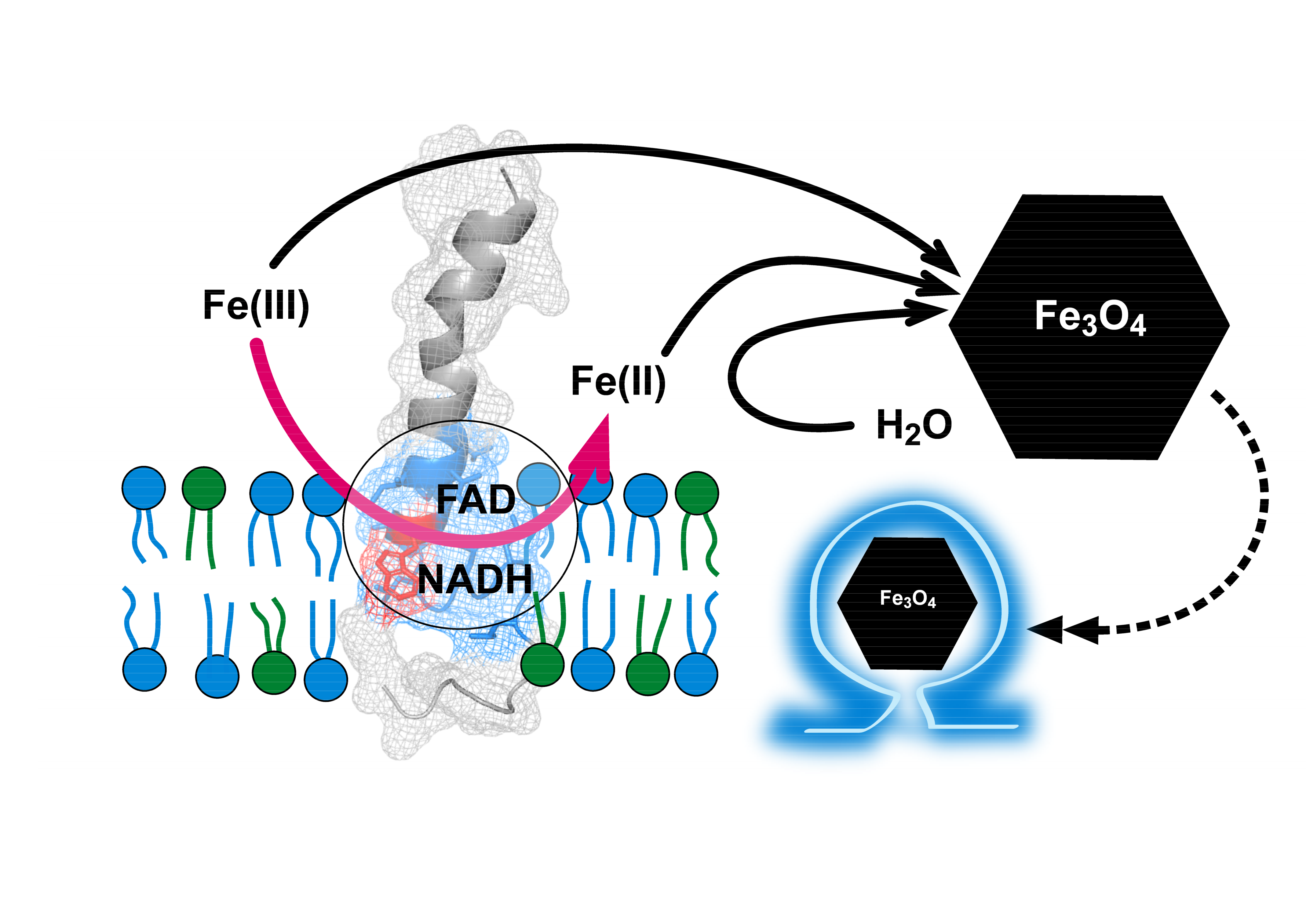
Magnetosomes of magnetotactic bacteria consist of magnetic nanocrystals with defined morphologies enclosed in vesicles originated from cytoplasmic membrane invaginations. Although many proteins are involved in creating magnetosomes, a single magnetosome protein, Mms6, can direct the crystallization of magnetite nanoparticles in vitro. The in vivo role of Mms6 in magnetosome formation is debated and the observation that Mms6 binds ferric and not ferrous iron raises the question of how Mms6 could promote the crystallization of magnetite, which contains both ferric and ferrous iron. Here we show that Mms6 is a ferric reductase that reduces ferric to ferrous iron using NADH and FAD as electron donor and cofactor, respectively. Reductase activity is elevated when Mms6 is integrated into either liposomes or bicelles. Analysis of Mms6 mutants suggests that the C-terminal domain binds iron and the N-terminal domain contains the catalytic site. Although Mms6 forms multimers that involve C-terminal and N-terminal domain interactions, a fusion protein with Mms6, which remains a monomer, displays reductase activity, which suggests that the catalytic site is fully in the monomer. These results are consistent with a hypothesis that Mms6, a membrane protein, promotes the formation of magnetite by a mechanism that involves reducing iron.