1. Introduction
Benzaldehyde (BZH) is a substance mainly used as a food supplement because of its organoleptic activity, such us cinnamon flavouring. Moreover, this product presents a great activity as antioxidant [
1,
2]. Peroxynitrite, on the other hand, is present in cells and animal tissues. Both compounds can react with each other in food or food preserves. The control of these degradation/oxidation processes is therefore of great interest to the food industry. The obtained reaction products show, depending on the concentration, high levels of toxicity. More than 300 aldehydes have been identified in more than 300 different foods or food components. More specifically, benzaldehyde is found in 150 of the 300 different foods or food components, such as alcoholic beverages, dairy products, meat, fruits, vegetables, coffee, tea, cocoa and cinnamon [
3]. According to Krings et al. [
4] benzaldehyde is considered the second most important food flavouring agent. It is a key ingredient in natural fruit flavourings. Benzaldehydes appear on a list of Generally Regarded as Safe (GRAS) flavouring substances in Europe [
5].
On the other hand, the importance of peroxynitrite (ONOO
-) chemistry is focused on biological systems where it possesses the ability to react with almost all biomolecules. Peroxynitrite can produce disruption of cellular structures, inactivation of enzymes and ion channels by oxidation and nitration of proteins, and DNA damage [
6].
Thus, peroxynitrite can acts as a radical former [
7] or as a hydroxylating, nitrosating agent and oxidizing agent, due to its redox potential, 1.4V at pH=7, one of the strongest that has been studied [
8]. As a novelty, in this study HOONO presents reactivity as a nucleophilic organic species. To analyze which are the reactive chemical species and their role in the reaction mechanism, the decomposition of peroxynitrite must be considered. In this way, Koppenol et al. [
9] have proposed a decomposition mechanism that implies two ways: the radical formation and the production of one activate intermediate. The degree of radical formation is not fixed for every reaction involving peroxynitrite, but it depends on the reaction conditions and the characteristics and concentration of the oxidizable substrates [
10,
11,
12].
The chemistry of peroxynitrite under physiological conditions has been extensively studied in the biomedical field due to its formation/reaction in the oxidative/nitrosative stress [
13,
14]. In contrast, bromatology it has not been so well developed. This research in bromatology is therefore of great interest in understanding the action mechanism of peroxynitrite with different foods or their additives, in which it can induce oxidative reactions. These reactions can modify the physicochemical properties of the canned food. There are two ways to form a peroxynitrite precursor species (NO), in biological tissues [
15]:
The main pathway to produce nitric oxide is through nitric oxide synthase in uncured meats. This enzyme reduces its activity due to low pH, low temperature storage, salting and/or thermal processing operations typical for muscle foods.
The postmortem loss of calcium can stimulate nitric oxide synthase activity, increasing nitric oxide formation and creating favourable conditions for peroxynitrite production.
In plant tissues, peroxynitrite will be produced when lipid oxidation occurs, mainly caused by injuries due to chilling, wounds, SO
2 fumigation, ozone exposure, high salt concentrations or dehydration [
15].
Once formed, peroxynitrite can affect the oxidative stability of foods leading to the appearance of rancidity, loss of colour and alterations in the functional properties of proteins, decreasing the quality of the food [
16]. This oxidation process can be modified by changes in pH, salt, carbon dioxide concentration and different processing and storage temperatures [
15].
The purpose of this study is to know in detail the decomposition process of different benzaldehydes in the presence of peroxynitrite to analyze the loss of their organoleptic and antioxidant properties during storage. In addition, a quantitative structure-activity relationship (QSAR) was analyzed to confirm the proposed mechanism.
In advance, benzaldehydes are oxidized by peroxynitrite through a mechanism formed by three competitive pathways: a radicalic attack, a peracid oxidation and a Cannizzaro reaction type, described in acidic media for the first time in this work.
The radical mechanism, for both, aldehydes and other organic species oxidation has been mentioned by [
17,
18,
19,
20].
To improve this study, the reactivity-structure correlation of different substituted benzaldehydes has been analyzed. The chosen aldehydes are present as flavorings in alcoholic and non-alcoholic beverages, candies, ice creams, jellies, cheeses, pastries… [
21]. Thus, the selected ones were 4-hydroxybenzaldehyde (pHBZH), 4-methylbenzaldehyde (pMeBZH), 4-methoxybenzaldehyde (pMoxBZH), 4-nitrobenzaldehyde (pNBZH), 4-trifluoromethylbenzaldehyde (pTFBZH) and 2-hydroxybenzaldehyde (oHBZH).
The goal of this work is analyzed for the first time in acid media a Cannizzaro type mechanism present in the reaction, as well as the study of the QSAR.
2. Materials and Methods
2.1. Readgents
2.1.1. Peroxynitrite synthesis
The Peroxynitrite synthesis used in this work was described [
22]. This method was selected because it improves the yield (almost 100%) and does not generate species that modify the initial reaction rate.
The reaction must be carried out in a basic medium with equimolecular amounts of H2O2, since nitrites (NO2- ) are highly unstable in both neutral and acidic media. This way, the final hydrogen peroxide concentration would be negligible. The obtained products are peroxynitrite and 2-ethoxyethanol. Although the last one can react with ONOO- , this reaction is very slow.
