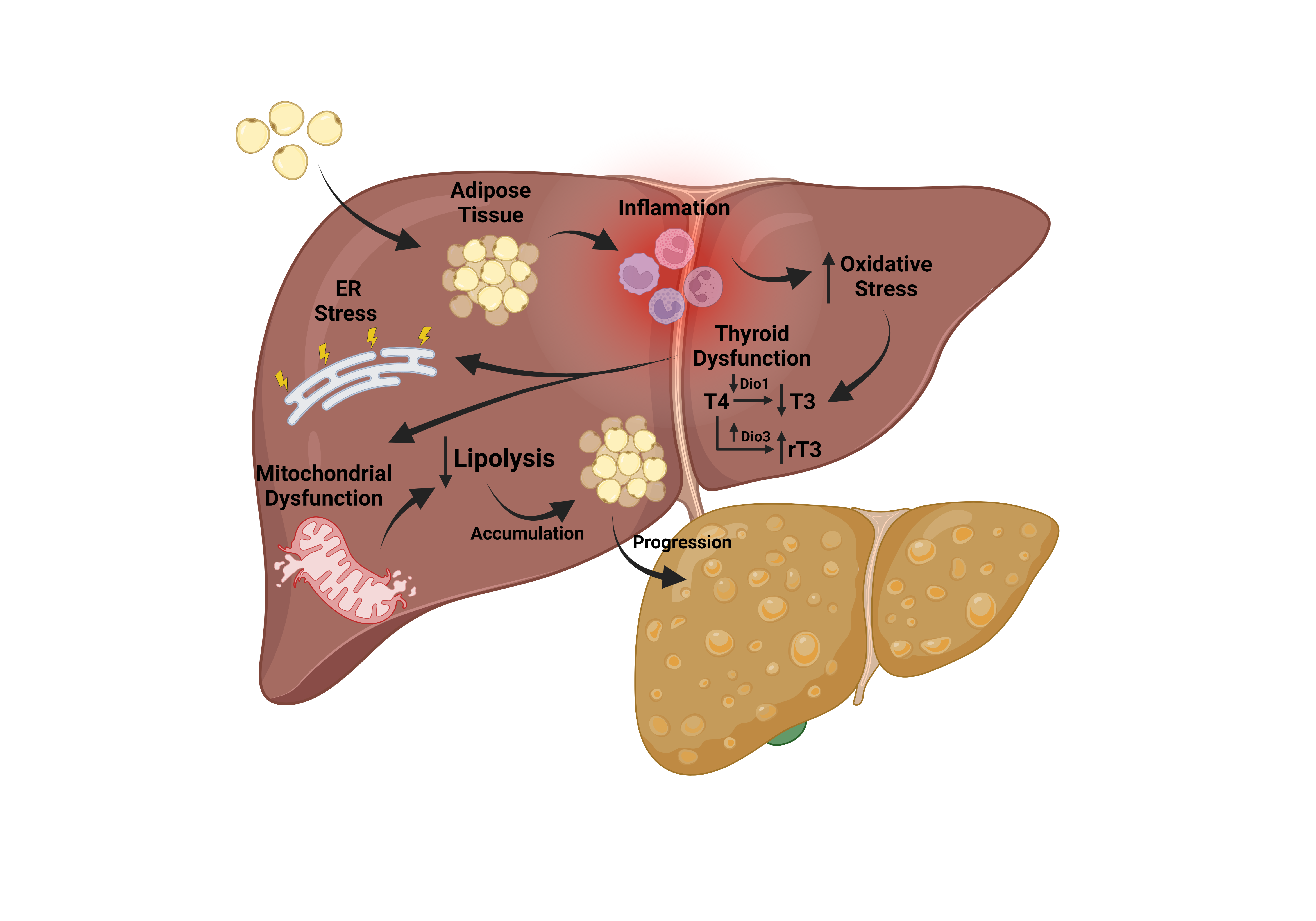
Metabolic dysfunction associated fatty liver disease (MAFLD) has gained worldwide attention as a public health problem. Nonetheless, lack of enough mechanistic knowledge restrains effective treatments. It is known that T3 regulate hepatic lipid metabolism, and mitochondrial function. Liver dysfunction of T3 contributes to MAFLD, but its role is not fully understood. Objective: To evaluate the role of T3 dysfunction in the progression of MAFLD in an animal model. Method-ology: Male/adult Sprague Dawley rats (n=20) were allocated to a control group (standard di-et–2.93kcal/g) and diet group (CDHF–4.3kcal/g). Euthanasia took place in the 28th week. D3 ac-tivity and expression, UCP2 and D1 expression, REDOX status, mitochondrial, Krebs cycle and endoplasmic reticulum homeostasis in liver tissue were measured. Results: We observed an in-crease in D3 activity/expression (P<0.001) related to increased TBARS and carbonyls and GSH reduction in the MAFLD group (P<0.05). There was a T3-dependent decrease in UCP2 expression (P=0.01), mitochondrial capacity, respiratory activity with increased endoplasmic reticulum stress in the MAFLD group (P < 0.001). Surprisingly, in and environment with lower T3 levels we observed an augmented alpha-ketoglutarate dehydrogenase (KGDH) and glutamate dehydro-genase (GDH) enzymes activity (P<0.05). Conclusion: Induced D3, triggered by changes in the REDOX state, decreases T3 availability, and hepatic mitochondrial capacity. The Krebs cycle en-zymes were altered as well as reticulum stress. Taken together, these results shed new light on the role of T3 metabolism in MAFLD and new treatment opportunities.