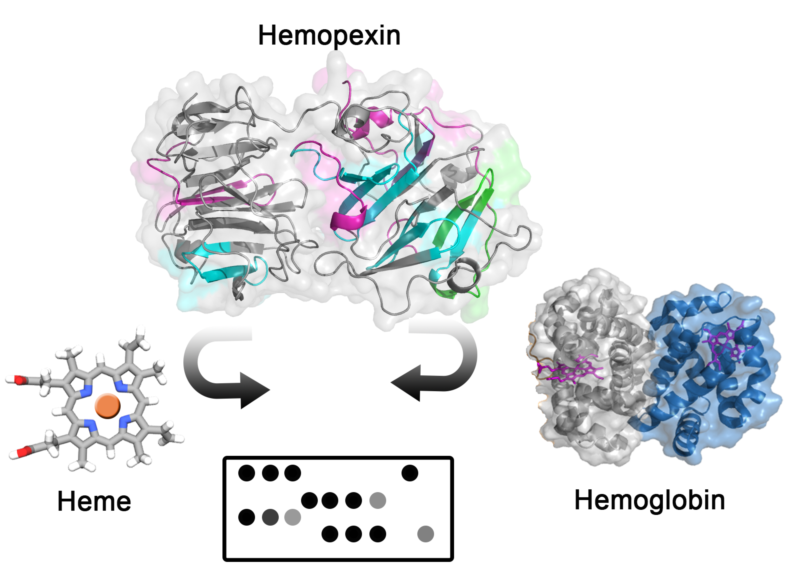
Abstract: Background: Hemopexin (Hx) is a plasma glycoprotein that scavenges heme (Fe(III) protoporphyrin IX), Hx have important implication in hemolytic disorders and hemorrhagic condition because the release of hemoglobin increase labile heme, which is potentially toxic producing oxidative stress. Hx has been considered for therapeutic use and diagnostics. In this work, we analyzed and mapped interaction sequences of Hx with hemin and hemoglobin (2) Methods: Spot-synthesis technique was used to map human hemopexin (P02790) binding to hemin and human hemoglobin, a library of 15 amino acid peptides with a 10-amino acid overlap was designed to represent the entire coding region (aa 1-462) of hemopexin and synthesized onto cellulose membranes. In silico approach was performed to analyze amino acid frequency in identified interaction regions, and molecular docking was applied for protein-protein interaction (3) Results: Seven linear peptide sequences in Hx were identified to bind hemin (H1-H7), and five were described for Hb (Hb1-Hb5) interaction, with just two sequences shared between hemin and Hb. Amino acid composition of identified sequences demonstrated that Histidine residues are relevant for heme binding, H105, H293, H373, H400, H429, and H462 was distributed in H1-H7 peptide sequences, but other residues may also play an important role. Molecular docking analysis demonstrated Hx association with the β-chain of Hb, with several hot spot amino acids that coordinated interaction. (4) Conclusions: This study highlights new insights on Hx-hemin binding motifs and protein-protein interactions with Hb. Binding sequences and identified specific peptides can be used for therapeutic purposes and diagnostics, as hemopexin is under investigation to treat different diseases, and there is an urgent need for diagnostics of labile heme for monitoring hemolysis.