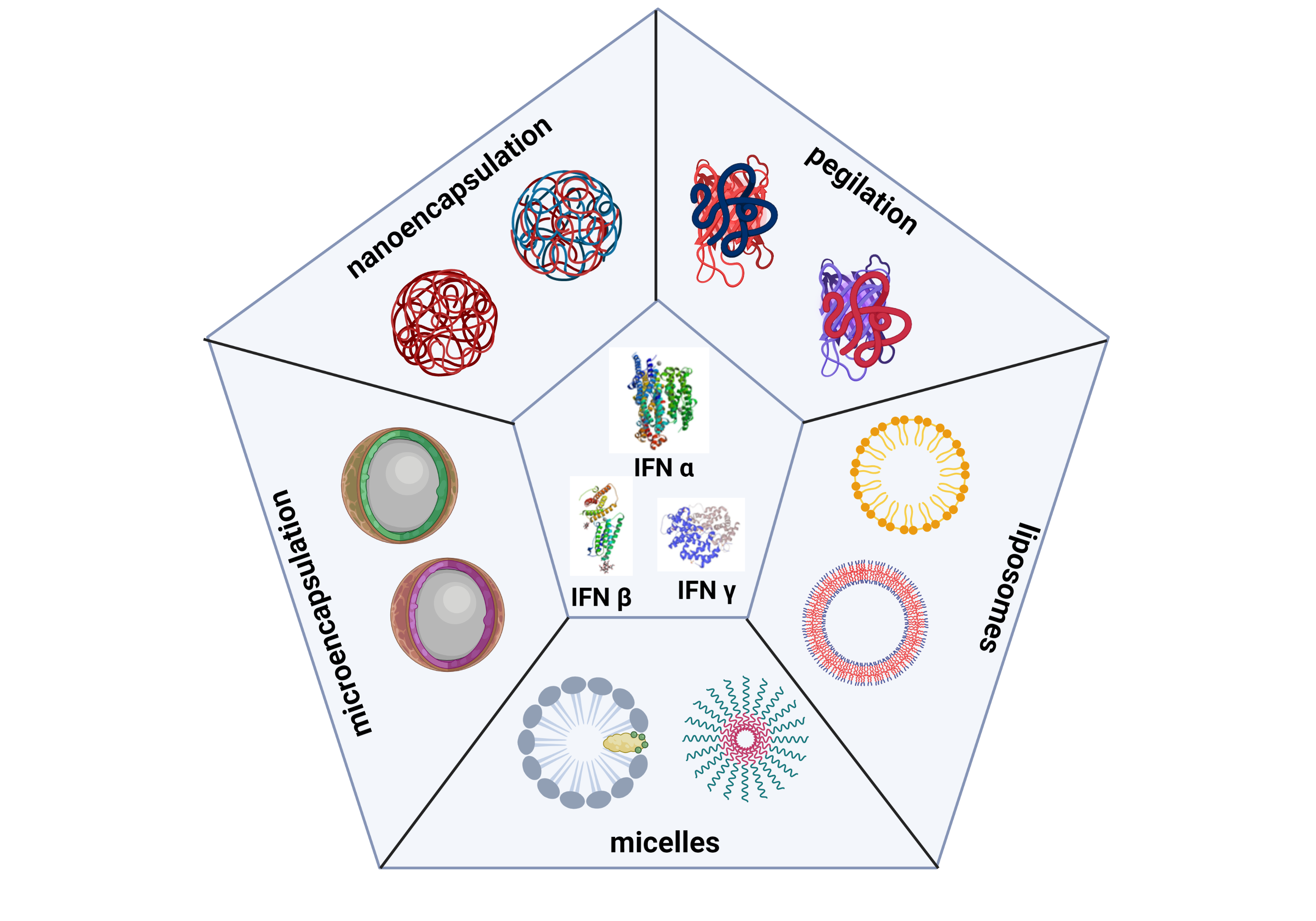
Interferons (IFNs) are cytokines involved in the immune response that act on innate and adaptive immunity. These proteins are natural cell-signaling glycoproteins expressed in response to viral infections, tumors, and biological inducers and constitute the first line of defense of vertebrates against infectious agents. They have been marketed for more than 30 years with considerable impact on the global therapeutic protein market thanks to their diversity in terms of biological activities. They have been used as single agents or with combination treatment regimens, demonstrating promising clinical results, resulting in 22 different formulations approved by regulatory agencies. The 163 clinical trials with currently active IFNs reinforce their importance as therapeutics for human health. However, their application has presented difficulties due to the molecules’ size, sensitivity to degradation, and rapid elimination from the bloodstream. For some years now, work has been underway to obtain new drug delivery systems to provide adequate therapeutic concentrations for these cytokines, decrease their toxicity and prolong their half-life in the circulation. Although different research groups have presented various formulations that encapsulate IFNs, to date, there is no formulation approved for use in humans. The current review exhibits an updated summary of all encapsulation forms presented in the scientific literature for these cytokines IFNα, IFNß, and IFNγ, from the year 1996 to the year 2021, considering parameters such as: encapsulating matrix, route of administration, and encapsulation.