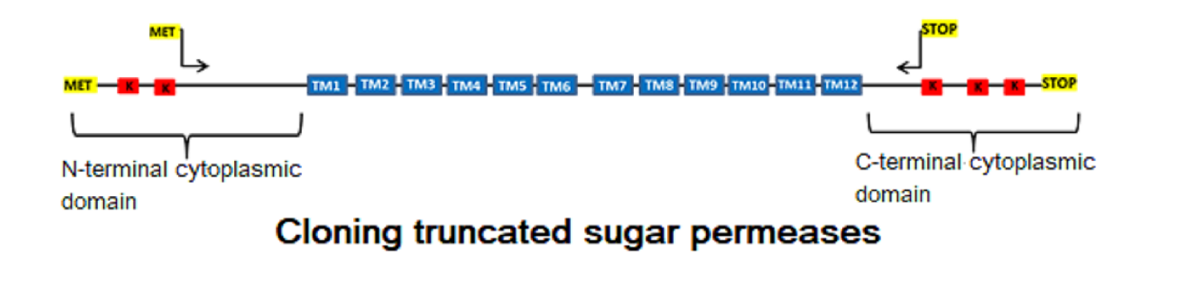
: In our previous work we had developed an hxt-null Saccharomyces cerevisiae strain displaying high xylose reductase, xylitol dehydrogenase and xylulokinase activities that proved to be useful as a chassis strain to study new xylose transporters, as SsXUT1 from Scheffersomyces stipitis. Spathaspora passalidarum and Spathaspora arborariae have in their genomes genes with high sequence similarity (78-80%) to SsXUT1. To characterize these putative transporter genes (SpXUT1 and SaXUT1, respectively) they were expressed in the same chassis strain as SsXUT1. Surprisingly, the cloned genes could not restore the ability to grow in several monosaccharides tested, although the strains expressing the SsXUT1 and SpXUT1 permeases, after growth on maltose, showed the presence of 14C-glucose and 14C-xylose transport activity. An important feature of these permeases is that SsXUT1 lacks lysine residues in its N-terminal domain with high-confidence ubiquitinylation potential, and has only one at the C-terminal domain, while the SpXUT1 transporter had several of such residues at its C-terminal domain. When the SpXUT1 gene was cloned in a truncated version lacking such lysine residues, the permease allowed grow on glucose or xylose, and even promoted xylose fermentation by the hxt-null strain. In another approach, we deleted two arrestins known to be involved in sugar transporter ubiquitinylation and endocytosis (ROD1 and ROG3), but only the rog3Δ strain allowed modest growth on these sugars. Taken together, these results suggest that to allow efficient sugar transporter expression in S. cerevisiae the lysines involved in transporter endocytosis should be removed from the sequence of the permease.