Submitted:
17 January 2023
Posted:
18 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
| Compound | Concentration | Exposed stage | In vivo parameters evaluated | Parameter methodology | Reference |
| Ulexite | 5, 10, 20 y 40 mg/l | 14 d.p.f | 1. Protein concentration, 2. Activity of SOD, 3. Activity of CAT, 4. Activity of GPx, 5. Activity of MPO, 6. Activity of paraoxonase and arylesterase, 7. Ratio of paraxone hydrolysis 8. Lipid peroxidation, 9. Activity of Caspase-3, 10. Determination of DNA damage by level 8-OHdG |
1. Bradford method, 2. The optical density of reaction with xanthine and xanthine oxidase, 3. Aebi method, 4. Oxidation of NADPH measuring its absorbance, 5. O-dianisidine oxidation, 6. Commercial kits, 7. Absorbance a 37 C, 8. TBARS method, 9. Elisa kit, 10. Commercial kit | (Alak et al., 2021) |
| MeO-PEG-b -PMOT | 1mM, 3 mM, 10 mM, 30 mM | 5 d.p.f (embryos) |
1. Mortality, 2. gstp1 expression, 3. Biodistribution in cells, 4. Morphology | 1. Observation under microscope 2. Dimethyl maleate and RNA probes, 3. expose to labeled RNP and exposed to electron fluorescence, 4. microscope observation | (Vong et al., 2016) |
| Diphenyl diselenide | 3.0 mg/Kg DD of food3.0 mg/Kg DD of food | 4-6 m.p.f (adult fis) | 1. Blood glucose, 2. Tissue preparation, 3. Lipid peroxidation, 4. Carbonylated proteins, 5. non-protein thiol levels, 6. Catalase assay, 7. Superoxide dismutase assay, 8. Glutathione peroxidase, 9 s-transferase glutathione, 10. RT-PCR | 1. Glucometer, 2. Homogenize the brain with Tris-HCl, 3. TBARS method, 4. Spectroscopy with the solution treated with DNPH, denaturation buffer, ethanol, ethyl acetate, 5. Spectroscopy on samples treated with TSA and DTNB 6. Mix with potassium phosphate buffer and H2O2, measured H2O2 reduction by spectroscopy, 7. adrenochrome formation by spectroscopy, 8. NADPH oxidation spectroscopy, 9. CDNB glutathione reduction spectroscopy, 10. for antioxidant enzyme genes |
(Santos et al., 2020 |
| Abamectina | 0.5, 10, 15, 20 y 25 μg/l |
7 dpf (young fish) | 1. Superoxide dismutase activity, 2. Iron-reducing antioxidant power, 3. glutathione oxidase activity, 4. reduced glutathione, 5. protein concentration, 6. gene expression | 1. NBT reduction spectroscopy, 2. Sample spectroscopy with TPTZ and FeCl3 mixture, 3. NADPH reduction spectroscopy, 4. Beutler method sample spectroscopy with Rasul and DTNB solution treatment, 5. NBT method Bradford, Spectroscopy of the sample with ethanol, phosphoric acid, and Coomassie blue, 6. Real-time PCR with cyp1a, vtg, and β-actin primers |
(Hanachi et al., 2021) |
