Submitted:
20 January 2023
Posted:
25 January 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials & Methods
Results
Discussion
Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noble, S.; Balfour, J. A. Meloxicam. Drugs 1996, 51(3), 424-430, Doi 10.2165/00003495-199651030-00007.
- Fleischmann, R.; Iqbal, I.; Slobodin, G. Meloxicam. Expert opinion on pharmacotherapy, 2002, 3(10), 1501-1512. Doi 10.1517/14656566.3.10.1501.
- Davis, R.; Brogden, R. N. Nimesulide. Drugs, 1994, 48(3), 431-454. Doi 10.2165/00003495-199448030-00008.
- Ward, A.; Brogden, R. N. Nimesulide. Drugs, 1988, 36(6), 732-753. Doi 10.2165/00003495-198836060-00004.
- Salade, D. A.; Arote, K. S.; Patil, P. H.; Patil, P. S.; Pawar, A. R. A Review on Pharmaceutical Cleaning Validation. Asian Journal of Pharmaceutical Analysis, 2022, 12(3), 197-202. Doi 10.52711/2231-5675.2022.00033.
- Sarwar, A.; McSweeney, C.; Smith, M.; Timmermans, J.; Moore, E. Investigation of an alternative approach for real-time cleaning verification in the pharmaceutical industry. Analyst, 2020, 145(22), 7429-7436. Doi 10.1039/D0AN01219J.
- Starek, M.; Krzek, J. A review of analytical techniques for determination of oxicams, nimesulide and nabumetone. Talanta, 2009, 77(3), 925-942. Doi 10.1016/j.talanta.2008.09.022.
- Mahale, N. B.; Badhan, P. J.; Nikam, K. R.; Chaudhari, S. R. Comparative in vitro evaluation of commercial Nimesulide tablets. International Journal of pharmaceutical sciences and research, 2011, 2(10), 2610-2612. Doi 10.13040/IJPSR.0975-8232.2(10).2610-12.
- da Fonseca, L. B.; Labastie, M.; de Sousa, V. P.; Volpato, N. M. Development and validation of a discriminative dissolution test for nimesulide suspensions. AAPS PharmSciTech, 2009, 10(4), 1145-1152. Doi 10.1208/s12249-009-9320-4.
- Singh, S.; Sharda, N.; & Mahajan, L. Spectrophotometric determination of pKa of nimesulide. International journal of pharmaceutics, 1999, 176(2), 261-264. Doi 10.1016/S0378-5173(98)00304-4.
- Nagaraja, P.; Yathirajan, H. S.; Arunkumar, H. R.; Vasantha, R. A. Novel coupling reagents for the sensitive spectrophotometric determination of nimesulide in pharmaceutical preparations. Journal of pharmaceutical and biomedical analysis, 2002, 29(1-2), 277-282. Doi 10.1016/S0731-7085(02)00060-2.
- Altinöz, S.; Dursun, Ö. Ö. Determination of nimesulide in pharmaceutical dosage forms by second order derivative UV spectrophotometry. Journal of pharmaceutical and biomedical analysis, 2000, 22(1), 175-182. Doi 10.1016/S0731-7085(99)00264-2.
- Florea, M.; Monciu, C. M.; Andritoiu, M. L.; Bacanu, L. G. Spectrophotometric determination of nimesulide through ion-pair complex formation with hexadecyltrimethylammonium bromide. Farmacia, 2008, 56(6), 639-646.
- Saber, A. L.; El-Sayed, G. O. Extractive spectrophotometric determination of anti-inflammatory drug nimesulide in pharmaceutical formulations and human plasma. Journal of Food and Drug Analysis, 2011, 19(4), 429-436. Doi 10.38212/2224-6614.2208.
- Lakshmi, C. S.; Reddy, M. N. Spectrophotometric estimation of nimesulide and its formulations. Microchimica Acta, 1999, 132(1), 1-6. Doi 10.1007/PL00010067.
- Perju, A. C.; Mândrescu, M.; Spac, A. F.; Dorneanu, V. Nimesulide spectrophotometric determination in the visible region. Revista Medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi, 2007, 111(2), 535-539.
- Kamalapurkar, O.S.; Harikrishna ,Y. UV spectrophotometric estimation ofnimesulide. Eastern Pharmacist, 1997, 40(478), 145-146.
- Chen, X. Determination of nimesulide in suppositories by UV spectrometry.
- Zhongguo Xiandai Yingyong Yaoxue, 2007, 24(2), 151-153.
- Chandran, S.; Saggar, S.; Priya, K.P.; Saha, R.N. New ultraviolet spectrophotometric method for the estimation of nimesulide. Drug Development and Industrial Pharamacy, 2000, 26(2), 229-234. Doi 10.1081/DDC-100100350.
- Upadhyay, K.; Asthana, A.; Tiwari, N.; Mathew, S. B. Determination of nimesulide in pharmaceutical and biological samples by a spectrophotometric method assisted with the partial least square method. Research on Chemical Intermediates, 2013, 39(8), 3553-3563. Doi 10.1007/s11164-012-0862-9.
- Pino, K. F. D.; Oliveira, L. N.; Silva, M. J.; Caon-Filho, O.; Dadamos, T. R. L. Spectrophotometric Determination of Nimesulide, Tribulus terrestris, and Amoxicillin in an Alkaline Medium, in Clinical and Commercial Samples. Journal of Applied Spectroscopy, 2019, 85(6), 1151-1157. Doi 10.1007/s10812-019-00774-9.
- Chowdary, K. R.; Kumar, K. G.; & Rao, G. D. New spectrophotometric methods for the determination of nimesulide. Indian journal of pharmaceutical sciences, 1999, 61(2), 86-89.
- Bhatti, M. K.; Hayat, M. M.; Nasir, R.; Ashraf, M.; Hussain, B.; Ahmad, I. Development and validation of spectrophotometric method for the determination of nimesulide in bulk and tablet dosage forms by biuret reagent method. Journal of the Chemical Society of Pakistan, 2012, 34(3), 713-716.