The experimental procedure is as follows:
Synthesis of 2-ethoxyethylnitrite: 2,78 ml of sulfuric acid 2M are added dropwise to a 12 ml of ethoxyethanol 2.5 M solution with 40 gr sodium nitrite at 0 ºC. The reaction finish when no more nitrogen oxides is released. After one hour, the alkyl nitrite phase is refrigerated in an opaque container with 2 mm molecular sieves.
Synthesis of peroxynitrite anion: When neaded, peroxynitrite is prepared by mixing 0,2 ml of 2-ethoxyethylnitrite (precursor) with 15 ml of hydrogen peroxide 0,109 M and 15 ml of sodium hydroxide 2 M in 70 ml of H2O. The resulting anion must be stored at -18 °C. Peroxynitrite concentration is determined daily by spectrophotometry at 302 nm.
2.1.2. Other reagents.
The rest of the reagents used during this work have "for analysis" purity, and all the solutions are prepared with double-distilled water.
2.2. Procedure and measurements
The experimental results were obtained when two solutions were introduced in a Stopped Flow SX-20MV spectrometer from Applied Photophysics, equipped with an anaerobic kit. The first solution contains, the required concentration of benzaldehyde, ionic strength Na2SO4 and H2SO4. The second one, the necessary concentration of peroxynitrite, sodium hydroxide, Na2SO4. Both solutions were previously bubbled with Argon for 15 minutes to ensure the removal of dissolved CO2 and O2.
The control chemical species were benzaldehydes, and their concentrations were measured at 250 nm, its maximum adsorption wavelength.
The pH was checked in all kinetic experiments by using a Crison (micropH 2000) pH meter.
2.3. Kinetic analysis
The experimental data were analyzed using the initial rate method. This method avoids the following problems:
Product interference.
Self-decomposition of reactants.
Inhibition or autocatalysis effects.
Presence of competitive reactions.
The collected the absorbance-time date, are fitted to a four-degree polynomial [
23] and analyzed statistically in the Origin2019b program, where A is the optical absorbance, t is time and a, b, c, d & e are fitting parameters (eq. 1).
In order to determine the initial reaction rate, a Lambert-Beer law should be considered. Then:
The experimental values and errors obtained in this kinetic study represent an arithmetic mean of five reactions carried out under the same experimental conditions to ensure the reproducibility of the study.
3. Results
In the next paragraphs, will be used benzaldehyde as an example to explain the experimental results, the rest of the data for the other aldehydes studied are included for consultation in the supporting information.
3.1. Influence of substrate and oxidant contentrations on the reaction rate.
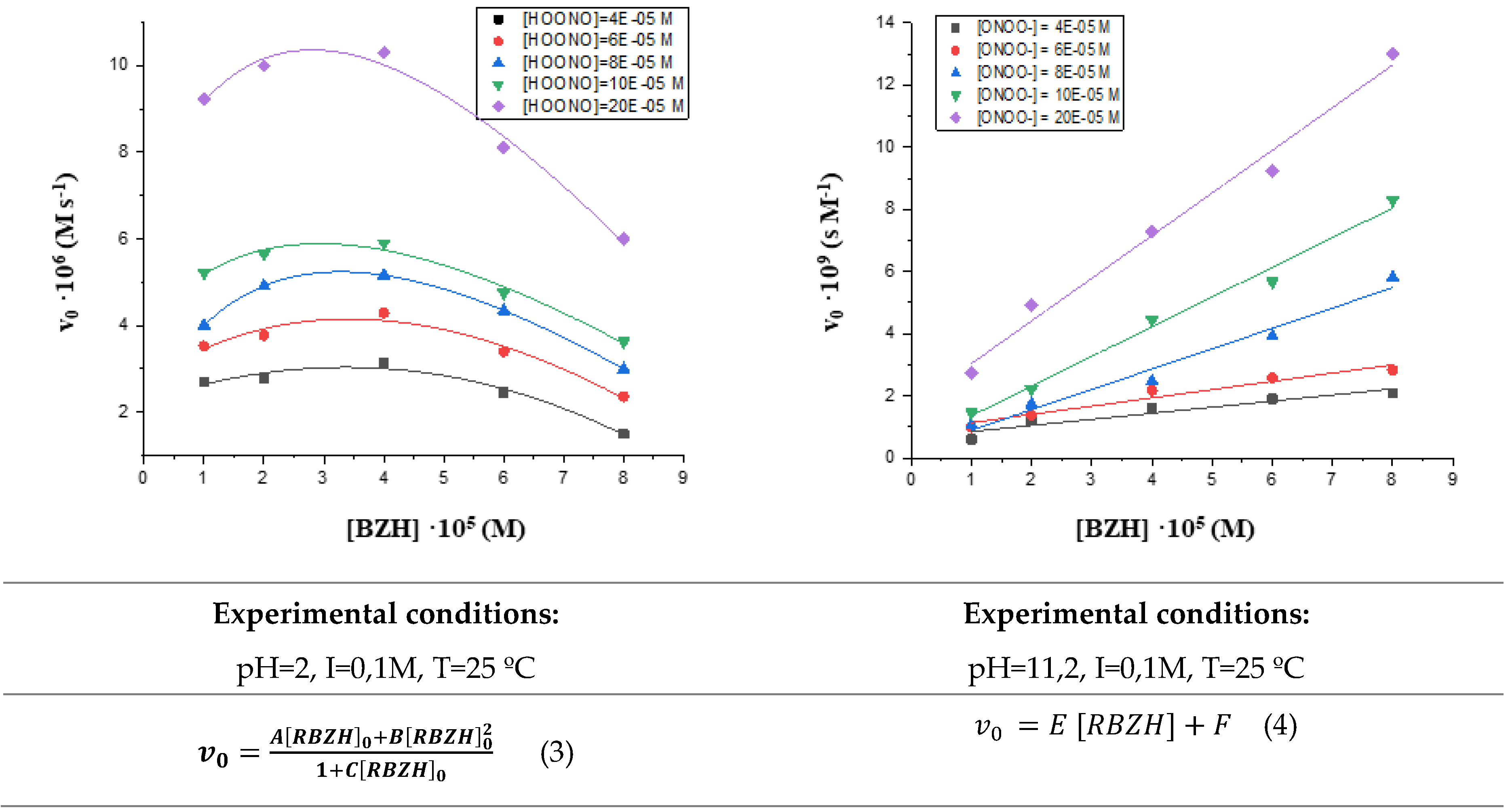
A series of experiments were programmed to analyse the influence of substrate concentration on the reaction rate in which only benzaldehyde concentration was varied, keeping pH, ionic strength and temperature constant.