| Fluralaner | 2.00 and 0.20 mg/L 7 dpf (young fish) | 7 d.p.f (young fish) | 1. Acute toxicity, 2. Bioconcentration and elimination, 3. Antioxidant enzymatic response (CAT, GSH-PX, GTS, SOD y CarE) |
1. Fluralaner concentration in water with fish exposed to the compound and death time of each fish, 2. Analysis of water in bioconcentration and elimination period, 3. Use of Nanjing Jiancheng kits | (Jia et al.,2018) |
| Monobutil pftalato | 0, 0.5, 5, 10 mg/L | adult fish | 1. Activity of SOD, GSH-Px, CAT, MDA, NaKAtpase, Ca Mg ATPase, ALT, AST, 2. PCR, 3. Histological analysis, 4. Apoptosis 5. Viability of hepatocytes | 1. Use of Nanjing Jiancheng kits, 2. RT-PCR of gene expression (sod, cat, gpx, Nrf2, HO-1), 3. Liver extraction, fixation of the treated sample, and observation under microscope 4. Nanjing Jiancheng Kit and flow cytometer 5. MTT method, spectroscopy after treatment with dimethyl sulfoxide. | (Jiao et al., 2020) |
| Vitamin E | 2.62, 52.34, y 101.27 mg/kg |
15 d.p.f | 1. Superoxide dismutase activity, 2. GSH-PX activity 3. Peroxidase activity, 4. PCR, 5. Western blot 6. Fatty acid analysis, 7. Histological analysis |
1. Xanthine oxidase method, 2. NADPH oxidation spectroscopy, 3. Spectroscopy, 4. qPCR for nt10b, GSK-3β, PPARγ, β-catenin, and β-actin genes, 5. SDS PAGE and antibody gel for β-catenin, β-Actin, and GSK-3β, 6. Esterification of fatty acids with methanol and hexane, then GC-MS chromatography, 7. Fixation and microscope observation |
(Liu et al., 2020) |
| Ketoprofeno | 1, 10 y 100 μg/ml | 3 h.p.f | 1. Toxicity, 2. Marker of oxaloacetyl transaminase and glutamine pyruvate transaminase, 3. LDH, 4. Membrane-bound ATPase, 5. SOD activity, 6. Cat activity, 7. GSH activity, 8. GPx activity, 9. GST activity, 10. Lipid peroxidation, 11 histopathology |
1. Observation of movement, breathing, and swimming, 2. Reitman and Frankel method, 3. Tietz method, 4. Shiosaca method using UV spectrum, 5. Marklun method, 6. Aebi method H2O2 consumption, 7. Ellman's method, 8. Habig's method, CDNB production, 9. Devasagayam's method, MDA production, 10. Plate fixation and observation by trinocular microscope. | (Rangasamy et al., 2018) |
| Resveratrol | 10 μg/mL | 3 h.p.f | 1. Generation of intracellular ROS, 2. Apoptosis, 3. Superoxide dismutase activity, 4. PCR, 5. EROD assay |
1. Fluorescence microscope for reaction with DCFH-DA, 2. Using acridine orange, 3. Nanjing kit, 4. Fluorescence microscope with studies 8-OHdG, γH2AX, cleaved caspase 3, 4. qPCR for Gapdh genes and Elfa, 5. Fluorescence from the reaction with 7-ethoxyresorufin | (Ren et al., 2020) |