- El-henawy, M. M. E; Ragab, G. H.; Amin, A. E. S.; Sultan A., F. Spectrophotometric determination of nimesulide in pure and in pharmaceutical formulations using ion-associate complex formation. Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry, 2014, 2(4), 240-248.
- Nagaraja, P.; Arun Kumar, H. R.; Vasantha, R. A.; Yathirajan, H. S. Spectrophotometric determination of nimesulide by oxidative coupling with N-bromosuccinimide and promethazine hydrochloride. Oxidation communications, 2003, 26(1), 116-120.
- Mamatha, N.; Radhika, K.; Kumar, S. A. S. Sensitive and validated UV spectrophotometric methods for the estimation of nimesulide in pharmaceutical and bulk formulations. World Journal of Pharmaceutical Research, 2014, 3(3), 4241-4247.
- Reddy, M. N.; Reddy, K. S.; Shankar, D. G.; Sreedhar, K. Spectrophotometric determination of nimesulide. Indian journal of pharmaceutical sciences, 1998, 60(3), 172-173.
- Patil, S. V. Electroanalytical and UV- spectroscopic study for analysis of nimesulide in pharmaceutical samples. International Journal of Chemical Studies, 2022, 10(1), 7-12.
- Shakkor, S. J. Spectrophotometric Determination of Reduced Nimesulide using 8-Hydroxyquinolinol Reagent in Pharmaceutical Preparations. Kirkuk University Journal-Scientific Studies, 2015, 10(1), 143-157.
- Soni, M. K.; Sharma, K. An Eco-friendly spectrophotometric analysis of poorly water-soluble drug (Nimesulide) using the mixed hydrotropic concept. Asian Journal of Pharmaceutical and Health Sciences, 2020, 9(4), 2181-2184.
- Shrivastava, D.; Dwivedi, S. Estimation of nimesulide using mixed solvency approach. International Journal of Pharmacy & Life Sciences, 2020, 11(5), 6629-6634.
- Murthy, T. K.; Reddy, M. N.; Reddy, M. D.; Sankar, D. G. Spectrophotometric determination of flutamide, nimesulide and meloxicam. Asian Journal of Chemistry, 2001, 13(3), 915-918.
- Garcı́a, M. S.; Sánchez-Pedreño, C.; Albero, M. I.; Martı́, J. Spectrophotometric methods for determining meloxicam in pharmaceuticals using batch and flow-injection procedures. European journal of pharmaceutical sciences, 2000, 9(3), 311-316. Doi 10.1016/S0928-0987(99)00069-X.
- Oliveira, É. D. F. S.; Azevedo, R. D. C. P.; Bonfilio, R.; Oliveira, D. B. D.; Ribeiro, G. P.; Araújo, M. B. D. Dissolution test optimization for meloxicam in the tablet pharmaceutical form. Brazilian Journal of Pharmaceutical Sciences, 2009, 45(1), 67-73. Doi 10.1590/S1984-82502009000100008.
- Mandrescu, M.; Spac, A. F.; Dorneanu, V. Spectrophotometric determination of meloxicam. Revista de chimie, 2009, 60(2), 160-163.
- Hassan, E. M. Spectrophotometric and fluorimetric methods for the determination of meloxicam in dosage forms. Journal of pharmaceutical and biomedical analysis, 2002, 27(5), 771-777. Doi 10.1016/S0731-7085(01)00530-1.
- Al-Momani, I. F. Indirect flow-injection spectrophotometric determination of meloxicam, tenoxicam and piroxicam in pharmaceutical formulations. Analytical sciences, 2006, 22(12), 1611-1614. Doi 10.2116/analsci.22.1611.
- Alam, M. N.; Rahman, N.; Azmi, S. N. H. Optimized and validated spectrophotometric method for the determination of uranium (VI) via complexation with meloxicam. Journal of hazardous materials, 2008, 155(1-2), 261-268. Doi 10.1016/j.jhazmat.2007.11.055.
- Saha, R. K. Spectrophotometric micro determination of silver (I) using meloxicam as a new analytical reagent. Oriental Journal of chemistry, 2016, 32(1), 499-507. Doi 10.13005/ojc/320157.
- Taha, E. A.; Salama, N. N.; Abdel Fattah, L. S. Stability-indicating methods for determination of meloxicam and tenoxicam in the presence of their degradation products. Spectroscopy letters, 2002, 35(4), 501-516. Doi 10.1081/SL-120013886.
- Dhandapani, B.; Eswara, M. S.; Susrutha, N.; Rama, S.; Rani, S.; Sarath, T.; Celestin, R. Spectrophotometric estimation of meloxicam in bulk and its pharmaceutical formulations. International Journal of Pharma Sciences and Research, 1(4), 217-221.
- Gurupadayya, B. M.; Trinath, M. N.; Shilpa, K. Spectrophotometric determination of meloxicam by sodium nitroprusside and 1,10-phenanthroline reagents in bulk and its pharmaceutical formulation. Indian Journal of Chemical Technology, 2013, 20(2), 111-115.
- Reddy, M. N.; Murthy, T. K.; Rajita, K.; Shankar, D. G. New spectrophotometric methods for the determination of meloxicam. Indian Journal of Pharmaceutical Sciences, 2001, 63(3), 245-247.
- Joseph-Charles, J.; Bertucat, M. Determination of meloxicam in tablet formulations by ultraviolet spectrophotometry and high-performance liquid chromatography. Analytical letters, 1999, 32(10), 2051-2059. Doi 10.1080/00032719908542951.
- Abed, R. I.; Hadi, H. Determination of meloxicam using direct and indirect flow injection spectrophotometry. Current Pharmaceutical Analysis, 2021, 17(2), 254-264. Doi 10.2174/1573412916666200224103731.
- Zawilla, N. H.; Mohammad, M. A. A.; Aly, S. E. M. Determination of meloxicam in bulk and pharmaceutical formulations. Journal of pharmaceutical and biomedical analysis, 2003, 32(6), 1135-1144. Doi 10.1016/S0731-7085(03)00232-2.
- Nemutlu, E.; Sedef, K. I. R. Validated determination of meloxicam in tablets by using UV spectrophotometry. Hacettepe University Journal of The Faculty of Pharmacy, 2004, 24(1), 13-24.