The results are shown in the
Figure 1, this figure shows on the left the graph corresponding to the experimental data in an acid medium and on the right, those corresponding to a basic medium. At the bottom of each graph, the equation of the best mathematical fit obtained is shown.
3.2. Influence of peroxynirite concentration on the initial reaction rate.
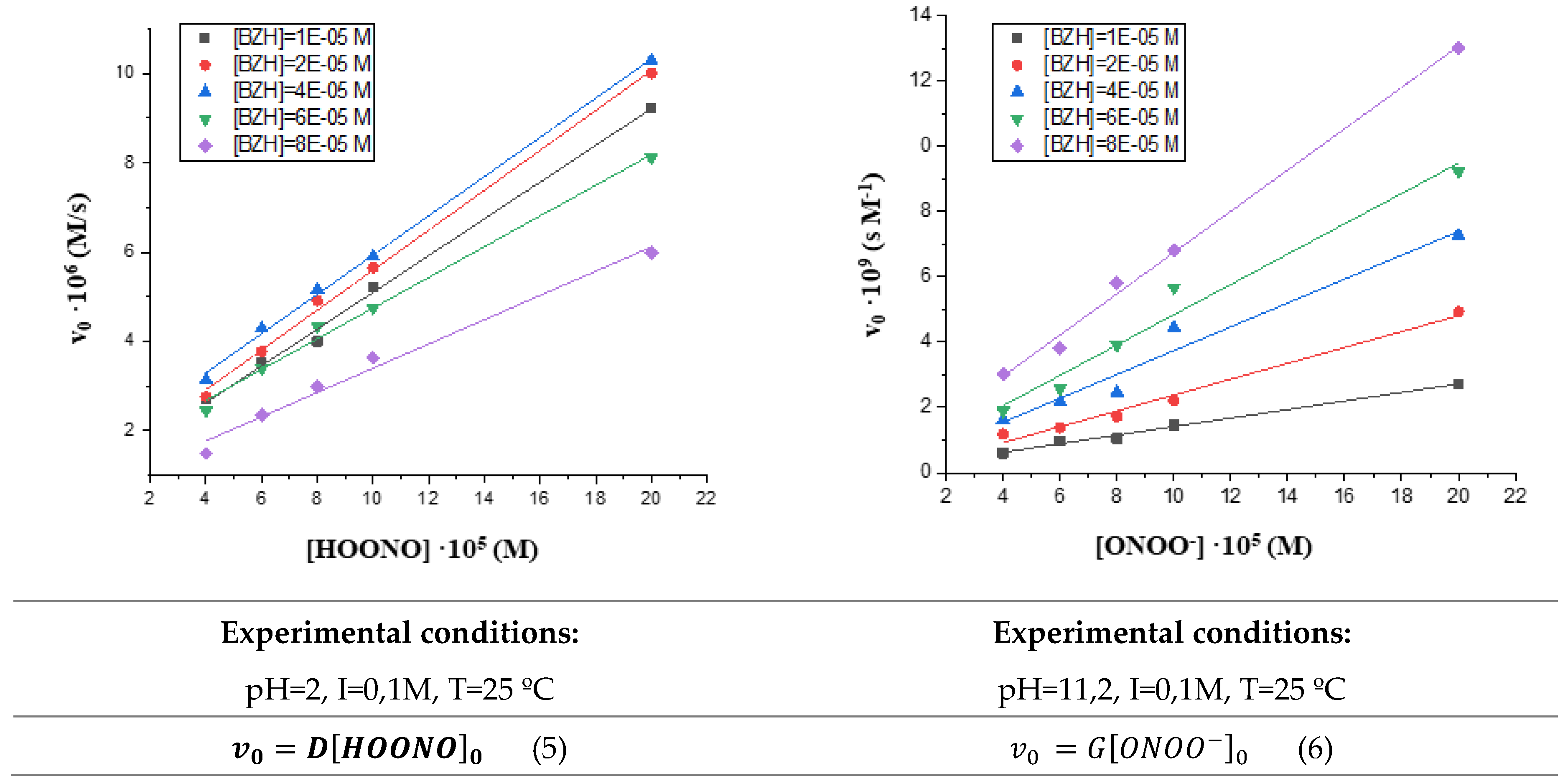
To perform this analysis, a series of experiments were carried out where only the peroxynitrite concentration was varied. The results are shown in the
Figure 2. This figure shows, as
Figure 1, on the left the graph of the experimental data in an acid medium and on the right, those corresponding to a basic medium. At the bottom of each graph, the equation of the best mathematical fit obtained is shown.
