Submitted:
30 January 2023
Posted:
07 February 2023
You are already at the latest version
Abstract
Keywords:
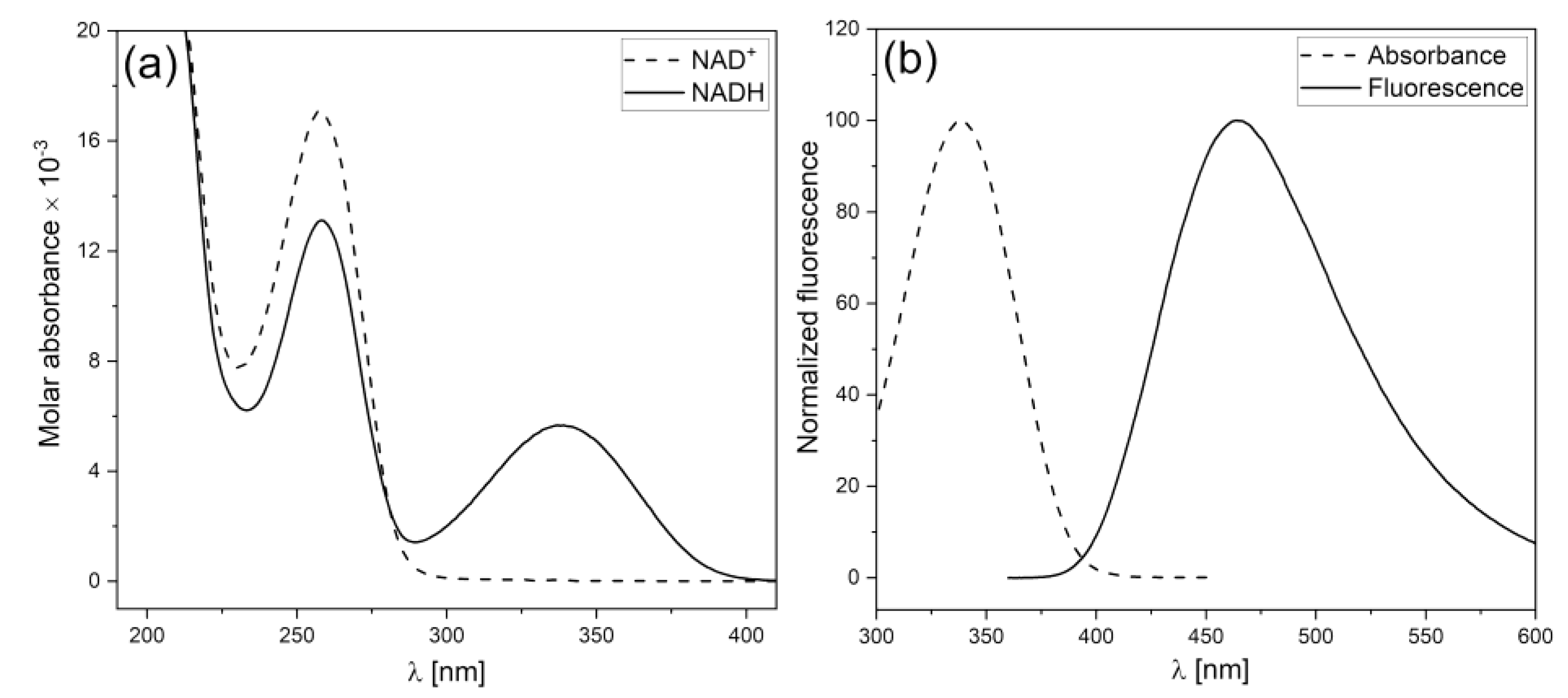
1. Fundamentals of the FMSF methodology
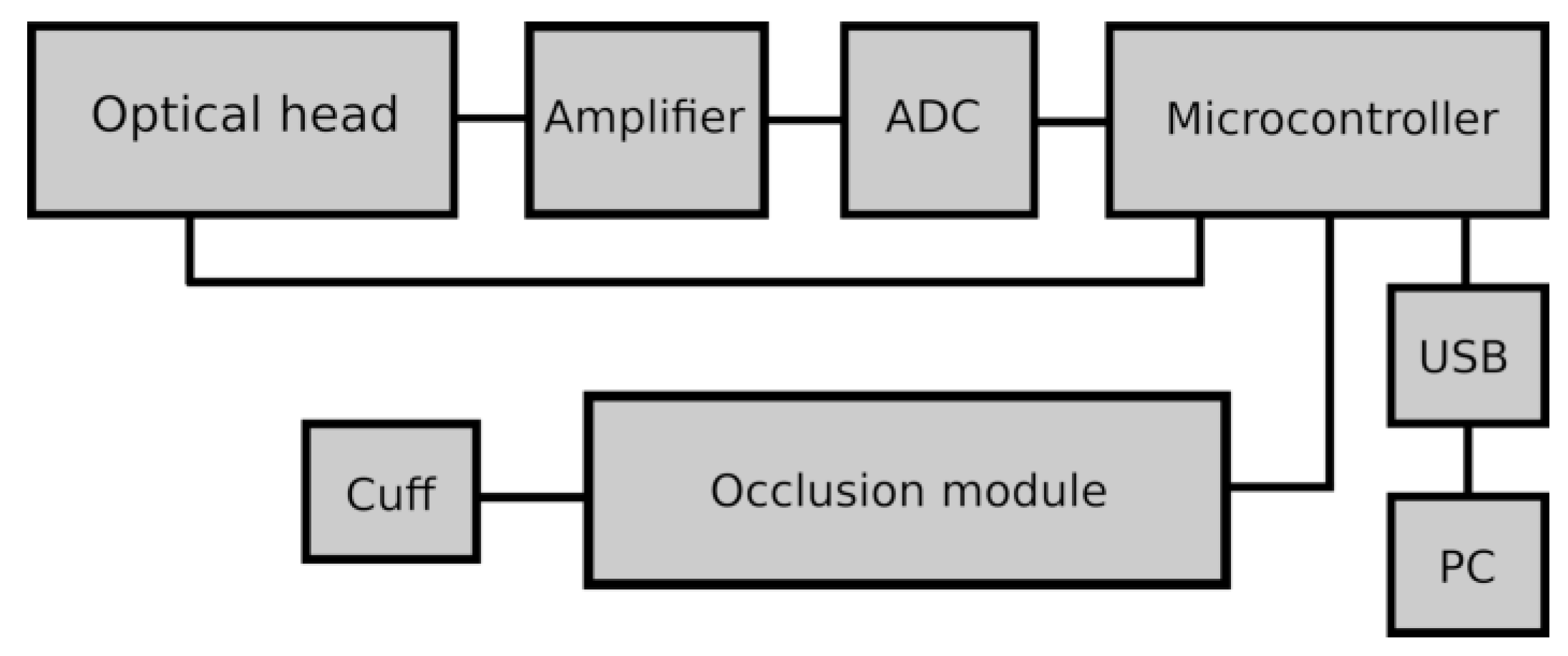
2. Technical aspects of the FMSF measurement
3. Definition of the measured FMSF parameters
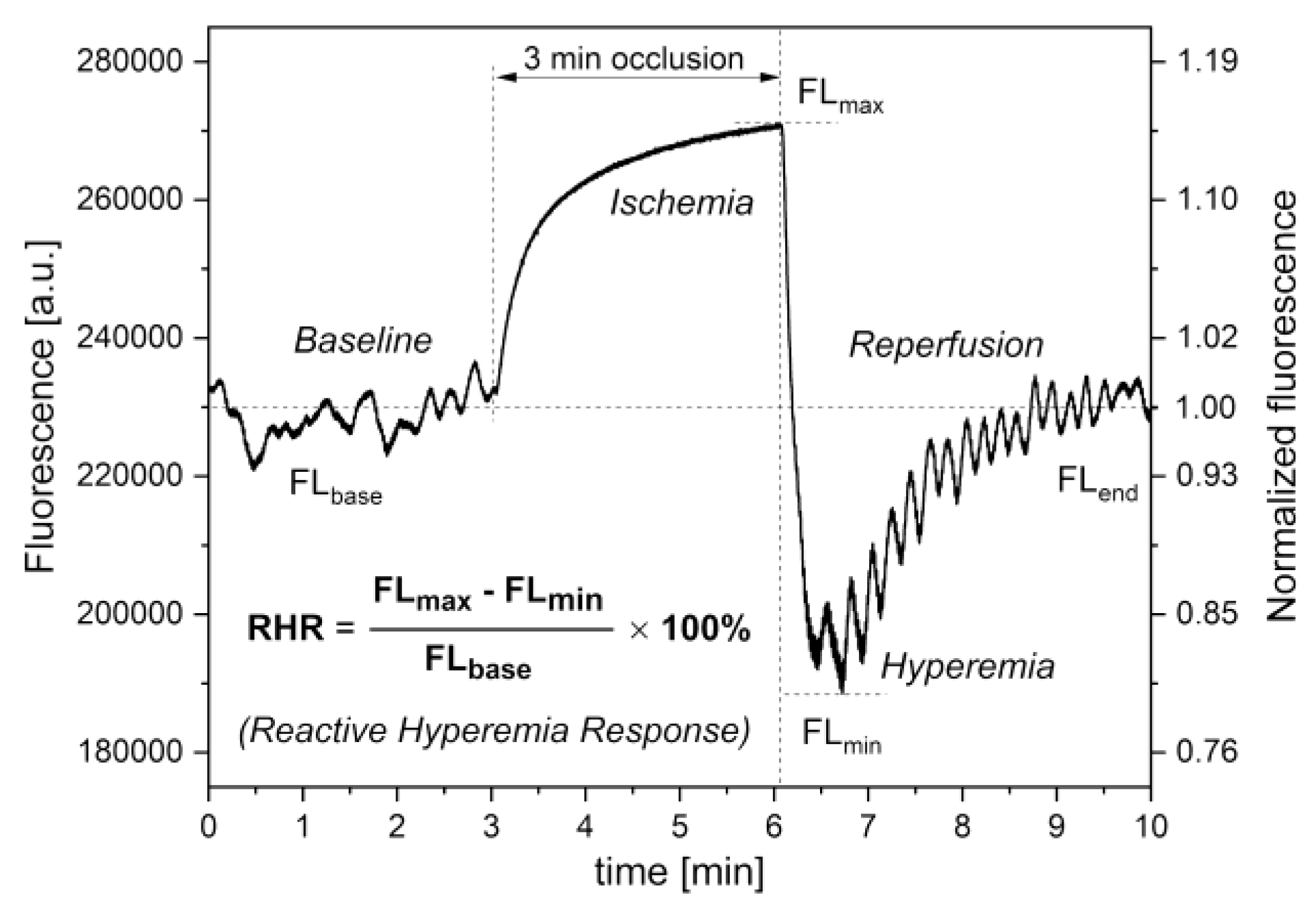
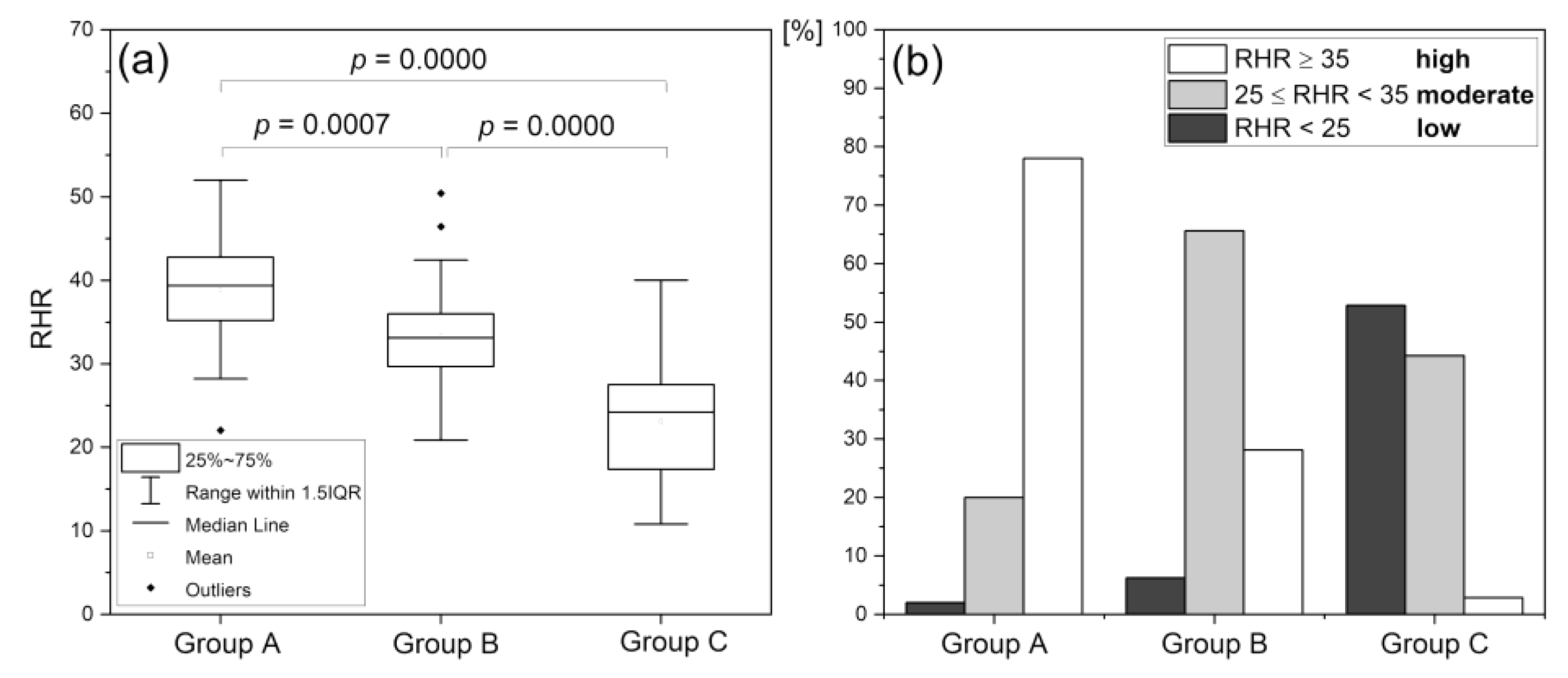
Reactive Hyperemia Response (RHR)
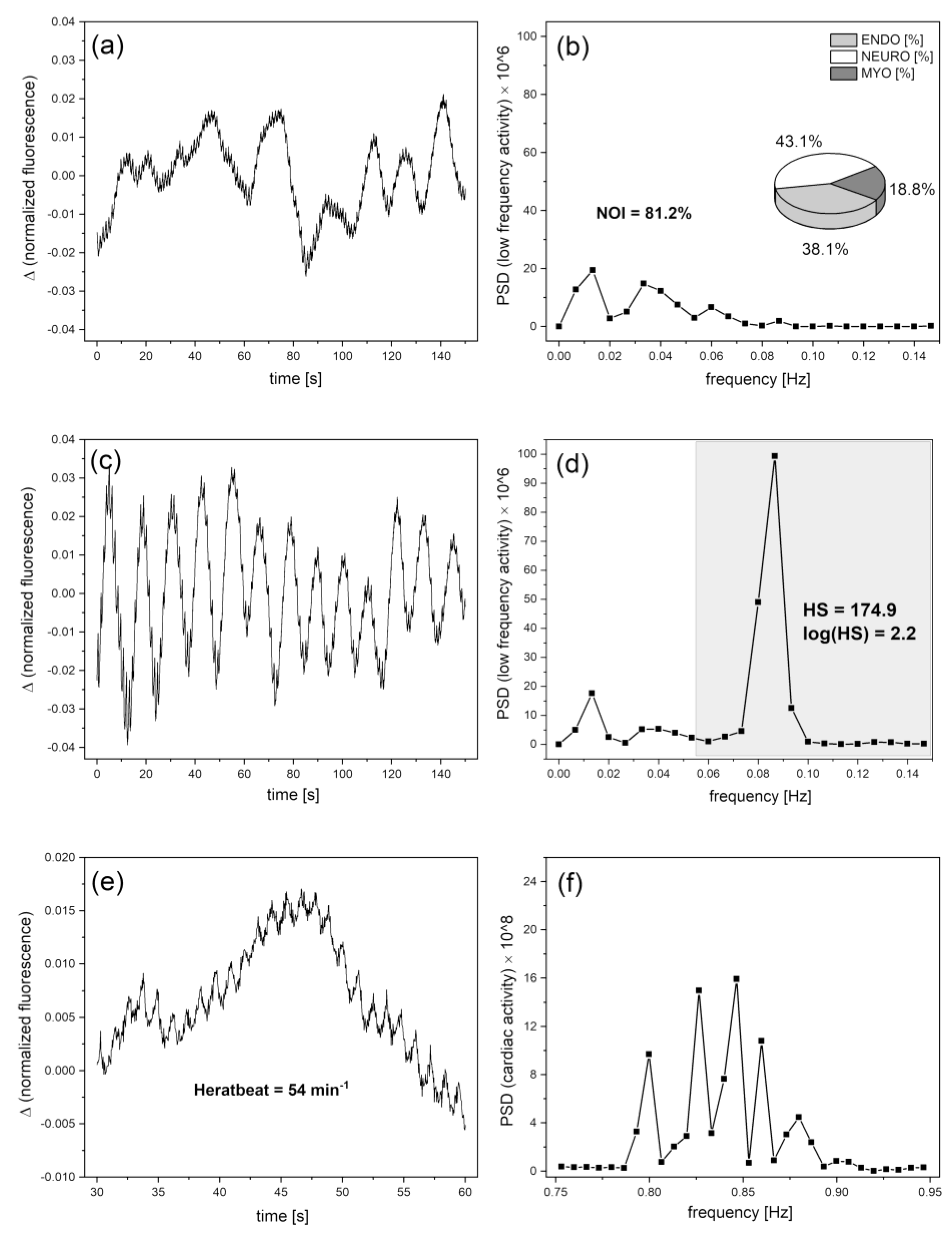
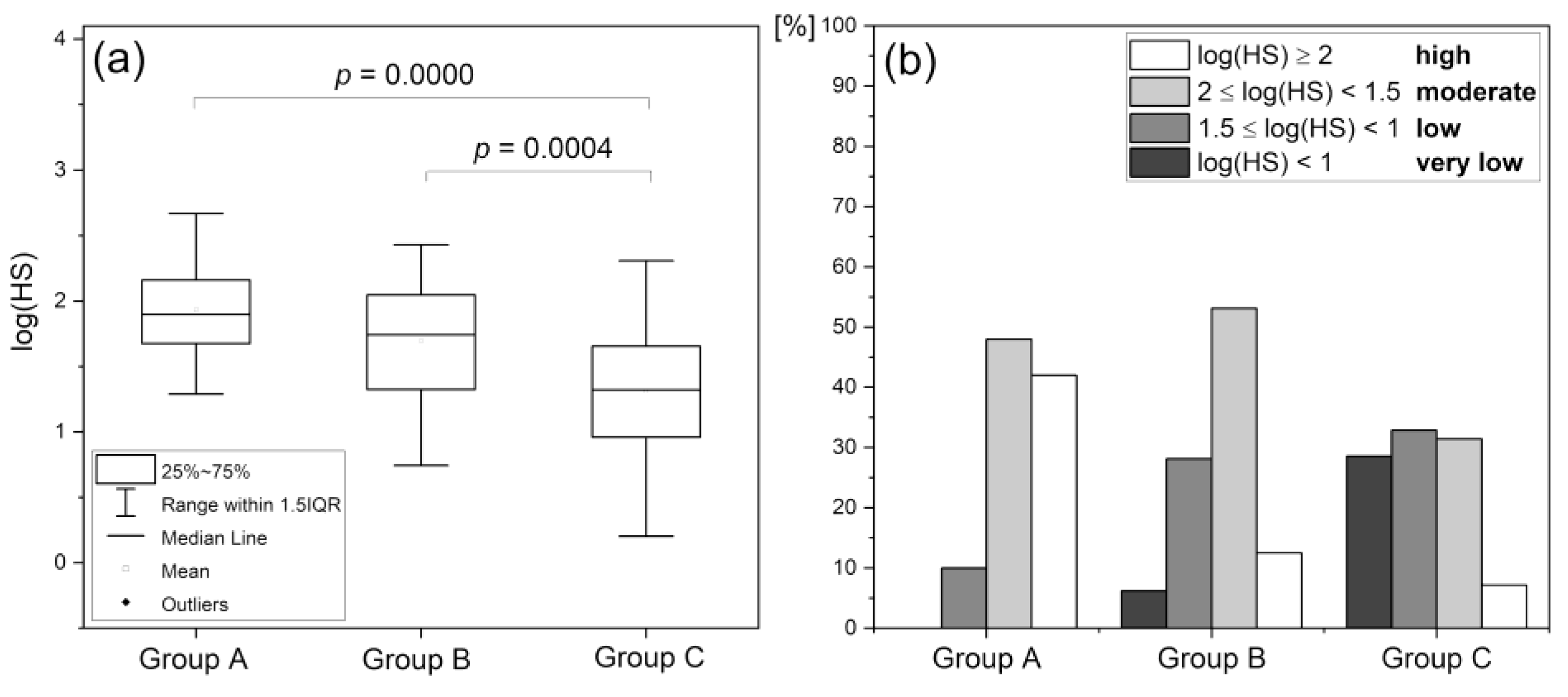
Hypoxia Sensitivity (HS)
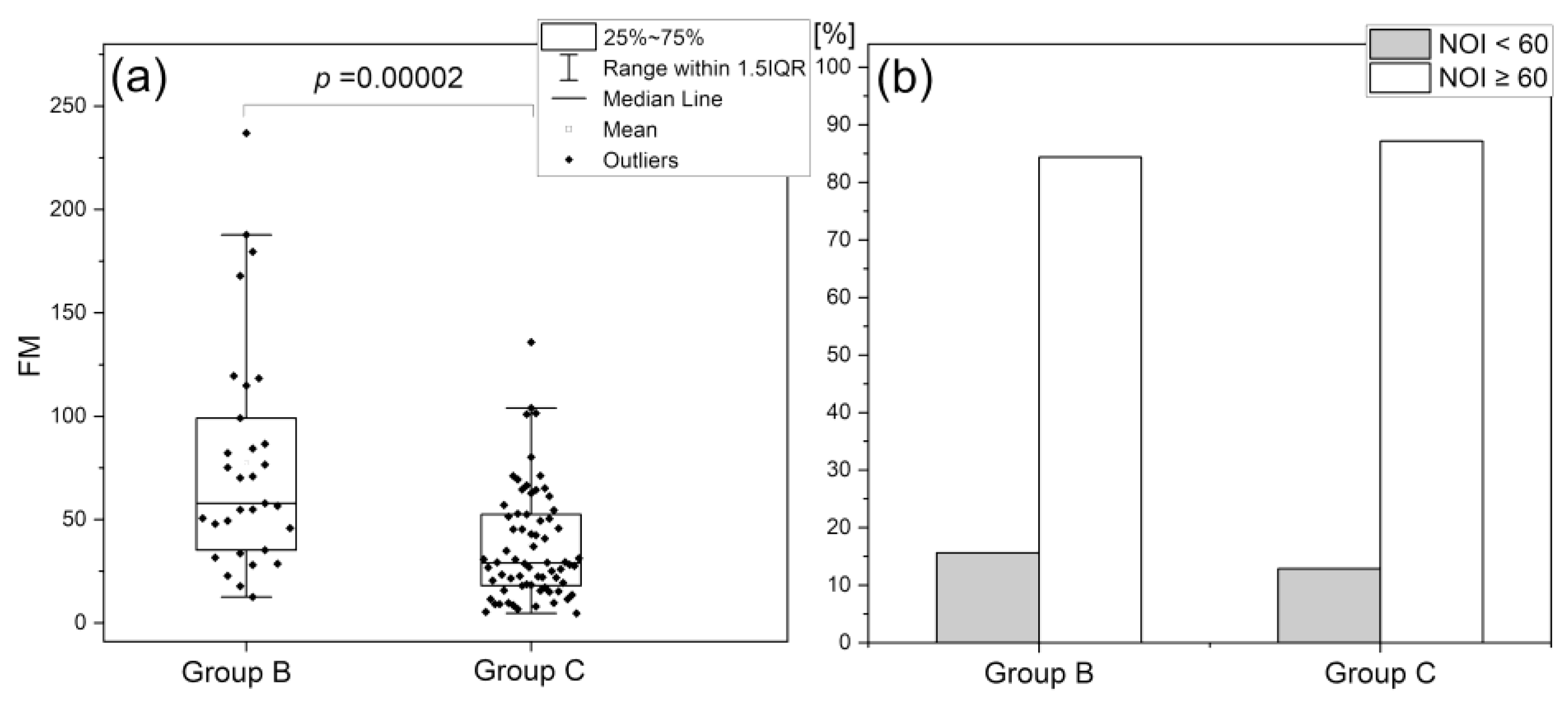
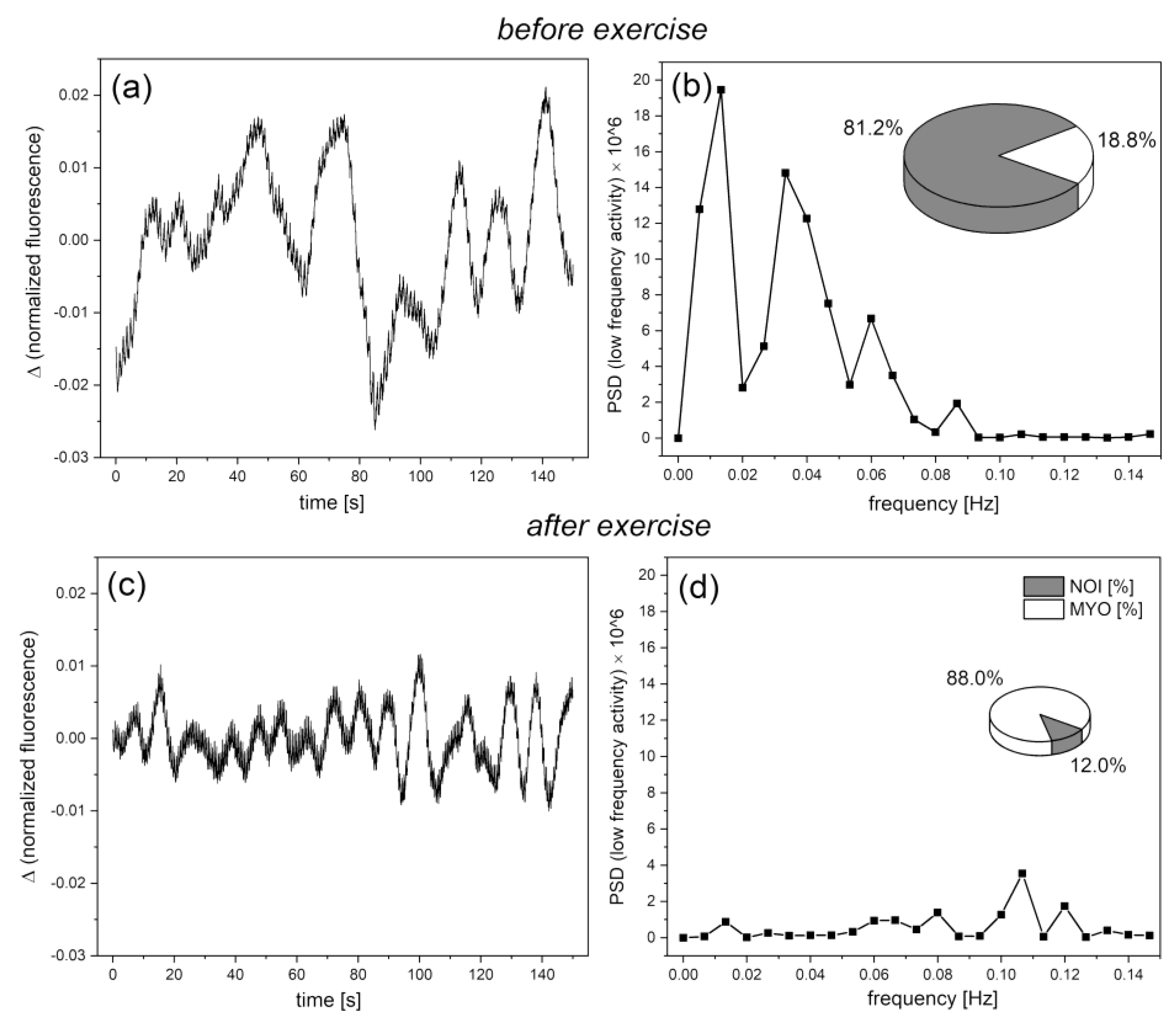
Normoxia Oscillatory Index (NOI)
Cardiac oscillations
4. Diagnostic potential of the FMSF method
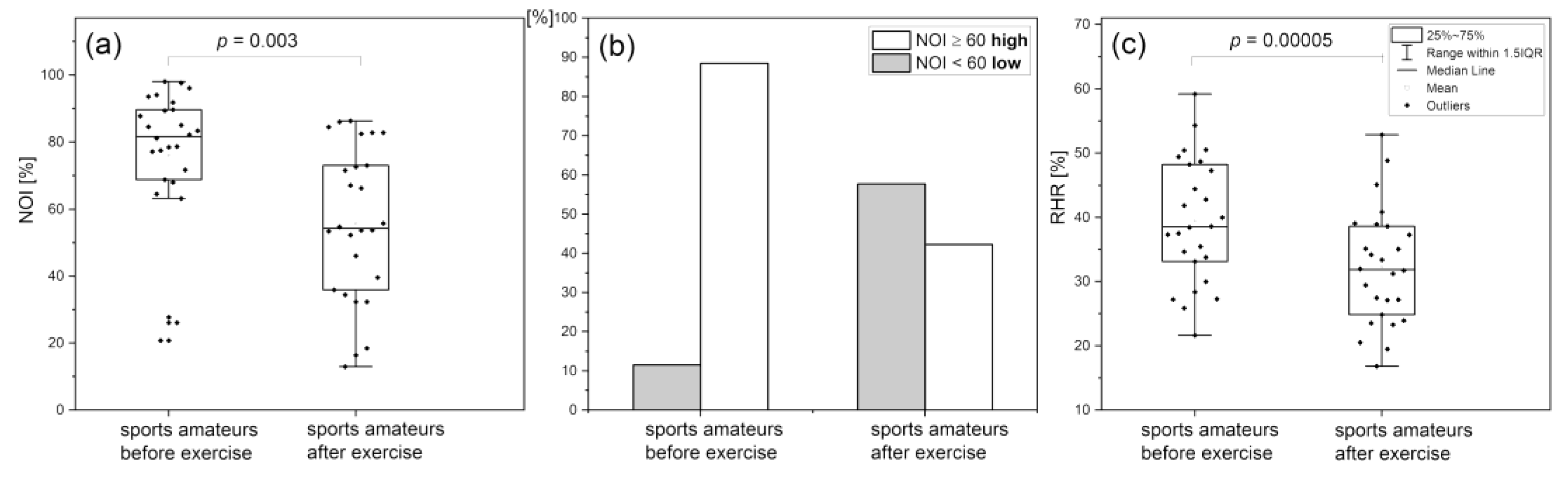
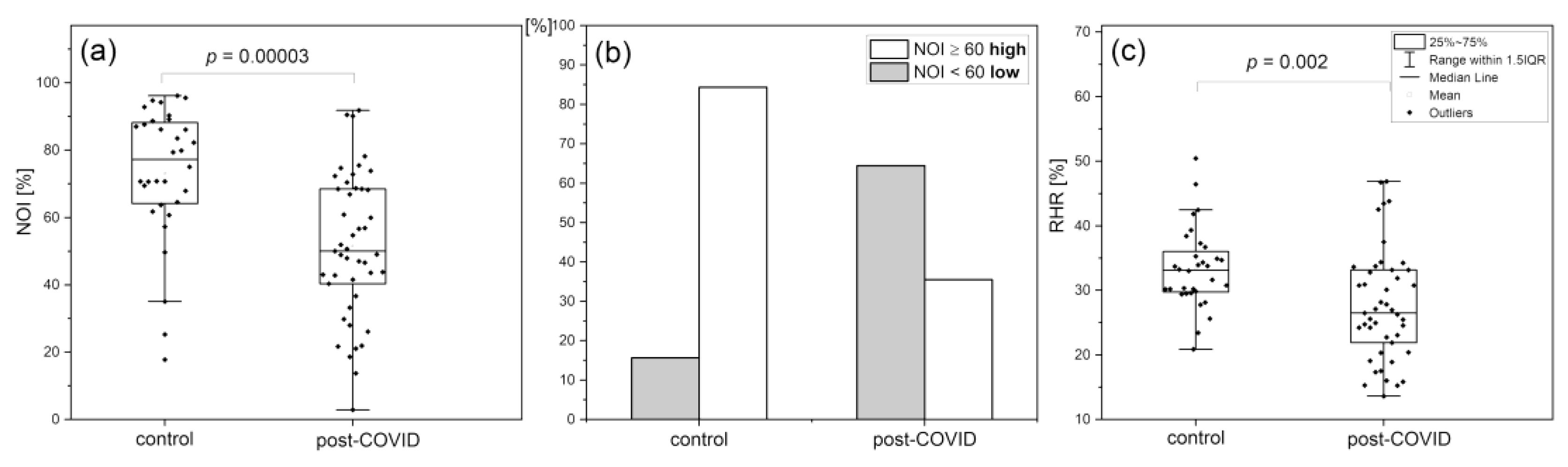
Reactive Hyperemia Response (RHR)
Hypoxia Sensitivity (HS)
Normoxia Oscillatory Index (NOI)
5. Concluding remarks
- -
- assessing dysfunction of the vascular circulation, especially the macrocirculation;
- -
- predicting the risk of developing cardiovascular diseases often comorbid with diabetes mellitus.
- -
- assessing microcirculatory dysfunction in diabetes, cardiovascular disease, peripheral arterial disease, hypertension;
- -
- predicting healing in difficult-to-heal wounds (including diabetic foot ulcers);
- -
- assessing microcirculatory status to determine exercise tolerance in healthy and non-healthy people.
- -
- assessing fatigue due to stress from various sources,
- -
- monitoring microcirculation in the recovery and rehabilitation process.
Author Contributions
Funding
Conflicts of Interest
References
- Vita, J.A.; Keaney, J.F. Endothelial Function. Circulation 2002, 106, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Gryglewski, R.J. Pharmacology of vascular endothelium. FEBS J. 2005, 272, 2956–2967. [Google Scholar] [CrossRef] [PubMed]
- Chłopicki, S.; Gryglewski, R. Angiotensin converting enzyme (ACE) and HydroxyMethylGlutaryl-CoA (HMG-CoA) reductase inhibitors in the forefront of pharmacology of endothelium. Pharmacol Rep. 2005, 57, 86–96. [Google Scholar] [PubMed]