- Vasiliki, V.; Pinto, P. C. A. G.; Saraiva, M. L. M. F. S.; Lima, J. L. F. C. Sequential injection determination of meloxicam in pharmaceutical formulations with spectrophotometric detection. Canadian Journal of Analytical Sciences and Spectroscopy, 2007, 52(6), 351-358.
- Hasan, S. H.; Othman, N. S.; Surchi, K. M. Development and Validation of a UV-Spectrophotometric Method for Determination of Meloxicam in Bulk and in Tablet Formulations. International Journal of Pharma Sciences and Research, 2015, 6(7), 1040-1045.
- Hasan, S. H.; Othman, N. S.; Surchi K., M. Spectrophotometric Method for Determination of Meloxicam in Pharmaceutical Formulations Using N-bromosuccinimide as an Oxidant. International Journal of Pharma Sciences and Research, 2014, 5(12), 963-969.
- Induri, M.; Mantripragada, B. R.; Yejella, R. P.; Kunda, P. R.; Nannapaneni, D. T.; Boddu, R. Dissolution studies and quantification of meloxicam in tablet dosage form by spectrophotometry. Pakistan journal of pharmaceutical sciences, 2012, 25(1), 283-287.
- Elham, A. Simple Spectrophotometric Methods for the Determination of Meloxicam in Presence of Its Degradation Products. Chinese Journal of Pharmaceutical Analysis, 2004, 24(4), 390-394.
- Mahood, A. M.; Najm, N. H. Spectrophotometric Estamation of Meloxicam Using Charge Transfer Complex. In: IOP Conference Series: Materials Science and Engineering. Bristol: IOP Publishing, 2019, Vol. 571, No 1, Paper No 012081. Doi 10.1088/1757-899X/571/1/012081.
- Kasem, M. A.; Megahed, H. E.; Moustafa, M. E.; Ibrahim, H. A. Sensitive, Direct and Rapid Spectrophotometric Method for the Determination of Meloxicam through Ion-Associate Complex Formation. Journal of Basic and Environmental Sciences, 2014, 1, 92–101. [Google Scholar]
- Sundarapandian, M.; Venkataraman, S.; Xavierarulappa, R.; Boopathi, M.; Selvakumar, S. Spectrophotometric Determination of Meloxicam in Bulk Drug and Pharmacuetical Formulations. Asian Journal of Research in Chemistry, 2009, 2(4), 467-468.
- Pomykalski, A.; Hopkała, H. Comparison of classic and derivative UV spectrophotometric methods for quantification of meloxicam and mefenamic acid in pharmaceutical preparations. Acta poloniae pharmaceutica, 2011, 68(3), 317-323.
- Taha, E. A.; Salama, N. N.; Fattah, L. E. S. A. Spectrofluorimetric and spectrophotometric stability-indicating methods for determination of some oxicams using 7-chloro-4-nitrobenz-2-oxa-1,3-diazole (NBD-Cl). Chemical and pharmaceutical bulletin, 2006, 54(5), 653-658. Doi 10.1248/cpb.54.653.
- Chaudhary, K. B.; Bhardwaj, K.; Verma, G.; Kumar, P. Validated Analytical Method development for the determination of Meloxicam by UV Spectroscopy in API and Pharmaceutical dosage form. Asian Journal of Pharmaceutical Education and Research, 2018, 7(2), 60-69.
- Baban, S. O.; Jallal, A. F. Determination of meloxicam in pharmaceutical formulation by azo-coupling reaction with sulphanilic acid using both batch and flow-injection technique. Rafidain Journal of Science, 2011, 22(4), 121-132.
- Redasani, V. K.; Patel, C. F.; Chhajed, C. F.; Surana, S. S. Quantitative Determination of Meloxicam in bulk and in tablet by UV Spectrophotometry. International Journal of Pharmaceutics and Drug Analysis, 2014, 2(3), 246-250.
- Abbas, R. F.; Mahdi, N. I.; Waheb, A. A.; Aliwi, A. G.; Falih, M. S. Fourth Derivative and Compensated Area under the Curve Spectrophotometric Methods Used for Analysis Meloxicam in the Local Market Tablet. Al-Mustansiriyah Journal of Science, 2018, 29(3), 70-76.
- Chaplenko, A. A.; Monogarova, O. V.; Oskolok, K. V. Spectroscopic and colorimetric determination of meloxicam, lornoxicam, tenoxicam in drugs. International Journal of Pharmaceutical & Biological Archives, 2018, 9(1), 31-35.
- Kuchekar, B. S.; Late, S. G.; Shingavi, A. A.; Shinde, D. B. Spectrophotometric Estimation Of Melatonin And Meloxicam Using Folin-Ciocalteu Reagent. Indian Journal of Pharmaceutical Sciences, 2001, 63(4), 321-323.
- Adrian, R. Research concerning the probabilities of the errors which happen in making observations, etc. The Analyst; or Mathematical Museum, 1808, 1(4), 93-109.
- Ringbom, A. Über die Genauigkeit der colorimetrischen Analysenmethoden I. Zeitschrift für analytische Chemie, 1938, 115(9), 332-343. Doi 10.1007/BF01753937.
- Ayres, G. H. Evaluation of accuracy in photometric analysis. Analytical Chemistry, 1949, 21(6), 652-657. Doi 10.1021/ac60030a002.
- Youmans, H. L.; Brown, V. H. Selection of optimum ranges for photometric analysis. Analytical Chemistry, 1976, 48(8), 1152-1155. Doi 10.1021/ac50002a022.
- Sandell, E. B. Colorimetric Determination of Traces of Metals. New York: Interscience Publishers, 1944.
- Currie, L. A. Detection and quantification limits: origins and historical overview. Analytica Chimica Acta, 1999, 391(2), 127-134. Doi 10.1016/S0003-2670(99)00105-1.
- Shrivastava, A.; Gupta, V. B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles of young scientists, 2011, 2(1), 21-25. Doi 10.4103/2229-5186.79345.