| Compound | Concentration | Exposed stage | In vivo evaluator parameters | Parameters methodology | Reference |
|---|---|---|---|---|---|
| White chicken egg lysosome hydrolyzate 58,000 U/ml | 0, 156, 312, 625,1250, 2500, 5000 μg/ml | 48 hpf (larva) | 1. Lipid peroxidation, 2. Mortality, 3. Morphology | 1. TBARS method, 2. Stereomicroscope 3. lack of somite formation, failure of tail bud detachment from yolk sac by stereomicroscope | (Carrillo et al., 2016) |
| Nrf2a expression in GMO fish | 1 h.p.f | 1. Morphology, 2. Glutathione and redox cysteine, 3. PCR, 4. Apoptosis | 1.Light microscope, 2. HPLC, 3. qPCR for actb, b2m genes, 4. Acridine orange assay | (Sant et al., 2018) | |
| Lyophilisate of Lactobacillus rhamnosus and Bifidobacterium longum | 12% in food | 1 d.p.f | 1. Sperm motility, kinetics, and concentration, 2. Weight, 3. Behavior | 1. Phase contrast trinocular optical microscope 2. Microbalance, 3. Observation by cameras | (Valcarce et al., 2019) |
4. Discussion
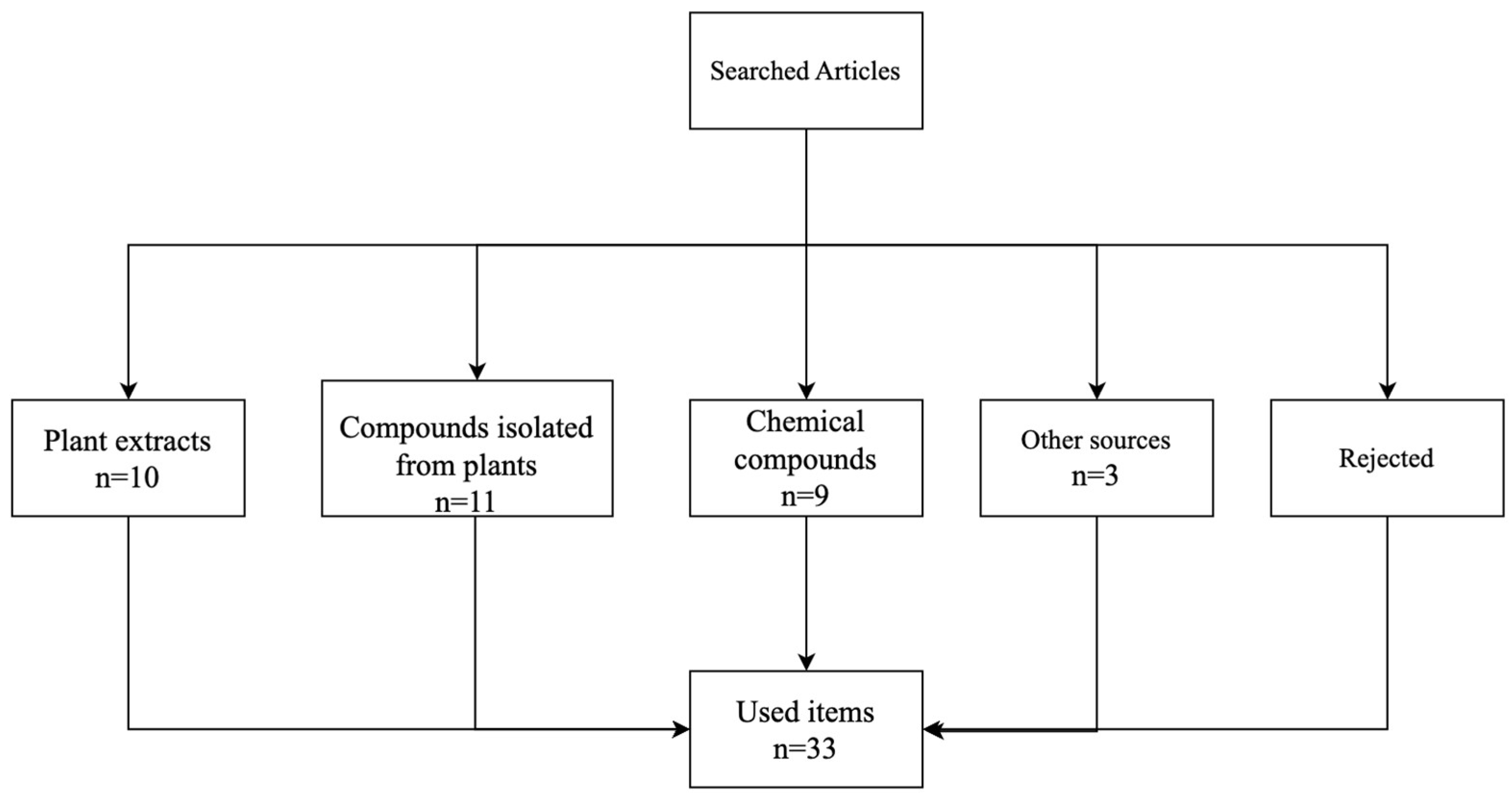
Bibliographical material
Plant extracts
Isolated compounds from plants
Chemical compounds
Other sources
5. Conclusions
References
- Camacho-Escobar MA, Ramos-Ramos DA, Ávila-Serrano NY, Sánchez-Bernal EI, López-Garrido SJ. Las defensas físico-químicas de las plantas y su efecto en la alimentación de los rumiantes. REVISTA TERRA LATINOAMERICANA. 2020, 38, 443–53. [CrossRef]
- Camacho-Romero, O.I.; Melgarejo-Gómez, S.; De-La-Rosa-Torres, C. Extracción y evaluación de los metabolitos secundarios de extractos etéreos del fruto Syzygium cumini (Jambool). Revista Tecnología en Marcha, 2017; 30, 113. [Google Scholar] [CrossRef]
- Castro GD. Dependencia de la dosis en los mecanismos de toxicidad y la evaluación de riesgo en toxicología. Acta Bioquimica Clinica Latinoamericana [Internet]. 2013 [cited 2022 Nov 21]; Available from: http://www.scielo.org.ar/scielo.php?pid=S0325-29572013000300010&script=sci_abstract.
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons. Curr. Cancer Drug Targets 2016, 12, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Moctezuma Viera, KR. Utilización de animales en la investigación biomédica y médica. Rev Iberoam Bioet. 2020, 12, 1–19. [Google Scholar] [CrossRef]
- Escobedo-Moratilla A, Barba R, Pérez-Urizar J. Modelos preclínicos in vitro e in vivo para la evaluación de la actividad biológica en estudios de biocomparabilidad. Gac Med Mex [Internet]. 2015 [cited 2022 Nov 26];151:376–86. Available from: https://www.anmm.org.mx/GMM/2015/n3/GMM_151_2015_3_377-386.pdf.