- Giannotti, G.; Landmesser, U. Endothelial Dysfunction as an Early Sign of Atherosclerosis. Herz Kardiovaskuläre Erkrank. 2007, 32, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.; Roustit, M.; Cracowski, J.L. Skin microvascular endothelial function as a biomarker in cardiovascular diseases? Pharmacol Rep. 2015, 67, 803–810. [Google Scholar] [CrossRef]
- Cracowski, J.-L.; Roustit, M. Human Skin Microcirculation. Comprehensive Physiology 2020, 1105–1154. [Google Scholar] [CrossRef] [PubMed]
- Ijzerman, R.G.; De Jongh, R.T.; Beijk, M.A.M.; Van Weissenbruch, M.M.; Delemarre-van de Waal, H.A.; Serné, EH.; et al. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest. 2003, 33, 536–542. [Google Scholar] [CrossRef]
- Rossi, M.; Matteucci, E.; Pesce, M.; Consani, C.; Franzoni, F.; Santoro, G.; et al. Peripheral microvascular dysfunction as an independent predictor of atherosclerotic damage in type 1 diabetes patients: A preliminary study. Clin Hemorheol Microcirc. 2013, 54, 381–391. [Google Scholar] [CrossRef]
- Gȩbicki, J.; Marcinek, A.; Zielonka, J. Transient Species in the Stepwise Interconversion of NADH and NAD+. Acc Chem Res. 2004, 37, 379–386. [Google Scholar] [CrossRef]
- McMullen, R.L.; Chen, S.; Moore, D.J. Spectrofluorescence of skin and hair. Int J Cosmet Sci. 2012, 34, 246–256. [Google Scholar] [CrossRef]
- Cicchi, R.; Vogler, N.; Kapsokalyvas, D.; Dietzek, B.; Popp, J.; Pavone, F.S. From molecular structure to tissue architecture: Collagen organization probed by SHG microscopy. J Biophotonics. 2013, 6, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Dunaev, A.V.; Dremin, V.V.; Zherebtsov, E.A.; Rafailov, I.E.; Litvinova, K.S.; Palmer, S.G.; et al. Individual variability analysis of fluorescence parameters measured in skin with different levels of nutritive blood flow. Med Eng Phys. 2015, 37, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Du, G.; Wang, Y.; Su, C.; Guo, L.; Chen, X. Noninvasive in vivo study of NADH fluorescence and its real-time intrinsic dynamical changes: Experiments and seven-layered skin model Monte Carlo simulations. J Innov Opt Health Sci. 2022, 15, 2230006. [Google Scholar] [CrossRef]