3.3. Influence of pH on the initial reaction rate.
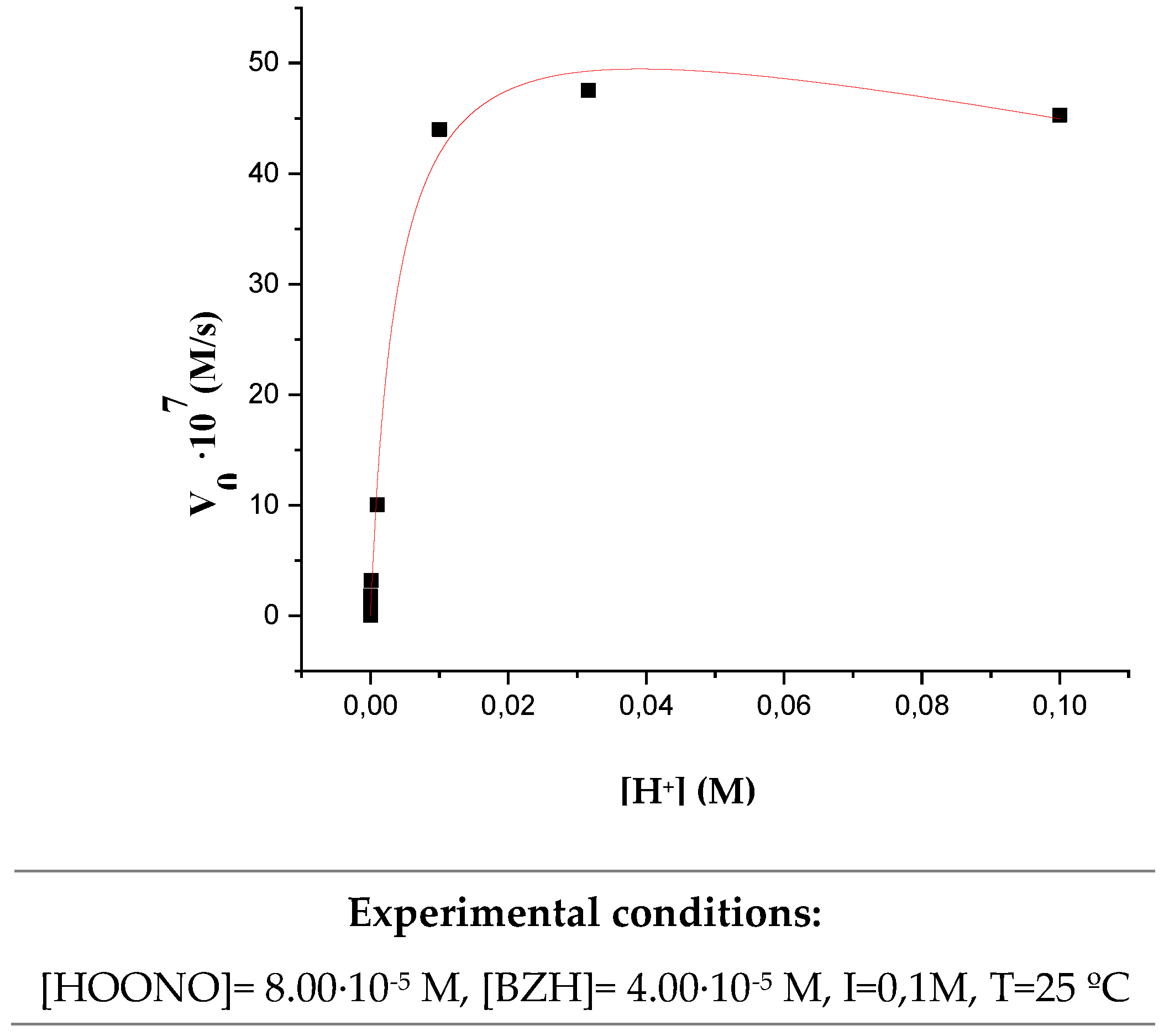
In order to study the effect of pH on the initial reaction rate, a series of experiments were carried out in which benzaldehyde (4.00·10
-5 M) and peroxynitrite (8.00·10
-5 M) concentrations, ionic strength (0,1 M) and temperature (25 ºC) were kept constant, varying the pH value from 1 to 12, where the best mathematical fit was:
These results can be observed en
Figure 3. In this figure the initial reaction rate increases with increasing proton concentration abruptly reaching a maximum from which the reaction rate decreases slightly with increasing proton concentration.
3.4. Influence of the ionic strength on the initial reaction rate.
No effect of ionic strength was observed, so that, at least one of the species involved in the velocity determining step is neutral. The effect of ionic strength on the kinetic coefficients depends on the magnitude and sign of the charges of the reactants [
24]. By applying the extended Debye-Hückel law it can be obtained: [
25], (eq. 8).