- Little, T. A. Method Validation Essentials, Limit of Blank, Limit of Detection, and Limit of Quantitation. BioPharm International, 2015, 28(4), 48-51.
| Solvent | Used reagents | Wavelength, nm | Linearity, mg/L | Accuracy, % | Precision, % | Reference |
|---|---|---|---|---|---|---|
| Methanol | None | 397 | Not specified | Not specified | Not specified | [8] |
| Water | NaOH | 397 | 5-30 | 2 | 1 | [9] |
| Water | Phosphate buffer | 393 | Not specified | Not specified | Not specified | [10] |
| Methanol | Iminodibenzyl | 600 | 0.1-7.5 | 0.2 | 0.1 | [11] |
| Methanol | 3-aminophenol | 470 | 0.4-12 | 0.3 | 0.2 | [11] |
| Ethanol | None | 262-291WXCSecond derivative | 2-90 | 2 | 1 | [12] |
| Chloroform | None | 248-268WXCSecond derivative | 2-50 | 3 | 1 | [12] |
| Water/chloroform | Hexadecyl-trimethyl-ammonium bromide | 404 | 6-20 | 1 | 0.7 | [13] |
| Water/chloroform | Bromocresol green | 412 | 2-18 | 5 | 0.6 | [14] |
| Water/chloroform | Bromocresol purple | 410 | 2-16 | 4 | 0.5 | [14] |
| Water/chloroform | Bromothymol blue | 407 | 2-18 | 3 | 0.5 | [14] |
| Water/chloroform | Brilliant blue G | 502 | 2-18 | 5 | 0.5 | [14] |
| Water/chloroform | Methyl orange | 482 | 2-14 | 3 | 0.7 | [14] |
| Water | p-N,N-dimethyl phenylene diamine dihydrochloride, chloramine-T | 540 | 10-50 | 0.8 | 0.6 | [15] |
| Water | p-N,N-dimethyl phenylene diamine dihydrochloride, 3-methyl-2-benzothiazolinone hydrazine hydrochloride | 600 | 12.5-75 | 1.2 | 0.4 | [15] |
| Water | HNO2, cresyl fast violet acetate | 565 | 2-12 | 2.2 | 0.2 | [15] |
| Water | p-methyl aminophenol sulphate, K2Cr2O7 | 550 | 20-120 | 0.8 | 0.5 | [15] |
| Water | Thymol | 476 | 5-40 | 2.4 | 2.2 | [16] |
| Water | NaOH | 397 | Not specified | Not specified | Not specified | [17] |
| Water | NaOH | 397 | Not specified | Not specified | Not specified | [18] |
| Water/aceto-nitrile | None | 300 | 10-50 | 1 | 0.4 | [19] |
| Acetonitrile | None | 300 | 10-50 | 0.4 | 0.4 | [19] |
| Methanol | Orcinol | 465 | 0.4-4 | 1.8 | 1.6 | [20] |
| Water | NaOH | 460 | 0.4-5.1 | 8 | Not specified | [21] |
| Methanol/water | Phloroglucinol, ammonium sulfamate | 400 | 4-20 | 2 | Not specified | [22] |