- Mayevsky, A.; Rogatsky, G.G. Mitochondrial function in vivo evaluated by NADH fluorescence: From animal models to human studies. Am J Physiol Physiol. 2007, 292, C615–C640. [Google Scholar] [CrossRef] [PubMed]
- Mayevsky, A.; Barbiro-Michaely, E. Use of NADH fluorescence to determine mitochondrial function in vivo. Int J Biochem Cell Biol. 2009, 41, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Balu, M.; Mazhar, A.; Hayakawa, C.K.; Mittal, R.; Krasieva, T.B.; König, K.; et al. In vivo multiphoton NADH fluorescence reveals depth-dependent keratinocyte metabolism in human skin. Biophys J. 2013, 104, 258–267. [Google Scholar] [CrossRef]
- Pouli, D.; Balu, M.; Alonzo, C.A.; Liu, Z.; Quinn, K.P.; Rius-Diaz, F. Imaging mitochondrial dynamics in human skin reveals depth-dependent hypoxia and malignant potential for diagnosis. Sci Transl Med. 2016, 8, 367ra169. [Google Scholar] [CrossRef] [PubMed]
- Roustit, M.; Cracowski, J.L. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci. 2013, 34, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef]
- Modena, M.G.; Bonetti, L.; Coppi, F.; Bursi, F.; Rossi, R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002, 40, 505–510. [Google Scholar] [CrossRef]
- Brevetti, G.; Silvestro, A.; Schiano, V.; Chiariello, M. Endothelial Dysfunction and Cardiovascular Risk Prediction in Peripheral Arterial Disease. Circulation 2003, 108, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Gokce, N.; Keaney, J.F.; Hunter, L.M.; Watkins, M.T.; Nedeljkovic, Z.S.; Menzoian, J.O.; et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003, 41, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Mancini, G.B.J.; Kuramoto, L.; Schulzer, M.; Frohlich, J.; Ignaszewski, A. The prognostic importance of endothelial dysfunction and carotid atheromaburden in patients with coronary artery disease. J Am Coll Cardiol. 2003, 42, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Crouse, J.R.; Hsu, F.-C.; Burke, G.L.; Herrington, D.M. Brachial Flow-Mediated Dilation Predicts Incident Cardiovascular Events in Older Adults. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef]