3.5. Influence of temperature on the initial reaction rate.
Moreover, the influence of temperature on the initial reaction rate was analyzed in the range between 10 and 30 ºC. It is observed in
Table 1, that the initial reaction rate increases with temperature.
3.6. Product determination.
The technique selected for the determination of products was proton nuclear magnetic resonance spectroscopy, 1H-NMR.
For product determination, 25 experiments were performed under kinetic conditions with the following characteristics: [RBZH] = 4.00·10-5 M, [HOONO] = 8.00·10-5 M, I = 0.1 M, pH = 2 and 11.2, 25 ºC. The reaction was stopped after 15 min by adding ethyl ether to extract the organic products. The organic phase is dried with magnesium sulphate, filtered and the solvent is removed.
The products were determined by 1H-NMR. In addition to benzaldehyde, the presence of benzoic acid and benzyl alcohol were detected in acidic medium and only benzoic acid in basic medium. The obtained experimental relation [benzyl alcohol]/[benzoic acid] was 0,06.
4. Discussion
The proposed mechanism that could justifies all the experimental results must consider the peroxynitrite decomposition [
9] as source of reactive species: peroxynitrous acid, peroxynitrite anion and radical species such as
٠NO
2 and
٠OH. Thus, in the proposed mechanism, these species must be considered to react with benzaldehyde.
Moreover, benzyl alcohol has been detected by 1H-RMN, what justifies the proposed of a Cannizzaro type reaction in acidic media.
Considering these species as well as the mathematical adjustments of the experimental results, it is proposed the reaction scheme (the detailed mechanism can be found in the supporting information).
The reaction mechanism begins with the formulation of acid-base equilibria for peroxynitrite (9) and benzaldehyde (10).
Subsequently, three competitive stages are described:
Firstly, the oxidation of the substrate by the radicals formed by peroxynitrite decomposition (11 and 12).
The second is the direct oxidation of the aldehyde by the peroxynitrite anion (13).
The third is the Canizzaro-type reaction in an acid medium (14-16).
The first two stages have been studied for other aldehydes in the literature [
18,
26], while the third one has never been described.
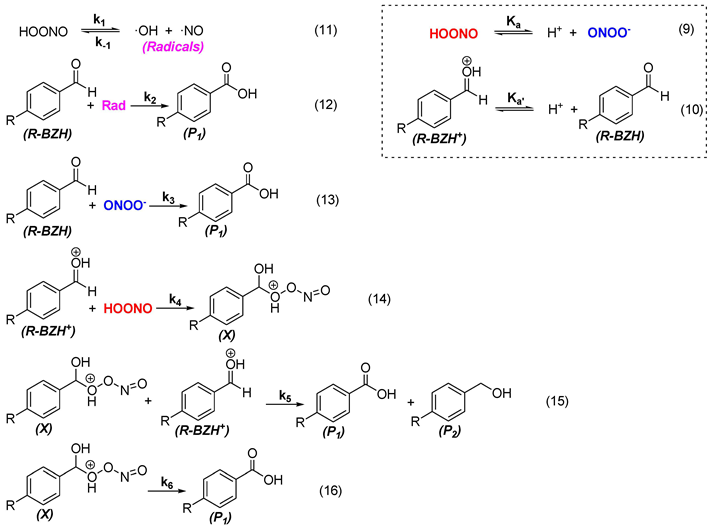
Due to its novelty, this work is focused on the Cannizaro-type reaction, which involves a nucleophilic attack of peroxynitous acid over the protonated aldehyde to form the X adduct (14). This specie can either decompose to yield benzoic acid (16) or react with another benzaldehyde molecule to produce benzoic acid and benzyl alcohol (15).
In this way, the main product observed experimentally was benzoic acid, which generates the rancidity of the added additive, altering its organoleptic properties and, in high concentrations, it can be harmful to health.
The theoretical rate equation obtained from the proposed mechanism is the following:
Applying the steady state theory to X adduct and considering the mass balance equation with respect to peroxynitrite, it can be written:
where A:
In equations (17 and 18) three terms can be observed. The first two correspond to the radical attack and the oxidation of benzaldehyde and the third one to the Cannizzaro-type reaction.
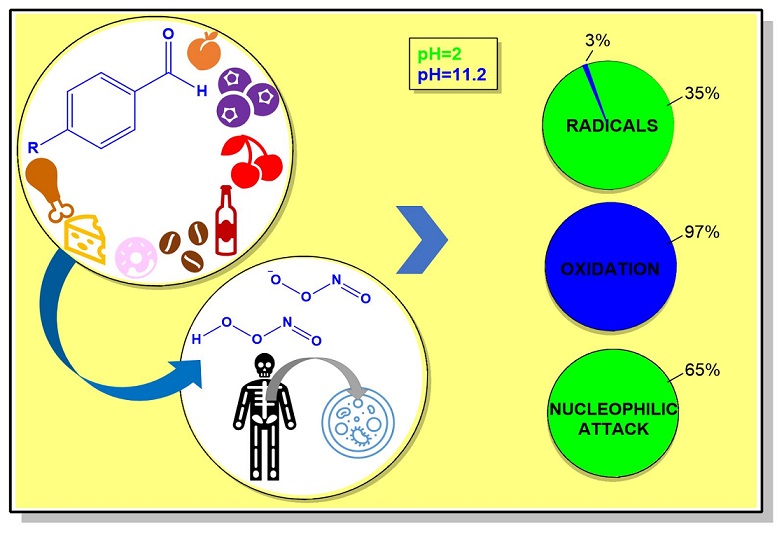
To evaluate the reaction percentage that follows each one of the three competitive stages, a free radical scavenger was used. Hydroquinone, known for its efficiency as a radical inhibitor [
27], was added until no variation of initial rate was observed, approximately a 3:1 ratio of [hydroquinone]/[peroxynitrite]. When this fact occurred, the radical pathway is completely inhibited so that the reaction percentages for each competitive stages can be calculated as shown in
Table 2.
