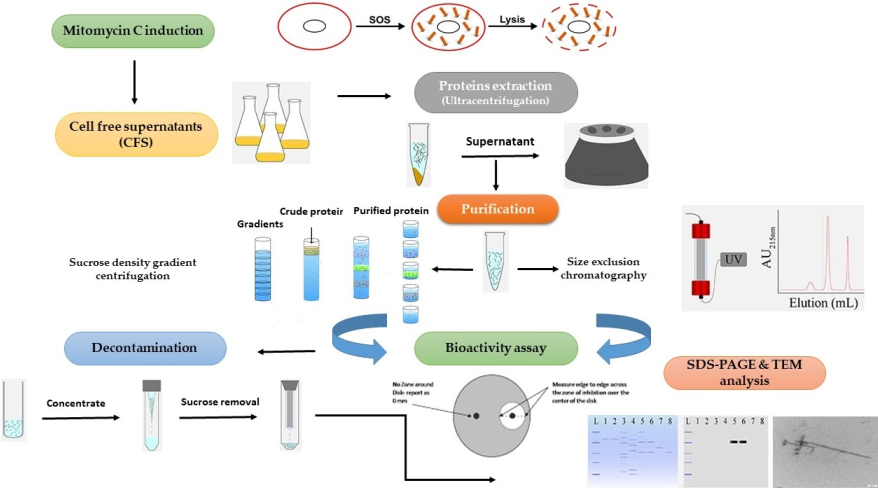
Brevibacillus laterosporus (Bl) is a Gram-positive and spore-forming bacterium belonging to the Brevibacillus brevis phylogenetic cluster. Globally, insect pathogenic strains of the bacterium have been isolated, characterised, and some activities patented. Two isolates, Bl 1821L and Bl 1951, exhibiting pathogenicity against the diamondback moth and mosquitoes, are under development as a biopesticide in New Zealand. However, due to the suspected activity of putative antibacterial proteins (ABPs), the endemic isolates often grow erratically. Various purification methods including size exclusion chromatography, sucrose density gradient centrifugation, polyethylene glycol precipitation, and ammonium sulphate precipitation employed in this study enabled the isolation of two putative antibacterial proteins of ~30 kD and ~48 kD from Bl 1821L and one putative antibacterial protein of ~30 kD from Bl 1951. Purification of the uninduced cultures of Bl 1821L and Bl 1951 also yielded the protein bands of ~30 kD and ~48 kD on SDS-PAGE which indicated their spontaneous induction. Disc diffusion assay was used to determine the antagonistic activities of the putative ABPs. Subsequent transmission electron microscope (TEM) examination of purified putative antibacterial protein-containing solution showed the presence of encapsulin (~30 kD) and polysheath (~48 kD) like structures. Although only the ~30 kD protein was purified from Bl 1951, both structures were seen in this strain under TEM. Furthermore, while assessing the antibacterial activity of some fractions of Bl 1951 against Bl 1821L in size exclusion chromatography method, population of Bl 1821L persister cells was noted. Overall, this work added a wealth of knowledge for the purification of the HMW proteins (bacteriocins) of the Gram-positive bacteria including Bl.