- Huang, A.L.; Silver, A.E.; Shvenke, E.; Schopfer, D.W.; Jahangir, E.; Titas, M.A.; et al. Predictive Value of Reactive Hyperemia for Cardiovascular Events in Patients With Peripheral Arterial Disease Undergoing Vascular Surgery. Arterioscler Thromb Vasc Biol. 2007, 27, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; et al. Predictive Value of Brachial Flow-Mediated Dilation for Incident Cardiovascular Events in a Population-Based Study. Circulation 2009, 120, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kitta, Y.; Obata, J.; Nakamura, T.; Hirano, M.; Kodama, Y.; Fujioka, D.; et al. Persistent Impairment of Endothelial Vasomotor Function Has a Negative Impact on Outcome in Patients With Coronary Artery Disease. J Am Coll Cardiol. 2009, 53, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Gori, T.; Parker, J.D.; Münzel, T. Flow-mediated constriction: Further insight into a new measure of vascular function. Eur Heart J. 2011, 32, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Gori, T.; Muxel, S.; Damaske, A.; Radmacher, M.-C.; Fasola, F.; Schaefer, S.; et al. Endothelial function assessment: Flow-mediated dilation and constriction provide different and complementary information on the presence of coronary artery disease. Eur Heart J. 2012, 33, 363–371. [Google Scholar] [CrossRef]
- Spiro, J.R.; Digby, J.E.; Ghimire, G.; Mason, M.; Mitchell, A.G.; Ilsley, C.; et al. Brachial artery low-flow-mediated constriction is increased early after coronary intervention and reduces during recovery after acute coronary syndrome: Characterization of a recently described index of vascular function. Eur Heart J. 2011, 32, 856–866. [Google Scholar] [CrossRef]