At pH = 2 the percentage of the radical pathway is similar to the one mention in the bibliographical data [
7]. Taking into account these results it can be evaluated certain rate constants as (k
3 = 0.717±0.005 M
-1 s
-1) (oxidation process) and the constants involves in Cannizzaro-type process. The
Table 3 shows these calculated values for k
4, the related to nucleophilic attack, and the relation between k
5 and k
6, that correspond with the benzaldehyde dismutation and its oxidation respectively.
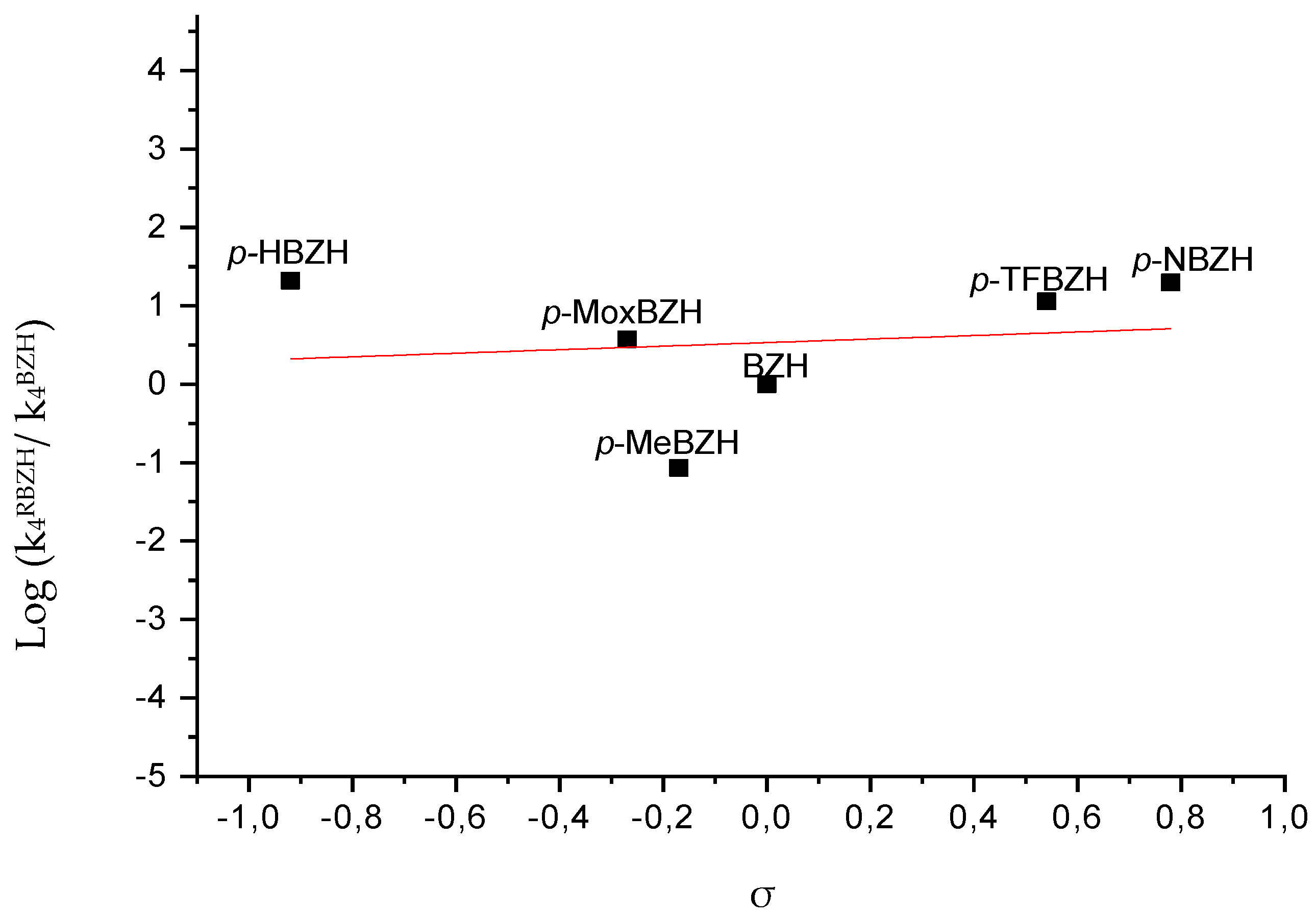
To know how the aromatic substituents, affect over the nucleophilic attack, the reactivity-structure correlation was analyzed using Hammet equation (20) [
29]. For this purpose, the nucleophile addition constants of the aldehyde in acid medium, k4 were plotted against constants σ (parameter that accounts for the inductive and conjugative effects of the substituents in the aromatic rings).
