactions during Breast Morphogenesis and Cancer Initiation. Cancers (Basel) 2022, 14, 5209. [Google Scholar] [CrossRef] [PubMed]
- Avşar Abdik, E. Differentiated Pre-Adipocytes Promote Proliferation, Migration and Epithelial-Mesenchymal Transition in Breast Cancer Cells of Different P53 Status. Mol Biol Rep 2021, 48, 5187–5198. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Cha, Y.J.; Koo, J.S. Adipocyte Biology in Breast Cancer: From Silent Bystander to Active Facilitator. Prog Lipid Res 2018, 69, 11–20. [Google Scholar] [CrossRef]
- Takehara, M.; Sato, Y.; Kimura, T.; Noda, K.; Miyamoto, H.; Fujino, Y.; Miyoshi, J.; Nakamura, F.; Wada, H.; Bando, Y.; et al. Cancer-Associated Adipocytes Promote Pancreatic Cancer Progression through SAA1 Expression. Cancer Sci 2020, 111, 2883–2894. [Google Scholar] [CrossRef]
- Kothari, C.; Diorio, C.; Durocher, F. The Importance of Breast Adipose Tissue in Breast Cancer. Int J Mol Sci 2020, 21, 5760. [Google Scholar] [CrossRef]
- Yao, H.; He, S. Multi-faceted Role of Cancer-associated Adipocytes in the Tumor Microenvironment (Review). Mol Med Rep 2021, 24, 866. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.; Kolla, M.; Meshulam, T.; Shafran, J.S.; Qiu, Y.; Casey, A.N.; Pompa, I.R.; Ennis, C.S.; Mazzeo, C.S.; Rabhi, N.; et al. Adipocyte-Derived Exosomes May Promote Breast Cancer Progression in Type 2 Diabetes. Sci Signal 2021, 14, eabj2807. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, W.H.; Koo, J.S. Adipocytes Can Induce Epithelial-Mesenchymal Transition in Breast Cancer Cells. Breast Cancer Res Treat 2015, 153, 323–335. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, B.; Zhao, Y.; Tao, Z.; Wang, Y.; Chen, G.; Hu, X. Mammary Adipocytes Protect Triple-Negative Breast Cancer Cells from Ferroptosis. J Hematol Oncol 2022, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; He, X.; Tong, C.; Li, H.; Xie, C.; Wu, Y.; Wang, L.; Yan, X.; Luo, D.; Tang, Y.; et al. Cancer-Associated Adipocytes Promote the Invasion and Metastasis in Breast Cancer through LIF/CXCLs Positive Feedback Loop. Int J Biol Sci 2022, 18, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.P.; Buelto, D.; Tago, E.; Owusu-Boaitey, K.E. Abnormal Mammary Adipose Tissue Environment of Brca1 Mutant Mice Show a Persistent Deposition of Highly Vascularized Multilocular Adipocytes. J Cancer Sci Ther 2011, 004. [Google Scholar] [CrossRef] [PubMed]
- Miran, I.; Scherer, D.; Ostyn, P.; Mazouni, C.; Drusch, F.; Bernard, M.; Louvet, E.; Adam, J.; Mathieu, M.-C.; Haffa, M.; et al. Adipose Tissue Properties in Tumor-Bearing Breasts. Front Oncol 2020, 10, 1506. [Google Scholar] [CrossRef]