- Cracowski, J.-L.; Minson, C.T.; Salvat-Melis, M.; Halliwill, J.R. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006, 27, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Roustit, M.; Cracowski, J.-L. Non-invasive Assessment of Skin Microvascular Function in Humans: An Insight into Methods. Microcirculation 2012, 19, 47–64. [Google Scholar] [CrossRef]
- Sibrecht, G.; Bugaj, O.; Filberek, P.; Nizinski, J.; Kusy, K.; Zielinski, J.; et al. Flow-Mediated Skin Fluorescence Method for Non-Invasive Measurement of the NADH at 460 nm–a Possibility to Assess the Mitochondrial Function. Postępy Biol Komórki. 2017, 44, 333–352. [Google Scholar]
- Nilsson, H.; Aalkjaer, C. Vasomotion: Mechanisms and physiological importance. Mol Interv. 2003, 3, 79–89, 51. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Carpi, A.; Galetta, F.; Franzoni, F.; Santoro, G. The investigation of skin blood flowmotion: A new approach to study the microcirculatory impairment in vascular diseases? Biomed Pharmacother. 2006, 60, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Aalkjær, C.; Boedtkjer, D.; Matchkov, V. Vasomotion–what is currently thought? Acta Physiol. 2011, 202, 253–269. [Google Scholar] [CrossRef]
- Stefanovska, A.; Bracic, M.; Kvernmo, H.D. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans Biomed Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef]
- Kvandal, P.; Landsverk, S.A.; Bernjak, A.; Stefanovska, A.; Kvernmo, H.D.; Kirkebøen, K.A. Low-frequency oscillations of the laser Doppler perfusion signal in human skin. Microvasc Res. 2006, 72, 120–127. [Google Scholar] [CrossRef]
- Bernjak, A.; Clarkson, P.B.M.; McClintock, P.V.E.; Stefanovska, A. Low-frequency blood flow oscillations in congestive heart failure and after beta1-blockade treatment. Microvasc Res. 2008, 76, 224–232. [Google Scholar] [CrossRef]
- Clough, G.F.; Kuliga, K.Z.; Chipperfield, A.J. Flow motion dynamics of microvascular blood flow and oxygenation: Evidence of adaptive changes in obesity and type 2 diabetes mellitus/insulin resistance. Microcirculation 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Semenza, G.L. Pharmacologic Targeting of Hypoxia-Inducible Factors. Annu Rev Pharmacol Toxicol. 2019, 59, 379–403. [Google Scholar] [CrossRef]