These results are shown in
Figure 4. In this figure as sigma increases, the rate constant of the nucleophilic addition of peroxynitrous acid to the aldehydic group of benzaldehydes, k4, increases slightly.
The value obtained for the Hammett ρ parameter was 0.23±0.05. This slightly positive value implies the generation of negative charge in the transition state. Then as σ increases this process slows down, due to the presence of activating groups, so, consequently, less alcohol is produced.
In short, depending on the structure of the used benzaldehydes, a greater or lesser amount of benzoic acids derivatives will be produced, a substance that should be a minority product in foodstuffs.
5. Conclusions
The proposed mechanism involves the formation of two undesirable products: benzoic acid and benzyl alcohol.
Benzoic acid is a synthetic preservative that produced benzoates in a basic medium is used to prevent the yeasts, bacteria, and some fungi growth. Some plant additives contain a much higher concentration such as cloves, cinnamon, sultanas, prunes, cranberries, and red fruits. It is used in soft drinks, juices, liqueurs, pastries, and convenience foods, and even in some brands of ibuprofen, toothpaste and mouthwash.
Benzyl alcohol, on the other hand, is a natural, colorless and mill smelling solvent used as a carrier for many additives is obtained by hydrolysis of benzoyl chloride [
30] and its occurs naturally in some fruits and plants. It is used in the production of artificial flavours and perfumes.
In terms of side effects, benzoic acid in low doses can cause asthma, urticaria and allergic reactions, where its long-term accumulation could favor the appearance of tumors, presenting a high level of toxicity. In contrast, of benzyl alcohol can cause drowsiness, abdominal pain, nausea, vomiting and diarrhea, in large doses [
31].
The degradation of aldehydes additives transforms its organoleptic properties and produces toxic benzoic acid derivatives. This is the main reason why this work has been focused on the study of this oxidation/degration process.
Considering the obtained results, three reaction pathways have been proposed to justify the experimental data, a radical attack and a direct oxidation by peroxynitrite anion that produce benzoic acid and finally a nucleophilic attack through Cannizzaro type reaction that yields benzoic acid and benzyl alcohol, which have never been described in literature in acid media before. The amount of toxic acid could be reduced by increasing the proportion of the third pathway.
On the other hand, as it can be deduced from the Hammett correlation study, the presence of activating groups can favor the formation of acids as it slows down the nucleophilic attack.
Consequently, these factors must be considered for a better conservation of its organoleptic properties and, therefore being able to avoid food rancidity and the appearance of undesirable toxic products.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Initial rates of all aldehydes in both media. 1H-NMR analysis. Mechanism in acid and basic medium and theoretical rate equations. Quantitative structure-activity relationships.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, LGR, FJP and JCM; methodology, CIA, LGR, IM, FJP and JT; software, CIA, LGR, IM, FJP and JT; validation, CIA, LGR, IM, FJP and JT; formal analysis, CIA, LGR, IM, FJP and JT; investigation, CIA and IM; resources, CIA and IM; data curation, CIA, LGR, IM, FJP and JT.; writing—original draft preparation, CIA, LGR, IM, FJP and JT; writing—review and editing, CIA, LGR, JCM, IM, FJP and JT.; supervision, LGR, FJP and JT; project administration, LGR, JCM, FJP and JT; funding acquisition, LGR, JCM, FJP and JT All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, J. B. , Lim, S. H., Sim, H. S., Park, J. H., Kwon, H. J., Nam, H. S., Kim, M. D., Baek, H. H. & Ha, S. J. (2017). Changes in antioxidant activities and volatile compounds of mixed berry juice through fermentation by lactic acid bacteria. Food Science and Biotechnology 26(2), 441–446. [CrossRef]
- Ullah, I. , Khan, A. L., Ali, L., Khan, A. R., Waqas, M., Hussain, J., Lee, I. J. & Shin, J. H. (2015). Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. Journal of Microbiology 53(2), 127–133. [CrossRef]
- Rothe, M. (1991). Volatile Compounds in Foods and Beverages. Herausgegeben von H. Maarse. 764 Seiten, zahlr. Abb. und Tab. Marcel Dekker, Inc., New York, Basel, Hong Kong 1991. Preis 172.50 $. Food / Nahrung, 35, 10, 1080–1080. [CrossRef]
- Krings, U. & Berger, R. G. (1998). Biotechnological production of flavours and fragrances. Applied Microbiology and Biotechnology 49(1), 1–8. [CrossRef]
- Europea, C. (1999). Decisión de la Comisión, de 23 de febrero de 1999, por la que se aprueba un repertorio de sustancias aromatizantes utilizadas en o sobre los productos alimenticios elaborado con arreglo al Reglamento (CE) nº 2232/96 del Parlam.