- Acevedo Fernández JJ, Angeles Chimal JS, Rivera HM, Petricevich López VL, Nolasco Quintana NY, Collí Magaña DY, et al. Modelos in vitro para la evaluación y caracterización de péptidos bioactivos. In: Bioactividad de péptidos derivados de proteínas alimentarias. OmniaScience; 2013. p. 29–82.
- Fina BL, Lombarte M, Rigalli A. Research a natural phenomenon: Studies in vivo, in vitro or in silico? | Investigación de un fenómeno natural: Estudios in vivo, in vitro o in silico?. Actualizaciones En Osteologia [Internet]. 2013 [cited 2022 Nov 26];9, 294–9. Available from: http://www.osteologia.org.ar/files/pdf/rid34_Fina.pdf.
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent Adult Zebrafish as a Tool for In Vivo Transplantation Analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, MB. The Zebrafish : A Tool in Education Resumen Introducción. Revista de Educación En Biología. 2016, 19, 11–18. [Google Scholar]
- Kettleborough, R.N.W.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Arteaga C, Bustillos A, Gómez-Catalán J. Migración de neutrófilos en larvas de pez cebra expuestos a extractos de sofrito de tomate. Arch Latinoam Nutr. 2020, 70, 182–90. [CrossRef]
- Boeri, P.; Piñuel, L.; Dalzotto, D.; Monasterio, R.; Fontana, A.; Sharry, S.; Barrio, D.A.; Carrillo, W. Argentine Patagonia barberry chemical composition and evaluation of its antioxidant capacity. J. Food Biochem. 2020, 44, e13254. [Google Scholar] [CrossRef]
- Cholan, P.M.; Han, A.; Woodie, B.R.; Watchon, M.; Kurz, A.R.; Laird, A.S.; Britton, W.J.; Ye, L.; Holmes, Z.C.; McCann, J.R.; et al. Conserved anti-inflammatory effects and sensing of butyrate in zebrafish. Gut Microbes 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Perumal S, Gopal Samy M v., Subramanian D. Developmental toxicity, antioxidant, and marker enzyme assessment of swertiamarin in zebrafish ( Danio rerio ). J Biochem Mol Toxicol. 2021, 35, e22843. [CrossRef]
- Poornima, S.; Nagarjun, N.; Ponmurugan, P.; Gnanamangai, B.M.; Narasimman, S. Toxicity and anti-inflammatory study of Parmotrema austrosinense extract against oxozalone induced intestinal inflammation in zebrafish (Danio rerio) model. Biocatal. Agric. Biotechnol. 2019, 21, 101278. [Google Scholar] [CrossRef]
- Pradeep, P.S.; Manisha, S.; Nayaki, J.M.A.; Sivaraman, D.; Selvaraj, R.; Seeni, S. Potential antioxidant and anti-inflammatory action of Hypericum hookerianum extracts in a liposome system evaluated with zebrafish embryos. J. Microencapsul. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- Mohamad Shariff NFS, Singgampalam T, Ng CH, Kue CS. Antioxidant activity and zebrafish teratogenicity of hydroalcoholic Moringa oleifera L. leaf extracts. British Food Journal 2020, 122, 3129–3137. [CrossRef]
- Udaya, S.; Babu, N.; Nanjappa, D.P.; Kalladka, K.; Chakraborty, G.; Chakraborty, A. Evaluation of Toxicity and Antioxidant Property of Cassia fistula Stem Bark Extracts in Zebrafish. J. Health Allied Sci. NU 2020, 10, 109–115. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J. Efficient extraction, antioxidant activities and anti-inflammation of polysaccharides from Notopterygium franchetii Boiss. Carbohydr. Polym. 2020, 248, 116783. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.; Boix, N.; Teixido, E.; Marizande, F.; Cadena, S.; Bustillos, A. The Zebrafish Embryo as a Model to Test Protective Effects of Food Antioxidant Compounds. Molecules 2021, 26, 5786. [Google Scholar] [CrossRef]