- Katarzynska, J.; Lipinski, Z.; Cholewinski, T.; Piotrowski, L.; Dworzynski, W.; Urbaniak, M.; et al. Non-invasive evaluation of microcirculation and metabolic regulation using flow mediated skin fluorescence (FMSF): Technical aspects and methodology. Rev Sci Instrum. 2019, 90, 104104. [Google Scholar] [CrossRef]
- Katarzynska, J.; Borkowska, A.; Czajkowski, P.; Los, A.; Szczerbinski, L.; Milewska-Kranc, A.; et al. Flow Mediated Skin Fluorescence technique reveals remarkable effect of age on microcirculation and metabolic regulation in type 1 diabetes. Microvasc Res. 2019, 124, 19–24. [Google Scholar] [CrossRef]
- Gebicki, J.; Katarzynska, J.; Marcinek, A. Effect of prolonged psychological stress on microcirculation oscillations: Diagnostic aspects. Vasc Health Risk Manag. (accepted). 2023. [Google Scholar] [CrossRef] [PubMed]
- Katarzyńska, J.; Zieliński, J.; Marcinek, A.; Gebicki, J. New Approach to Non-Invasive Assessment of Vascular Circulation Based on the Response to Transient Ischemia. Vasc Health Risk Manag. 2022, 18, 113–116. [Google Scholar] [CrossRef]
- Catrina, S.B.; Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef]
- Zheng, X.; Narayanan, S.; Xu, C.; Eliasson Angelstig, S.; Grünler, J.; Zhao, A.; et al. Repression of hypoxia-inducible factor-1 contributes to increased mitochondrial reactive oxygen species production in diabetes. Schipani E, Zaidi M, Schipani E, editors. Elife 2022, 11, e70714. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, A. , Gnudi, L. VEGF and angiopoietins in diabetic glomerulopathy: How far for a new treatment? Metabolism 2012, 61, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Botusan, I.R.; Sunkari, V.G.; Savu, O.; Catrina, A.I.; Grünler, J.; Lindberg, S.; et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA 2008, 105, 19426–19431. [Google Scholar] [CrossRef]
- Dae Ryong, C.; Nan Hee, K.; Jong Woo, Y.; Sang Kyung, J.; Won Yong, C.; Hyoung Kyu, K.; et al. Role of vascular endothelial growth factor in diabetic nephropathy. Kidney Int Suppl. 2000, 58, 104–112. [Google Scholar]
