Article
Version 1
Preserved in Portico This version is not peer-reviewed
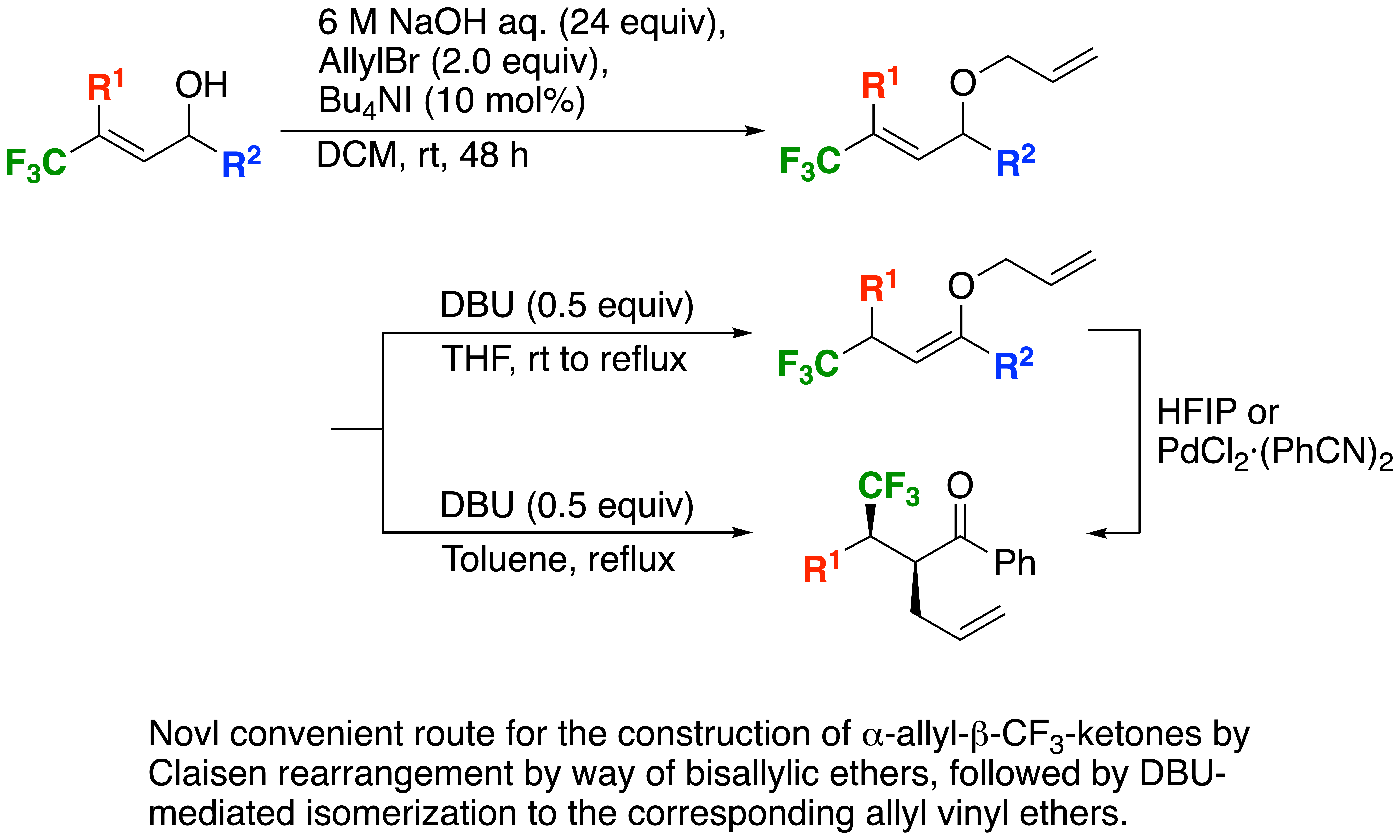
Base-Mediated Claisen Rearrangement of CF3-Containing Bisallyl Ethers
Version 1
: Received: 21 June 2021 / Approved: 23 June 2021 / Online: 23 June 2021 (11:16:15 CEST)
A peer-reviewed article of this Preprint also exists.
Hamada, Y.; Matsunaga, R.; Kawasaki-Takasuka, T.; Yamazaki, T. Base-Mediated Claisen Rearrangement of CF3-Containing Bisallyl Ethers. Molecules 2021, 26, 4365. Hamada, Y.; Matsunaga, R.; Kawasaki-Takasuka, T.; Yamazaki, T. Base-Mediated Claisen Rearrangement of CF3-Containing Bisallyl Ethers. Molecules 2021, 26, 4365.
Abstract
We have previously clarified that the strongly electron-withdrawing CF3 group nicely affected the base-mediated proton migration reactions of CF3-containinig propargylic or allylic alcohols to afford the corresponding a,b-unsaturated or saturated ketones, respectively, which was applied this time to the Claisen rearrangement after O-allylation of the allylic alcohols, followed by isomerization to the corresponding allyl vinyl ethers, enabling the desired rearrangement in a tandem fashion, or in a stepwise manner where a palladium catalyst attained an excellent diastereoselectivity.
Keywords
Claisen rearrangement; isomerization; trifluoromethyl; Cieplak rule
Subject
Chemistry and Materials Science, Analytical Chemistry
Copyright: This is an open access article distributed under the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Comments (0)
We encourage comments and feedback from a broad range of readers. See criteria for comments and our Diversity statement.
Leave a public commentSend a private comment to the author(s)
* All users must log in before leaving a comment