| Methanol/water | p-dimethylamino benzaldehyde | 415 | 4-24 | 2 | Not specified | [22] |
| Methanol | CuSO4, KNaC4H4O6, KI, NaOH | 400 | 25-200 | 0.8 | 2.1 | [23] |
| Ethanol/water | Bromocresol green | 643 | 2-14 | 0.5 | 1.2 | [24] |
| Ethanol/water | Bromocresol purple | 437 | 2-12 | 0.5 | 1.6 | [24] |
| Ethanol/water | Brilliant blue G | 554 | 2-13 | 1 | 1.3 | [24] |
| Methanol/water | N-bromo-succinimide, promethazine hydrochloride | 610 | 0.4-8 | Not specified | Not specified | [25] |
| Methanol | None | 297 | 10-50 | 2 | Not specified | [26] |
| Methanol/aceto-nitrile | None | 295 | 10-50 | 2 | Not specified | [26] |
| Methanol | Folin–Ciocalteu reagent | 600 | Not specified | Not specified | Not specified | [27] |
| Water | NaOH | 393 | 1.5-14 | Not specified | Not specified | [28] |
| Methanol/water | 8-hydroxy-quinolinol | 480 | 0.5-25 | 1.6 | 1.2 | [29] |
| Water | Sodium citrate, phenol | 390 | 10-40 | 3.6 | Not specified | [30] |
| Water | Sodium benzoate, phenol | 390 | 10-50 | 1.5 | Not specified | [31] |
| Water | KMnO4, Fast green FCF | 625 | Not specified | Not specified | Not specified | [32] |
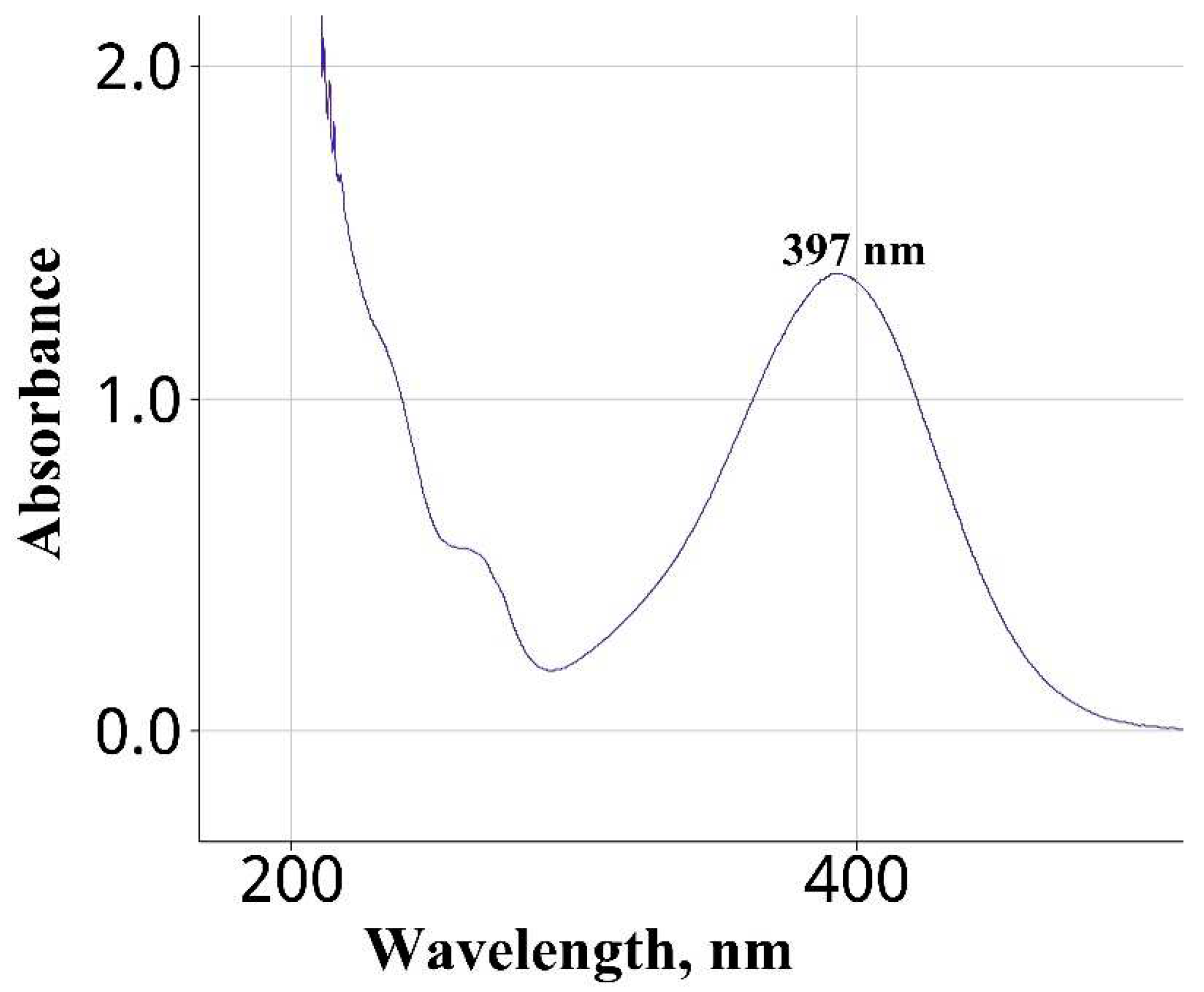
| Water | Na2CO3 | 397 | This work |
| Solvent | Used reagents | Wavelength, nm | Linearity, mg/L | Accuracy, % | Precision, % | Reference |
|---|---|---|---|---|---|---|
| Water | KMnO4, Fast green FCF | 625 | Not specified | Not specified | Not specified | [32] |
| Methanol | FeCl3 | 570 | 2-200 | 2.3 | Not specified | [33] |
| Water | NaOH | 362 | 0.5-20 | 1.9 | Not specified | [33] |
| Water | Phosphate buffer | 362 | Not specified | Not specified | Not specified | [34] |
| Methanol/aceto-nitrile | AlCl3 | 375 | 5-30 | 2.7 | 1.8 | [35] |
| Ethanol | HCl, NaOH | 340-384WXCDifference spectrum | 2-10 | 0.5 | 0.8 | [36] |
| Ethanol | HCl | 322-368WXCFirst derivative | 1-10 | 0.5 | 1.3 | [36] |
| HCl | 343-385WXCSecond derivative | 1-10 | 0.5 | 0.6 | [36] | |
| Water/chloroform | Saframin T | 518 | 4-12 | 1 | 0.4 | [36] |
| Water | N-bromo-succinimide, chloranilic acid | 530 | 10-160 | 8 | 1.2 | [37] |