- Tanaka, T. Expanding roles of the hypoxia-response network in chronic kidney disease. Clin Exp Nephrol. 2016, 20, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Los-Stegienta, A.; Borkowska, A.; Cypryk, K. Assessment of microvascular function using a novel technique Flow Mediated Skin Fluorescence (FMSF) in patients with diabetic kidney disease: A preliminary study. Microvasc Res. 2022, 144, 104417. [Google Scholar] [CrossRef] [PubMed]
- Katarzynska, J.; Borkowska, A.; Los, A.; Marcinek, A.; Cypryk, K.; Gebicki, J. Flow-Mediated Skin Fluorescence (FMSF) Technique for Studying Vascular Complications in Type 2 Diabetes. J Diabetes Sci Technol. 2020, 14, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.; Marcinek, A.; Zielinski, J. Assessment of Microcirculatory Status Based on Stimulation of Myogenic Oscillations by Transient Ischemia: From Health to Disease. Vasc Health Risk Manag. 2021, 17, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.; Katarzynska, J.; Cholewinski, T.; Sieron, L.; Marcinek, A. Flowmotion Monitored by Flow Mediated Skin Fluorescence (FMSF): A Tool for Characterization of Microcirculatory Status. Front Physiol. 2020, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Thangarajah, H.; Vial, I.N.; Grogan, R.H.; Yao, D.; Shi, Y.; Januszyk, M.; et al. HIF-1α dysfunction in diabetes. Cell Cycle. 2010, 9, 75–79. [Google Scholar] [CrossRef]
- Thangarajah, H.; Yao, D.; Chang, E.I.; Shi, Y.; Jazayeri, L.; Vial, I.N.; et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA 2009, 106, 13505–13510. [Google Scholar] [CrossRef]
- Lin, C.; Yin, G.; Ou, M.; Zheng, S. The effects of HIF-1α and VEGF on wound healing in diabetic mice. Biomed Res. 2017, 28, 8121–8124. [Google Scholar]
- Los-Stegienta, A.; Katarzynska, J.; Borkowska, A.; Marcinek, A.; Cypryk, K.; Gebicki, J. Differentiation of diabetic foot ulcers based on stimulation of myogenic oscillations by transient ischemia. Vasc Health Risk Manag. 2021, 17, 145–152. [Google Scholar] [CrossRef]
