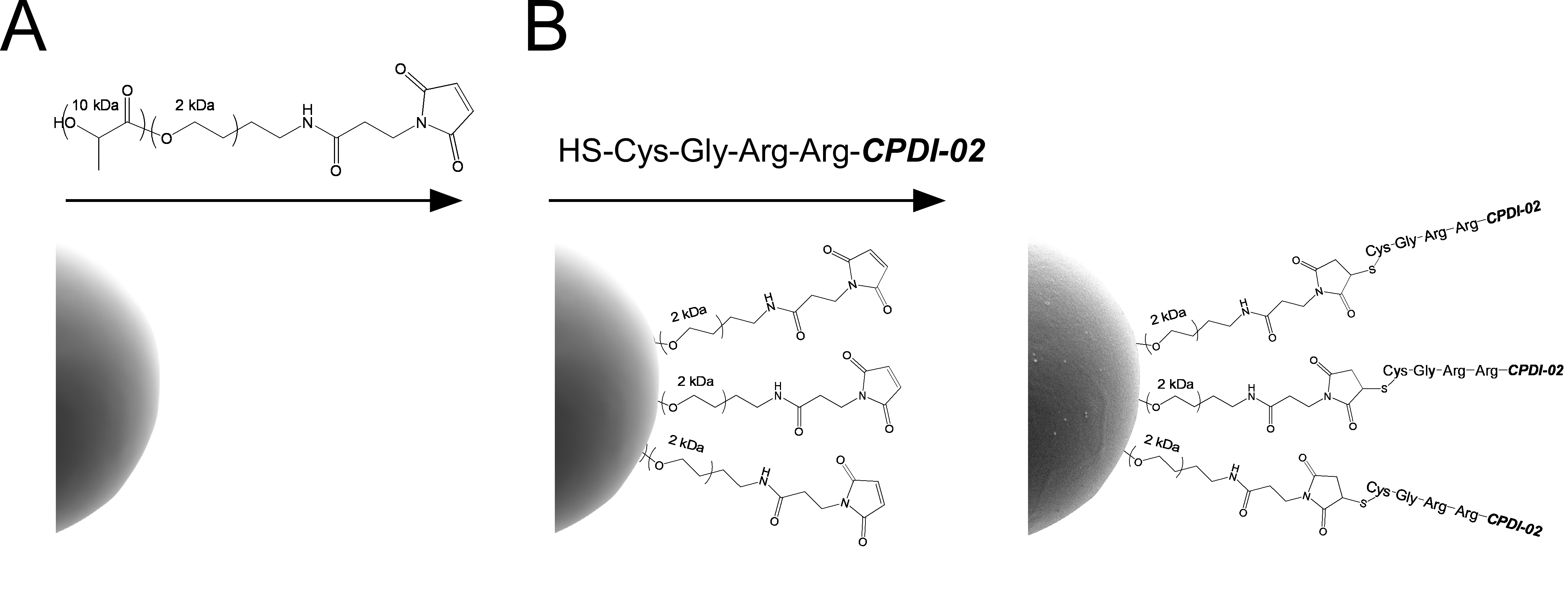
Generating long-lived mucosal and systemic antibodies through respiratory immunization with protective antigens encapsulated in nanoscale biodegradable particles could potentially decrease or eliminate the incidence of many infectious diseases but requires incorporation of a suitable mucosal immunostimulant. We previously found that respiratory immunization with a model protein antigen (LPS-free OVA) encapsulated in PLGA 50:50 nanoparticles (~380 nm diameter) surface modified with complement peptide-derived immunostimulant 02 (CPDI-02; formerly EP67) through 2kDa PEG linkers increases mucosal and systemic OVA-specific memory T-cells with long-lived surface phenotypes in young, naïve female C57BL/6 mice. Here, we determined if respiratory immunization with LPS-free OVA encapsulated in similar PLGA 50:50 microparticles (~1 μm diameter) surface modified with CPDI-02 (CPDI-02-MP) increases long-term OVA-specific mucosal and systemic antibodies. We found that, compared to MP surface modified with inactive, scrambled scCPDI-02 (scCPDI-02-MP), intranasal administration of CPDI-02-MP in 50 μL sterile PBS greatly increased titers of short-term (14 days post-immunization) and long-term (90 days post-immunization) antibodies against encapsulated LPS-free OVA in nasal lavage fluids, bronchoalveolar lavage fluids, and sera of young, naïve female C57BL/6 mice. Thus, surface modification of biodegradable microparticles with CPDI-02 is likely to increase long-term mucosal and systemic antibodies against encapsulated protein antigen after respiratory and possibly other routes of mucosal immunization.