- Tamir, S. , Burney, S. & Tannenbaum, S. R. (1996). DNA Damage by Nitric Oxide. Chemical Research in Toxicology, 9(5), 821–827. [CrossRef]
- Goldstein, S. & Czapski, G. (1995). The reaction of NO· with O2·− and HO2·−: A pulse radiolysis study. Free Radical Biology and Medicine, 19, 4, 505–510. [CrossRef]
- Radi, R. , Beckman, J. S., Bush, K. M. & Freeman, B. A. (1991). Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Archives of Biochemistry and Biophysics, 288, 2, 481–487. [CrossRef]
- Molina, C. , Kissner, R. & Koppenol, W. H. (2013). Decomposition kinetics of peroxynitrite: influence of pH and buffer. Dalton Transactions, 42, 27, 9898. [CrossRef]
- Contreras, R. , Galván, M., Oliva, M., Safont, V. S., Andrés, J., Guerra, D. & Aizman, A. (2008). Two state reactivity mechanism for the rearrangement of hydrogen peroxynitrite to nitric acid. Chemical Physics Letters, 457, 1-3, 216–221. [CrossRef]
- Berski, S. , Latajka, Z. & Gordon, A. J. (2011). Electron localization function and electron localizability indicator applied to study the bonding in the peroxynitrous acid HOONO. Journal of Computational Chemistry 32(8), 1528–1540. [CrossRef]
- Grubb, M. P. , Warter, M. L., Xiao, H., Maeda, S., Morokuma, K. & North, S. W. (2012b). No Straight Path: Roaming in Both Ground- and Excited-State Photolytic Channels of NO 3 → NO + O 2. Science, 335, 6072, 1075–1078. [CrossRef]
- Devasagayam, T. P. , Tilak, J. C., Boloor, K. K., Sane, K. S., Ghaskadbi, S. S. & Lele, R. D. (2004). Free radicals and antioxidants in human health: current status and future prospects. Journal of Association of Physicians of India, 52, 794–804, PMID: 15909857.
- Lobo, V. , Patil, A., Phatak, A. & Chandra, N. (2010). Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews, 4, 8, 118. [CrossRef]
- Brannan, R. G. , Connolly, B. J. & Decker, E. A. (2001). Peroxynitrite: a potential initiator of lipid oxidation in food. Trends in Food Science & Technology, 12, 5-6, 164–173. [CrossRef]
- Velasco, J. , Dobarganes, C. & Márquez-Ruiz, G. (2010). Oxidative rancidity in foods and food quality. ( Chemical Deterioration and Physical Instability of Food and Beverages, 3–32. [CrossRef]
- Uppu, R. M. , Winston, G. W. & Pryor, W. A. (1997). Reactions of Peroxynitrite with Aldehydes as Probes for the Reactive Intermediates Responsible for Biological Nitration. Chemical Research in Toxicology 10(12), 1331–1337. [CrossRef]
- Knudsen, F. S. , Penatti, C. A. A., Royer, L. O., Bidart, K. A., Christoff, M., Ouchi, D. & Bechara, E. J. H. (2000). Chemiluminescent Aldehyde and β-Diketone Reactions Promoted by Peroxynitrite. Chemical Research in Toxicology, 13, 5, 317–326. [CrossRef]
- Ischiropoulos, H. , Nelson, J., Duran, D. & Al-Mehdi, A. (1996). Reactions of nitric oxide and peroxynitrite with organic molecules and ferrihorseradish peroxidase: Interference with the determination of hydrogen peroxide. Free Radical Biology and Medicine, 20, 3, 373–381. [CrossRef]
- Nakao, L. S. , Ouchi, D. & Augusto, O. (1999). Oxidation of Acetaldehyde by Peroxynitrite and Hydrogen Peroxide/Iron(II). Production of Acetate, Formate, and Methyl Radicals. Chemical Research in Toxicology, 12, 10, 1010–1018. [CrossRef]
- Burdock, G. A. (2009). Fenaroli’s Handbook of Flavor Ingredients, (6th ed.). CRC Press.
- Leis, J. R. , Pen̄a, M. E. & Ríos, A. (1993). A novel route to peroxynitrite anion. J. Chem. Soc., Chem. Commun 16, 1298–1299. [CrossRef]
- Fernandez, S. L. (2016). Técnicas de ajuste de las curvas de concentración en cinética química. Tendencias en docencia e investigación en química. 1, 1, 323–329, Obtenido de http://hdl.handle.net/11191/5146.
- Laidler, K. J. (2003). Chemical Kinetics. 3Rd Edition. Pearson India.
- Skoog, D. A. (2021). Fundamentals of Analytical Chemistry (10th ed.). Cengage Learning.
- Shrestha, K. P. , Giri, B. R., Adil, M., Seidel, L., Zeuch, T., Farooq, A. & Mauss, F. (2021). Detailed Chemical Kinetic Study of Acetaldehyde Oxidation and Its Interaction with NOx. Energy & Fuels, 35, 18, 14963–14983. [CrossRef]
- Ingold, K. U. (1961). Inhibition of the Autoxidation of Organic Substances in the Liquid Phase. Chemical Reviews, 61, 6, 563–589. [CrossRef]
- Ritchie, C. D. & Sager, W. F. (2007). An Examination of Structure-Reactivity Relationships. Progress in Physical Organic Chemistry, 323–400. [CrossRef]
- Hammett, L. P. (1937). The Effect of Structure upon the Reactions of Organic Compounds. Benzene Derivatives. Journal of the American Chemical Society, 59, 1, 96–103. [CrossRef]
- Schwab, F. W. & Wichers, E. (1940). Preparation of benzoic acid of high purity. Journal of Research of the National Bureau of Standards, 25(6), 747. [CrossRef]
- Nair, B. (2001). Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. International Journal of Toxicology, 20, 23-50. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).