- Dawson, E.A.; Green, D.J.; Cable, N.T.; Thijssen, D.H.J. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol. 2013, 115, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- McClean, C.; Harris, R.A.; Brown, M.; Brown, J.C.; Davison, G.W. Effects of Exercise Intensity on Postexercise Endothelial Function and Oxidative Stress. Golbidi, S., editor. Oxid Med Cell Longev. 2015, 2015, 723679. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.A.; Graham, H.K.; Mollan, R.A.B.; Riddoch, C.; Sheridan, B.; Johnston, H. Calcium homeostasis and exercise. Int Orthop. 1989, 13, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Maïmoun, L.; Sultan, C. Effect of Physical Activity on Calcium Homeostasis and Calciotropic Hormones: A Review. Calcif Tissue Int. 2009, 85, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. , Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K. , Libby, P., Ridker, P.M. COVID-19—A vascular disease. Trends Cardiovasc Med. 2021, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep. 2021, 9, e14726. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.J.; Scheibenbogen, C. Dyspnea in Post-COVID Syndrome Following Mild Acute COVID-19 Infections: Potential Causes and Consequences for a Therapeutic Approach. Med. 2022, 58, 419. [Google Scholar] [CrossRef]
- Nguyen, T.; Johnston, S.; Clarke, L.; Smith, P.; Staines, D.; Marshall-Gradisnik, S. Impaired calcium mobilization in natural killer cells from chronic fatigue syndrome/myalgic encephalomyelitis patients is associated with transient receptor potential melastatin 3 ion channels. Clin Exp Immunol. 2017, 187, 284–293. [Google Scholar] [CrossRef]
- Perrin, R.; Riste, L.; Hann, M.; Walther, A.; Mukherjee, A.; Heald, A. Into the looking glass: Post-viral syndrome post COVID-19. Med Hypotheses. 2020, 144, 110055. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Voit-Bak, K.; Donate, T.; Rodionov, R.N.; Gainetdinov, R.R.; Tselmin, S.; et al. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis? Mol Psychiatry. 2022, 27, 34–37. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