- Koellensperger, E.; Bonnert, L.-C.; Zoernig, I.; Marmé, F.; Sandmann, S.; Germann, G.; Gramley, F.; Leimer, U. The Impact of Human Adipose Tissue-Derived Stem Cells on Breast Cancer Cells: Implications for Cell-Assisted Lipotransfers in Breast Reconstruction. Stem Cell Res Ther 2017, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; He, Y.; Yu, X. Bone Marrow Adipocyte: An Intimate Partner With Tumor Cells in Bone Metastasis. Front Endocrinol (Lausanne) 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- De Talhouet, S.; Peron, J.; Vuilleumier, A.; Friedlaender, A.; Viassolo, V.; Ayme, A.; Bodmer, A.; Treilleux, I.; Lang, N.; Tille, J.-C.; et al. Clinical Outcome of Breast Cancer in Carriers of BRCA1 and BRCA2 Mutations According to Molecular Subtypes. Sci Rep 2020, 10, 7073. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov 2015, 5, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, R.; Zuradelli, M.; Agostinetto, E.; Masci, G.; Losurdo, A.; De Sanctis, R.; Santoro, A. Platinum Salts in the Treatment of BRCA-Associated Breast Cancer: A True Targeted Chemotherapy? Crit Rev Oncol Hematol 2019, 135, 66–75. [Google Scholar] [CrossRef]
- Giannone, G.; Scotto, G.; Katsaros, D.; De Giorgi, U.; Farolfi, A.; Borella, F.; Cosma, S.; Ferrero, A.; Mangiacotti, S.; Villa, M.; et al. Hypersensitivity to Platinum Salts According to BRCA Status in Ovarian Cancer: A Retrospective Analysis of Clinical Outcomes and Systematic Review of Literature. Gynecol Oncol 2021, 162, 80–87. [Google Scholar] [CrossRef]
- Wattenberg, M.M.; Asch, D.; Yu, S.; O’Dwyer, P.J.; Domchek, S.M.; Nathanson, K.L.; Rosen, M.A.; Beatty, G.L.; Siegelman, E.S.; Reiss, K.A. Platinum Response Characteristics of Patients with Pancreatic Ductal Adenocarcinoma and a Germline BRCA1, BRCA2 or PALB2 Mutation. Br J Cancer 2020, 122, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Byrski, T.; Huzarski, T.; Dent, R.; Gronwald, J.; Zuziak, D.; Cybulski, C.; Kladny, J.; Gorski, B.; Lubinski, J.; Narod, S.A. Response to Neoadjuvant Therapy with Cisplatin in BRCA1-Positive Breast Cancer Patients. Breast Cancer Res Treat 2009, 115, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.N.; Wubbenhorst, B.; Wenz, B.M.; De Sloover, D.; Pluta, J.; Emery, L.; Barrett, A.; Kraya, A.A.; Anastopoulos, I.N.; Yu, S.; et al. BRCA Locus-Specific Loss of Heterozygosity in Germline BRCA1 and BRCA2 Carriers. Nat Commun 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Afghahi, A.; Timms, K.M.; Vinayak, S.; Jensen, K.C.; Kurian, A.W.; Carlson, R.W.; Chang, P.-J.; Schackmann, E.; Hartman, A.-R.; Ford, J.M.; et al. Tumor BRCA1 Reversion Mutation Arising during Neoadjuvant Platinum-Based Chemotherapy in Triple-Negative Breast Cancer Is Associated with Therapy Resistance. Clin Cancer Res 2017, 23, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Pilié, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-Art Strategies for Targeting the DNA Damage Response in Cancer. Nat Rev Clin Oncol 2019, 16, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Lord, C.J.; Serra, V.; Tutt, A.; Balmaña, J.; Castroviejo-Bermejo, M.; Cruz, C.; Oaknin, A.; Kaye, S.B.; de Bono, J.S. A Decade of Clinical Development of PARP Inhibitors in Perspective. Ann Oncol 2019, 30, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Bredow, K.; Blümcke, B.; Schneider, S.; Püsken, M.; Schmutzler, R.; Rhiem, K. Long-Term Survival of a BRCA2 Mutation Carrier Following Second Ovarian Cancer Relapse Using PARPi Therapy: A Case Report. Mol Clin Oncol 2022, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yu, X. Comparison between Talazoparib and Conventional Chemotherapy in the Treatment of HER2-Positive Breast Cancer Patients: A Retrospective Study. Front Immunol 2022, 13, 901636. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.M.; Moldovan, G.-L. Mechanisms of PARP1 Inhibitor Resistance and Their Implications for Cancer Treatment. NAR Cancer 2022, 4, zcac042. [Google Scholar] [CrossRef]
- Mustafina, O.E. The Possible Roles of Human Alu Elements in Aging. Front Genet 2013, 4, 96. [Google Scholar] [CrossRef]
- Morales, M.E.; White, T.B.; Streva, V.A.; DeFreece, C.B.; Hedges, D.J.; Deininger, P.L. The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair. PLoS Genet 2015, 11, e1005016. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Tookman, L.A.; Browne, A.K.; Connell, C.M.; Bridge, G.; Ingemarsdotter, C.K.; Dowson, S.; Shibata, A.; Lockley, M.; Martin, S.A.; McNeish, I.A. RAD51 and BRCA2 Enhance Oncolytic Adenovirus Type 5 Activity in Ovarian Cancer. Mol Cancer Res 2016, 14, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Reisländer, T.; Lombardi, E.P.; Groelly, F.J.; Miar, A.; Porru, M.; Di Vito, S.; Wright, B.; Lockstone, H.; Biroccio, A.; Harris, A.; et al. BRCA2 Abrogation Triggers Innate Immune Responses Potentiated by Treatment with PARP Inhibitors. Nat Commun 2019, 10, 3143. [Google Scholar] [CrossRef] [PubMed]
- Loizzi, V.; Dellino, M.; Cerbone, M.; Arezzo, F.; Cazzato, G.; Damiani, G.R.; Pinto, V.; Silvestris, E.; Kardhashi, A.; Cicinelli, E.; et al. The Role of Hormonal Replacement Therapy in BRCA Mutated Patients: Lights and Shadows. Int J Mol Sci 2023, 24, 764. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.F. Nonsurgical Prevention Strategies in BRCA1 and BRCA2 Mutation Carriers. Breast Care (Basel) 2021, 16, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Nehme, R.; Diab-Assaf, M.; Decombat, C.; Delort, L.; Caldefie-Chezet, F. Targeting Adiponectin in Breast Cancer. Biomedicines 2022, 10, 2958. [Google Scholar] [CrossRef]
- Zong, D.; Adam, S.; Wang, Y.; Sasanuma, H.; Callén, E.; Murga, M.; Day, A.; Kruhlak, M.J.; Wong, N.; Munro, M.; et al. BRCA1 Haploinsufficiency Is Masked by RNF168-Mediated Chromatin Ubiquitylation. Mol Cell 2019, 73, 1267–1281.e7. [Google Scholar] [CrossRef]
- Sullivan-Reed, K.; Bolton-Gillespie, E.; Dasgupta, Y.; Langer, S.; Siciliano, M.; Nieborowska-Skorska, M.; Hanamshet, K.; Belyaeva, E.A.; Bernhardy, A.J.; Lee, J.; et al. Simultaneous Targeting of PARP1 and RAD52 Triggers Dual Synthetic Lethality in BRCA-Deficient Tumor Cells. Cell Rep 2018, 23, 3127–3136. [Google Scholar] [CrossRef]
- Kayumov, M.; Jia, L.; Pardaev, A.; Song, S.-S.; Mirzaakhmedov, S.; Ding, C.; Cheng, Y.-J.; Zhang, R.I.; Bao, X.; Miao, Z.-H.; et al. Design, Synthesis and Pharmacological Evaluation of New PARP1 Inhibitors by Merging Pharmacophores of Olaparib and the Natural Product Alantolactone. Eur J Med Chem 2022, 240, 114574. [Google Scholar] [CrossRef]
