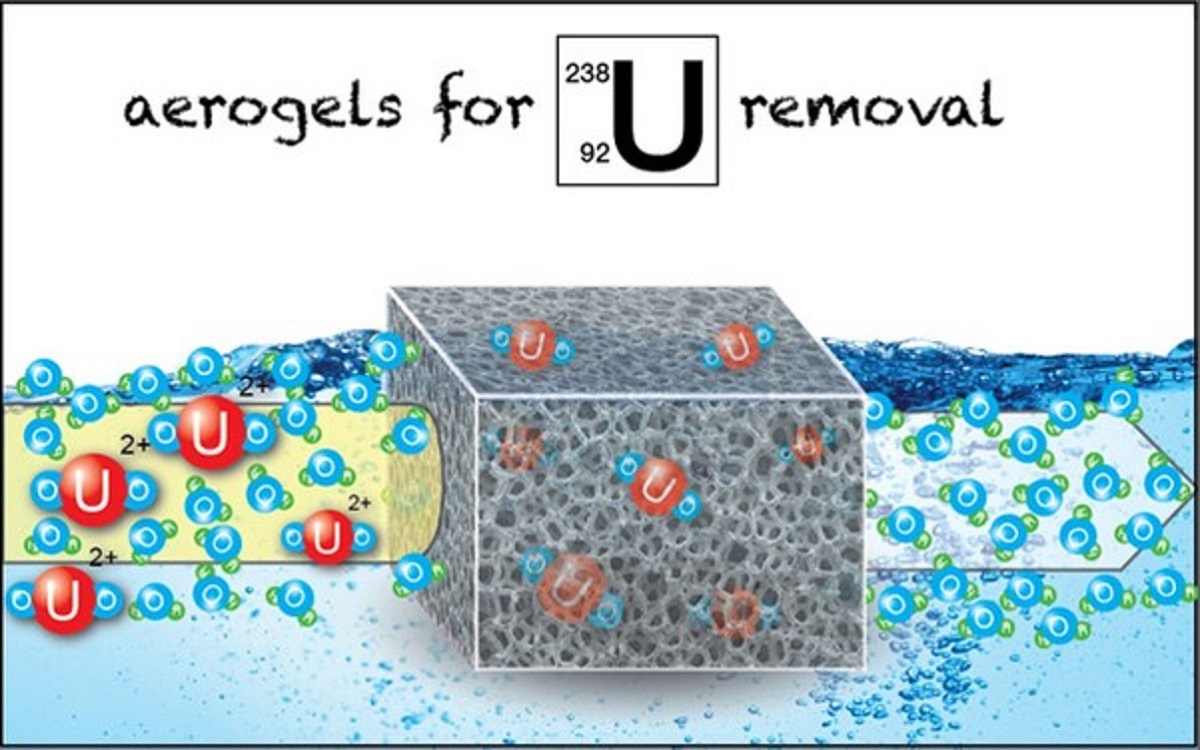
Aerogel-based adsorbents present extraordinary sorption capacity for hexavalent uranium that can be as high as 8.8 mol kg–1 (2088 g kg–1). The adsorption data follow generally the Langmuir isotherm model and the kinetic data are better described by the pseudo-second-order kinetic model, which is associated with chemisorption. Evaluation of the thermodynamic data reveals that the adsorption is generally an endothermic, entropy-driven process (ΔHo, ΔSo > 0). Spectroscopic studies (e.g., FTIR, XPS) indicate that the adsorption is based on the formation of in-ner-sphere complexes between surface active moieties and the uranyl cation. Regeneration and uranium recovery by acidification and complexation using carbonate or chelating ligands (e.g., EDTA) have been found to be successful. The application of aerogel-based adsorbents to uranium removal from industrial processes and uranium-contaminated waste waters was also successful, assuming that these materials could be very attractive as adsorbents in water treatment and uranium recovery technologies. However, the selectivity of the studied materials towards hexavalent uranium is limited suggesting further development of aerogel materials which could be modified by surface derivatization with chelating agents (e.g., salophen, iminodiacetate) presenting high selectivity for uranyl moieties.