- Cho, S.-H.; Heo, S.-J.; Yang, H.-W.; Ko, E.-Y.; Jung, M.S.; Cha, S.-H.; Ahn, G.; Jeon, Y.-J.; Kim, K.-N. Protective Effect of 3-Bromo-4,5-Dihydroxybenzaldehyde from Polysiphonia morrowii Harvey against Hydrogen Peroxide-Induced Oxidative Stress In Vitro and In Vivo. J. Microbiol. Biotechnol. 2019, 29, 1193–1203. [Google Scholar] [CrossRef]
- Endo, Y.; Muraki, K.; Fuse, Y.; Kobayashi, M. Evaluation of Antioxidant Activity of Spice-Derived Phytochemicals Using Zebrafish. Int. J. Mol. Sci. 2020, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Kim, K.-N.; Kang, S.-M.; Yang, X.; Kim, E.-A.; Song, C.B.; Nah, J.-W.; Jang, M.-K.; Lee, J.-S.; Jung, W.-K.; et al. Protective effect of dieckol isolated from Ecklonia cava against ethanol caused damage in vitro and in zebrafish model. Environ. Toxicol. Pharmacol. 2013, 36, 1217–1226. [Google Scholar] [CrossRef]
- Issac, P.K.; Guru, A.; Velayutham, M.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Arockiaraj, J. Oxidative stress induced antioxidant and neurotoxicity demonstrated in vivo zebrafish embryo or larval model and their normalization due to morin showing therapeutic implications. Life Sci. 2021, 283, 119864. [Google Scholar] [CrossRef] [PubMed]
- Roberto, V.P.; Surget, G.; Le Lann, K.; Mira, S.; Tarasco, M.; Guérard, F.; Poupart, N.; Laizé, V.; Stiger-Pouvreau, V.; Cancela, M.L. Antioxidant, Mineralogenic and Osteogenic Activities of Spartina alterniflora and Salicornia fragilis Extracts Rich in Polyphenols. Front. Nutr. 2021, 8, 719438. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, P.; Palanisamy, S.; Anjali, R.; Vinosha, M.; Elakkiya, M.; Marudhupandi, T.; Tabarsa, M.; You, S.; Prabhu, N.M. Isolation and structural characterization of sulfated polysaccharide from Spirulina platensis and its bioactive potential: In vitro antioxidant, antibacterial activity and Zebrafish growth and reproductive performance. Int. J. Biol. Macromol. 2019, 141, 809–821. [Google Scholar] [CrossRef]
- Kim, H.-S.; Wang, L.; Fernando, I.P.S.; Je, J.-G.; Ko, S.-C.; Kang, M.C.; Lee, J.M.; Yim, M.-J.; Jeon, Y.-J.; Lee, D.-S. Antioxidant efficacy of (−)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J. Appl. Phycol. 2020, 32, 3341–3348. [Google Scholar] [CrossRef]
- Xia, G.; Li, X.; Zhang, Z.; Jiang, Y. Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce. Open Life Sci. 2021, 16, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Le, H.D.; Kim, T.N.T.; The, H.P.; Nguyen, T.M.; Cornet, V.; Lambert, J.; Kestemont, P. Anti–Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.S.; Manisha, S.; Nayaki, J.M.A.; Sivaraman, D.; Selvaraj, R.; Seeni, S. Potential antioxidant and anti-inflammatory action of Hypericum hookerianum extracts in a liposome system evaluated with zebrafish embryos. J. Microencapsul. 2019, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.; Boix, N.; Teixido, E.; Marizande, F.; Cadena, S.; Bustillos, A. The Zebrafish Embryo as a Model to Test Protective Effects of Food Antioxidant Compounds. Molecules 2021, 26, 5786. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Wang, L.; Fernando, I.P.S.; Je, J.-G.; Ko, S.-C.; Kang, M.C.; Lee, J.M.; Yim, M.-J.; Jeon, Y.-J.; Lee, D.-S. Antioxidant efficacy of (−)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J. Appl. Phycol. 2020, 32, 3341–3348. [Google Scholar] [CrossRef]
- Xia, G.; Li, X.; Zhang, Z.; Jiang, Y. Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce. Open Life Sci. 2021, 16, 92–101. [Google Scholar] [CrossRef]