| Water/1,4-dioxan | UO2(NO3)2 | 398 | 5-60 | 1 | 1.5 | [38] |
| Water/ethanol | AgNO3 | 412 | 1-15 | Not specified | 1.3 | [39] |
| Methanol/water | 3-Methyl-2-benzothiazolinone-hydrazone hydrochloride, ceric ammonium sulphate | 450 | 2-20 | 1.0 | 0.5 | [40] |
| Water | NaOH | 269 | 5-30 | 0.3 | 4.2 | [41] |
| Water | FeCl3 | 476 | 50-250 | 0.5 | 2 | [41] |
| Water | Trisodium citrate | 269 | 5-30 | 2.3 | 5.7 | [41] |
| Water | Sodium nitroprusside, hydroxylamine | 363 | 4-20 | 3.8 | 1.5 | [42] |
| Methanol/water | FeCl3, 1,10-phen-anthroline | 343 | 10-50 | 1.5 | 0.9 | [42] |
| Water | FeCl3, K3[Fe(CN)6] | 770 | 0.25-2.5 | 1.2 | Not specified | [43] |
| Water | Folin–Ciocalteu reagent | 740 | 5-15 | 0.4 | Not specified | [43] |
| Water/1,4-dioxan/acetonitrile | HCl | 341 | 6-14 | 2.3 | 1.8 | [44] |
| Water | Procaine benzylpenicillin | 492 | 5-80 | Not specified | Not specified | [45] |
| Water | p-methyl aminophenol sulfate, NaIO4 | 656 | 15-225 | Not specified | Not specified | [45] |
| Methanol/water/ chloroform | Methylene blue | 654 | 1-5 | 1.2 | 2.3 | [46] |
| Acetonitrile | 2,3-dichloro-5,6-WXCdicyano-p-benzoquinone | 455 | 40-160 | 1 | 1 | [46] |
| Methanol/water | Borate buffer | 363 | 0.5-30 | 1 | 1.4 | [47] |
| Water | FeCl3, K3[Fe(CN)6] | 770 | 10-25 | 5 | Not specified | [48] |
| Methanol/water | HCl | 346 | 5-150 | 3 | 0.5 | [49] |
| Water | N-bromo-succinimide, indigocarmine | 610 | 0.2-50 | 1.5 | Not specified | [50] |
| Methanol/water | NaOH | 365 | 2-12 | 1.1 | 1.3 | [51] |
| Water | Phosphate buffer | 360 | 2-12 | 1.6 | 1.1 | [51] |
| Methanol | UO2CO3 | 406 | 10-100 | 1 | Not specified | [52] |
| Methanol | FeCl3 | 580 | 37.5-300 | 1 | Not specified | [52] |
| Ethanol | FeCl3, K3[Fe(CN)6] | 708 | 0.1-11 | 1.3 | 0.7 | [53] |
| Water | Orange G | 358 | 1-22 | 0.4 | 0.2 | [54] |
| Water | Methylene blue | 652 | 1-22 | 0.2 | 0.2 | [54] |
| Water | CuCl2 | 361 | 1-22 | 0.2 | 0.2 | [54] |
| Water/chloroform | Bromocresol green | 415 | 10-50 | 0.8 | Not specified | [55] |
| Water | NaOH | 361 | 4-14 | 1.2 | Not specified | [56] |
| Water | NaOH | 270 | 4-14 | 4.2 | Not specified | [56] |