- Gorodnova, T.V.; Sokolenko, A.P.; Kotiv, K.B.; Sokolova, T.N.; Ivantsov, A.O.; Guseynov, K.D.; Nekrasova, E.A.; Smirnova, O.A.; Berlev, I.V.; Imyanitov, E.N. Neoadjuvant Therapy of BRCA1-Driven Ovarian Cancer by Combination of Cisplatin, Mitomycin C and Doxorubicin. Hered Cancer Clin Pr. 2021, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Chatterjee, S.; Paul, S.; Das, B.; Dash, S.R.; Das, C.; Kundu, C.N. Olaparib Enhances the Resveratrol-Mediated Apoptosis in Breast Cancer Cells by Inhibiting the Homologous Recombination Repair Pathway. Exp Cell Res 2022, 420, 113338. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xu, H.; George, E.; Hallberg, D.; Kumar, S.; Jagannathan, V.; Medvedev, S.; Kinose, Y.; Devins, K.; Verma, P.; et al. Combining PARP with ATR Inhibition Overcomes PARP Inhibitor and Platinum Resistance in Ovarian Cancer Models. Nat Commun 2020, 11, 3726. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hurley, L.H. A First-in-Class Clinical G-Quadruplex-Targeting Drug. The Bench-to-Bedside Translation of the Fluoroquinolone QQ58 to CX-5461 (Pidnarulex). Bioorg Med Chem Lett 2022, 77, 129016. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Loibl, S.; Hu, C.; Hart, S.N.; Shimelis, H.; Moore, R.; Schem, C.; Tesch, H.; Untch, M.; Hilfrich, J.; et al. BRCA1/2 Mutations and Bevacizumab in the Neoadjuvant Treatment of Breast Cancer: Response and Prognosis Results in Patients With Triple-Negative Breast Cancer From the GeparQuinto Study. J Clin Oncol 2018, 36, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of P53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.T.; Kubota, E.; Hamill, J.D.; Klimowicz, A.; Ye, R.; Muzik, H.; Dean, M.; Tu, L.; Gilley, D.; Magliocco, A.M.; et al. Enhanced Cytotoxicity of PARP Inhibition in Mantle Cell Lymphoma Harbouring Mutations in Both ATM and P53. EMBO Mol Med 2012, 4, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Smeby, J.; Kryeziu, K.; Berg, K.C.G.; Eilertsen, I.A.; Eide, P.W.; Johannessen, B.; Guren, M.G.; Nesbakken, A.; Bruun, J.; Lothe, R.A.; et al. Molecular Correlates of Sensitivity to PARP Inhibition beyond Homologous Recombination Deficiency in Pre-Clinical Models of Colorectal Cancer Point to Wild-Type TP53 Activity. EBioMedicine 2020, 59, 102923. [Google Scholar] [CrossRef]
- Hong, T.; Lei, G.; Chen, X.; Li, H.; Zhang, X.; Wu, N.; Zhao, Y.; Zhang, Y.; Wang, J. PARP Inhibition Promotes Ferroptosis via Repressing SLC7A11 and Synergizes with Ferroptosis Inducers in BRCA-Proficient Ovarian Cancer. Redox Biol 2021, 42, 101928. [Google Scholar] [CrossRef]
- Feng, Z.; Kachnic, L.; Zhang, J.; Powell, S.N.; Xia, F. DNA Damage Induces P53-Dependent BRCA1 Nuclear Export. J Biol Chem 2004, 279, 28574–28584. [Google Scholar] [CrossRef]