- Roberto, V.P.; Surget, G.; Le Lann, K.; Mira, S.; Tarasco, M.; Guérard, F.; Poupart, N.; Laizé, V.; Stiger-Pouvreau, V.; Cancela, M.L. Antioxidant, Mineralogenic and Osteogenic Activities of Spartina alterniflora and Salicornia fragilis Extracts Rich in Polyphenols. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.-S.; Jeon, Y.-J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef]
- Cho, S.-H.; Heo, S.-J.; Yang, H.-W.; Ko, E.-Y.; Jung, M.S.; Cha, S.-H.; Ahn, G.; Jeon, Y.-J.; Kim, K.-N. Protective Effect of 3-Bromo-4,5-Dihydroxybenzaldehyde from Polysiphonia morrowii Harvey against Hydrogen Peroxide-Induced Oxidative Stress In Vitro and In Vivo. J. Microbiol. Biotechnol. 2019, 29, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Wang, L.; Fernando, I.P.S.; Je, J.-G.; Ko, S.-C.; Kang, M.C.; Lee, J.M.; Yim, M.-J.; Jeon, Y.-J.; Lee, D.-S. Antioxidant efficacy of (−)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J. Appl. Phycol. 2020, 32, 3341–3348. [Google Scholar] [CrossRef]
- Endo, Y.; Muraki, K.; Fuse, Y.; Kobayashi, M. Evaluation of Antioxidant Activity of Spice-Derived Phytochemicals Using Zebrafish. Int. J. Mol. Sci. 2020, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, B.; Hemalatha, D.; Shobana, C.; Nataraj, B.; Ramesh, M. Developmental toxicity and biological responses of zebrafish (Danio rerio) exposed to anti-inflammatory drug ketoprofen. Chemosphere 2018, 213, 423–433. [Google Scholar] [CrossRef]
- Carrillo, W.; Gómez-Ruiz, J.A.; Miralles, B.; Ramos, M.; Barrio, D.; Recio, I. Identification of antioxidant peptides of hen egg-white lysozyme and evaluation of inhibition of lipid peroxidation and cytotoxicity in the Zebrafish model. Eur. Food Res. Technol. 2016, 242, 1777–1785. [Google Scholar] [CrossRef]
- Vong, L.B.; Kobayashi, M.; Nagasaki, Y. Evaluation of the Toxicity and Antioxidant Activity of Redox Nanoparticles in Zebrafish (Danio rerio) Embryos. Mol. Pharm. 2016, 13, 3091–3097. [Google Scholar] [CrossRef]
- Ren, F.; Huang, Y.; Tao, Y.; Ji, C.; Aniagu, S.; Jiang, Y.; Chen, T. Resveratrol protects against PM2.5-induced heart defects in zebrafish embryos as an antioxidant rather than as an AHR antagonist. Toxicol. Appl. Pharmacol. 2020, 398, 115029. [Google Scholar] [CrossRef]
- Jiao, Y.; Tao, Y.; Yang, Y.; Diogene, T.; Yu, H.; He, Z.; Han, W.; Chen, Z.; Wu, P.; Zhang, Y. Monobutyl phthalate (MBP) can dysregulate the antioxidant system and induce apoptosis of zebrafish liver. Environ. Pollut. 2019, 257, 113517. [Google Scholar] [CrossRef]
- Alak, G.; Ucar, A.; Parlak, V.; Yeltekin, A.; Özgeriş, F.B.; Atamanalp, M.; Türkez, H. Antioxidant Potential of Ulexite in Zebrafish Brain: Assessment of Oxidative DNA Damage, Apoptosis, and Response of Antioxidant Defense System. Biol. Trace Element Res. 2020, 199, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.M.; de Macedo, G.T.; Prestes, A.S.; Ecker, A.; Müller, T.E.; Leitemperger, J.; Fontana, B.D.; Ardisson-Araújo, D.M.; Rosemberg, D.B.; Barbosa, N.V. Modulation of redox and insulin signaling underlie the anti-hyperglycemic and antioxidant effects of diphenyl diselenide in zebrafish. Free. Radic. Biol. Med. 2020, 158, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Sant, K.E.; Sinno, P.P.; Jacobs, H.M.; Timme-Laragy, A.R. Nrf2a modulates the embryonic antioxidant response to perfluorooctanesulfonic acid (PFOS) in the zebrafish, Danio rerio. Aquat. Toxicol. 2018, 198, 92–102. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Riesco, M.F.; Martínez-Vázquez, J.M.; Robles, V. Diet Supplemented with Antioxidant and Anti-Inflammatory Probiotics Improves Sperm Quality after Only One Spermatogenic Cycle in Zebrafish Model. Nutrients 2019, 11, 843. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