| Water | NaOH | 215 | 4-14 | 5.5 | Not specified | [56] |
| Water | NaOH | 386WXCFirst derivative | 4-14 | 1.3 | Not specified | [56] |
| Water | NaOH | 340WXCFirst derivative | 4-14 | 1.5 | Not specified | [56] |
| Water | NaOH | 273WXCFirst derivative | 4-14 | 3.4 | Not specified | [56] |
| Water | NaOH | 257WXCFirst derivative | 4-14 | 4 | Not specified | [56] |
| Water | NaOH | 409WXCSecond derivative | 4-14 | 1.5 | Not specified | [56] |
| Water | NaOH | 359WXCSecond derivative | 4-14 | 1.4 | Not specified | [56] |
| Water | NaOH | 316WXCSecond derivative | 4-14 | 3.7 | Not specified | [56] |
| Water | NaOH | 278WXCSecond derivative | 4-14 | 2.4 | Not specified | [56] |
| Water | NaOH | 269WXCSecond derivative | 4-14 | 1.4 | Not specified | [56] |
| Water | NaOH | 251WXCSecond derivative | 4-14 | 2.2 | Not specified | [56] |
| Water/acetone | 7-chloro-4-nitrobenz-2-oxa-1, 3-diazole | 460 | 0.5-4 | 1.7 | 1.3 | [57] |
| Ethanol | None | 365 | 2-18 | 2.3 | 1.3 | [58] |
| Water | NaNO2, HCl, sulphanilic acid | 365 | 1-20 | 3.5 | 2.3 | [59] |
| Water | NaOH | 269 | 5-30 | 1.6 | 1.4 | [60] |
| Water | NaOH | 253-279WXCArea under curve | 5-30 | 1.4 | 1.2 | [60] |
| Water | NaOH | 275WXCFirst derivative | 50-300 | 1.5 | 1.6 | [60] |
| Water | NaOH | 361WXCFourth derivative | 5-35 | 0.6 | 3.4 | [61] |
| Water | NaOH | 264-277, WXC352-378WXCArea under curve | 5-35 | 0.7 | 1.8 | [61] |
| Water/methanol | 7-chloro-4-nitrobenz-2-oxa-1, 3-diazole | 461 | 0.5-5 | 5 | 4 | [62] |
| Water | Folin-Ciocalteu reagent, Na2CO3 | 700 | 1.5-22.5 | 1.4 | Not specified | [63] |
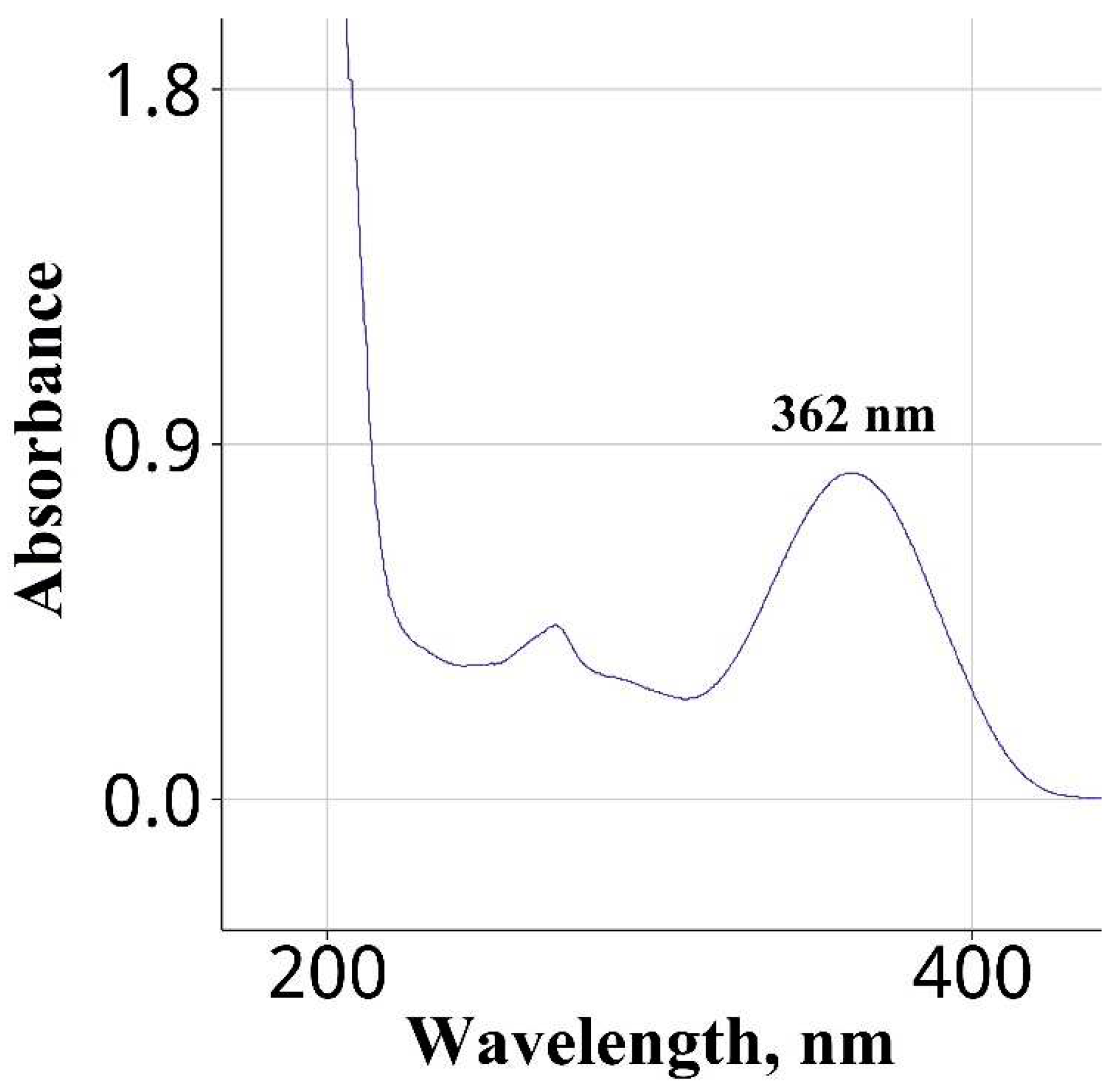
| Water | Na2CO3 | 362 | This work |
| Parameter | Value | |
|---|---|---|
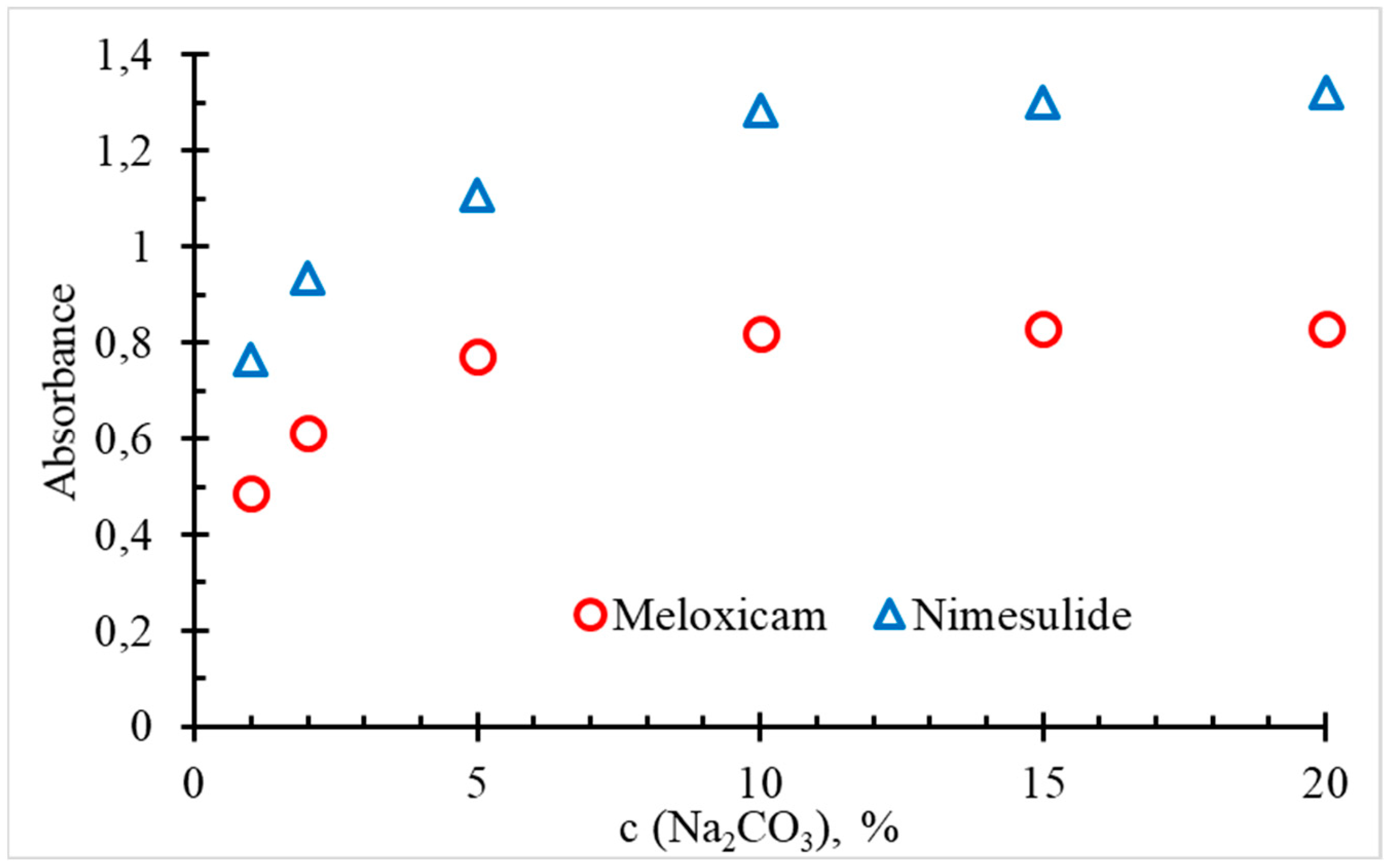
| Analysed pharmaceutical ingredient | Nimesulide | Meloxicam |
| Wavelength of maximum absorbance (nm) | 397 | 362 |
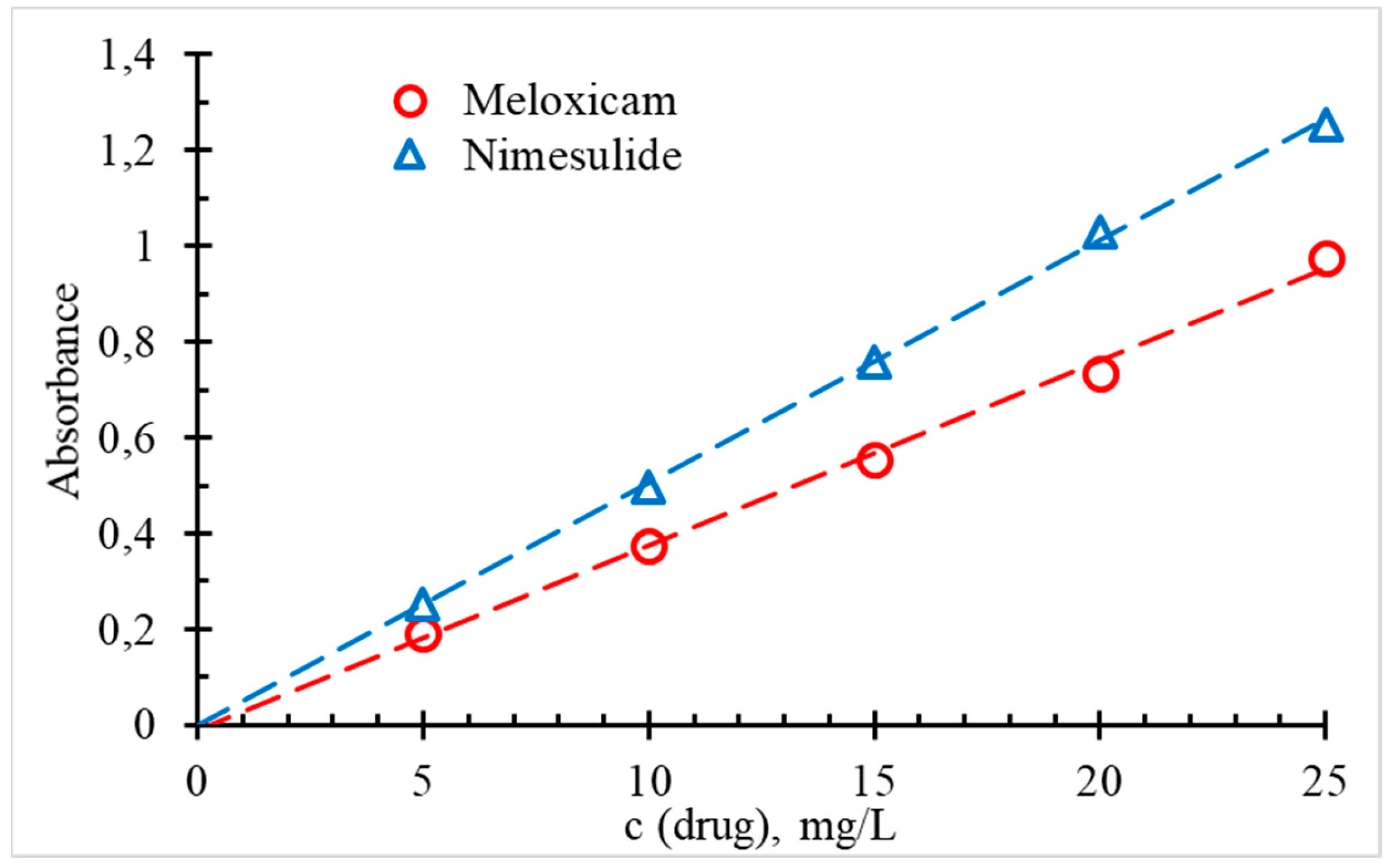
| Slope and its confidence interval (f = 4, p = 95%) (L/mg) | 0.051 ± 0,001 | 0.038 ± 0,001 |
| Intercept and its confidence interval (f = 4, p = 95%) | –0.002 ± 0,001 | –0.01 ± 0,01 |
| R2 value | 0.999 | 0.996 |