- Parfenyev, S.; Singh, A.; Fedorova, O.; Daks, A.; Kulshreshtha, R.; Barlev, N.A. Interplay between P53 and Non-Coding RNAs in the Regulation of EMT in Breast Cancer. Cell Death Dis 2021, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Barlev, N.A.; Sayan, B.S.; Candi, E.; Okorokov, A.L. The MicroRNA and P53 Families Join Forces against Cancer. Cell Death Differ 2010, 17, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Lezina, L.; Aksenova, V.; Fedorova, O.; Malikova, D.; Shuvalov, O.; Antonov, A.V.; Tentler, D.; Garabadgiu, A.V.; Melino, G.; Barlev, N.A. KMT Set7/9 Affects Genotoxic Stress Response via the Mdm2 Axis. Oncotarget 2015, 6, 25843–25855. [Google Scholar] [CrossRef] [PubMed]
- Portman, N.; Milioli, H.H.; Alexandrou, S.; Coulson, R.; Yong, A.; Fernandez, K.J.; Chia, K.M.; Halilovic, E.; Segara, D.; Parker, A.; et al. MDM2 Inhibition in Combination with Endocrine Therapy and CDK4/6 Inhibition for the Treatment of ER-Positive Breast Cancer. Breast Cancer Res 2020, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Bulatov, E.; Sayarova, R.; Mingaleeva, R.; Miftakhova, R.; Gomzikova, M.; Ignatyev, Y.; Petukhov, A.; Davidovich, P.; Rizvanov, A.; Barlev, N.A. Isatin-Schiff Base-Copper (II) Complex Induces Cell Death in P53-Positive Tumors. Cell Death Discov 2018, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, P.; Aksenova, V.; Petrova, V.; Tentler, D.; Orlova, D.; Smirnov, S.; Gurzhiy, V.; Okorokov, A.L.; Garabadzhiu, A.; Melino, G.; et al. Discovery of Novel Isatin-Based P53 Inducers. ACS Med Chem Lett 2015, 6, 856–860. [Google Scholar] [CrossRef]
- Fedorova, O.; Daks, A.; Petrova, V.; Petukhov, A.; Lezina, L.; Shuvalov, O.; Davidovich, P.; Kriger, D.; Lomert, E.; Tentler, D.; et al. Novel Isatin-Derived Molecules Activate P53 via Interference with Mdm2 to Promote Apoptosis. Cell Cycle 2018, 17, 1917–1930. [Google Scholar] [CrossRef]
| PARP Inhibitor |
Cancer Type | Co-Target | Co-Treatment | Phase | Register Number |
|---|---|---|---|---|---|
| Olaparib | BC,OC,FTC,EndA, UCC | mTORC1/2 or AKT |
Vistusertib or Capivasertib | 1b | NCT02208375 |
| Olaparib | OC, FTC, PPC | CTLA-4 | Tremelimumab | 2 | NCT02571725 |
| Talazoparib | TNBC | mTOR/PI3K | Gedatolisib | 2 | NCT03911973 |
| Olaparib | BC | CDK4,6 and HR |
Palbociclib, Fulvestrant | 1 | NCT03685331 |
| Niraparib | FTC,OC,EndA,PPC | PI3K | Copanlisib | 1 | NCT03586661 |
| Olaparib | TNBC | PD-L1 | Durvalumab | 2 | NCT05209529 |
| Niraparib | PanC | PD-1 | Dostarlimab | 2 | NCT04493060 |
| Talazoparib | melanoma | PD-1 | Nivolumab | 2 | NCT04187833 |
| Niraparib | rare tumors | PD-1 | Sintilimab | 2 | NCT04423185 |
| Olaparib | BC | VEGFR or ATR |
Cediranib or Ceralasertib | 2 | NCT04090567 |
| Fluzoparib | HER2- BC | VEGFR | Apatinib | 3 | NCT04296370 |
| Olaparib | OC, FTC, genital neoplasms |
multiple receptor tyrosine kinases | Anlotinib | 1 | NCT04566952 |
| Olaparib | serous OC | ATR | Ceralasertib | 2 | NCT03462342 |
| Rucaparib | mesothelioma | - | - | 2 | NCT03654833 |
| Olaparib | Pt-resistant OC | CDK4,6 | Abemaciclib | 1/1b | NCT04633239 |
| Olaparib | prostate cancer | LRHL | Leuprolide | 2 | NCT05498272 |
| Talazoparib | OC and other | BRD2,3,4 | ZEN-3694 | 2 | NCT05327010 |
| Veliparib | BC | BRCA1,2 | Temozolomide | 2 | NCT01009788 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





