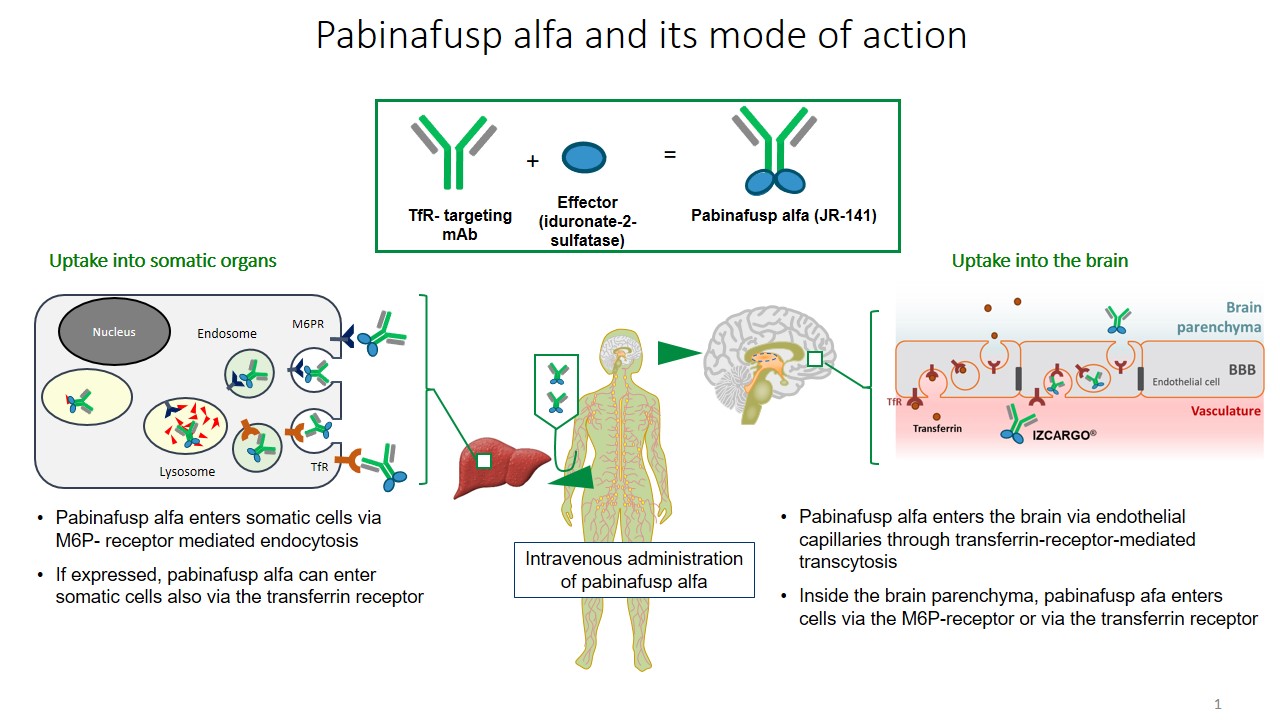
Enzyme replacement therapy (ERT) improves the somatic manifestations in mucopolysaccharidoses (MPS)).However, because intravenously administered enzymes cannot cross the blood brain barrier (BBB), ERT is ineffective against the progressive neurodegeneration and resultant severe central nervous system (CNS) symptoms observed in patients with neuronopathic MPS. Attempts to surmount this problem have been made with intrathecal and intracerebroventricular ERT intend to achieve CNS effects, but the burdens on patients are inimical to long-term multiple administrations. However, after pabinafusp alfa, a human iduronate-2-sulfatase fused with a BBB-crossing anti-transferrin receptor antibody, showed both central and peripheral efficacy in mice model, subsequent clinical trials in a total of 62 patients with MPS-II (Hunter syndrome) in Japan and Brazil substantiated this dual efficacy and provided an acceptable safety profile. To date, pabinafusp alfa is the only approved intravenous ERT effective against both the somatic and CNS symptoms of patients with MPS-II. This article summarizes the hitherto obtained preclinical and clinical evidence associated with this drug, and discusses the preclinical, translational and clinical challenges of evaluating, ameliorating and preventing the neurodegeneration in patients with MPS-II.