| Linearity range (mg/L) | 5 – 25 | 5 – 25 |
| Ringbom’s optimum range (mg/L) | 4 – 14 | 6 – 18 |
| Molar attenuation coefficient and its confidence interval (f = 4, p = 95%) (m2/mol) | 6100 ± 100 | 9100 ± 300 |
| Sandell’s sensitivity coefficient and its confidence interval (f = 4, p = 95%) (μg/cm2) | 0.019 ± 0.002 | 0.026 ± 0.004 |
| Limit of detection (mg/L) | 0.8 | 1.9 |
| Limit of quantification (mg/L) | 2.5 | 5.8 |
| Tested solutions of nimesulide | Mean measured concentration of nimesulide (mg/L) | Relative uncertainty (%) | Tested solutions of meloxicam | Mean measured concentration of meloxicam (mg/L) | Relative uncertainty (%) |
| Working solution, 15 mg/L | 15.08 | 0.5 | Working solution, 10 mg/L | 10.06 | 0.6 |
| Sample solution from tablets, 10 mg/L | 9.95 | 0.5 | Sample solution from tablets, 15 mg/L | 15.11 | 0.8 |
| Swab extract from working solution, 15 mg/L | 14.64 | 2.4 | Swab extract from working solution, 10 mg/L | 9.68 | 3.2 |
| Swab extract from sample solution from tablets, 10 mg/L | 9.71 | 2.9 | Swab extract from sample solution from tablets, 15 mg/L | 14.42 | 3.2 |
| Tested solutions of nimesulide | Standard deviation (mg/L) | Relative standard deviation (%) | Tested solutions of meloxicam | Standard deviation (mg/L) | Relative standard deviation (%) |
|---|---|---|---|---|---|
| Working solution, 15 mg/L (intra-day) | 0.211 | 1.4 | Working solution, 10 mg/L (intra-day) | 0.131 | 1.3 |
| Sample solution from tablets, 10 mg/L (intra-day) | 0.229 | 2.3 | Sample solution from tablets, 15 mg/L (intra-day) | 0.393 | 2.6 |
| Working solution, 15 mg/L (inter-day) | 0.318 | 2.1 | Working solution, 10 mg/L (inter-day) | 0.244 | 2.4 |
| Sample solution from tablets, 10 mg/L (inter-day) | 0.312 | 3.2 | Sample solution from tablets, 15 mg/L (inter-day) | 0.439 | 3.0 |
| Swab extract from working solution, 15 mg/L | 0.542 | 3.7 | Swab extract from working solution, 10 mg/L | 0.329 | 3.4 |
| Swab extract from sample solution from tablets, 10 mg/L | 0.427 | 4.4 | Swab extract from sample solution from tablets, 15 mg/L | 0.591 | 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





