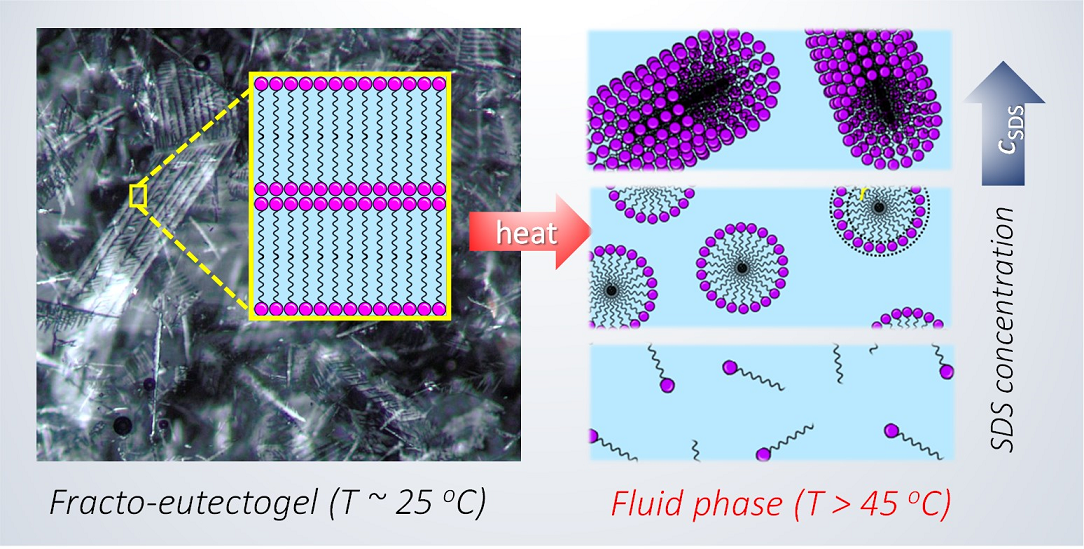
Understanding surfactant self-assembly in deep eutectic solvents (DES) is important to their potential use in industrial formulations. We have recently reported the formation of a fracto-eutectogel comprising SDS fractal aggregates at a concentration as low as 1.6 wt% in glyceline (a DES comprising glycerol and choline chloride) at room temperature. The building units of the fractals consisted of multilayers of self-assembled SDS lamellae arranged in a dendritic pattern. Here we report that this fractal phase transitions into a fluid phase above a critical gelation temperature, TCG ~ 45 oC, evident from polarized light microscopy (PLM) observations. Small-angle neutron scattering (SANS) reveals that this phase transition is underpinned by the nanoscopic morphological transformation of the SDS lamellae into cylindrical micelles at T > TGC. Fitting SANS profiles confirms that the morphology of the micelles is SDS-concentration (cSDS) dependent at T > TGC: cylindrical at cSDS > 0.6 wt% and spherical at cSDS = 0.6 wt%. At cSDS < 0.6 wt%, only isotropic scattering was observed in the SANS profiles. Such SDS self-assembly behaviors contrast with those we have previously observed in glycerol, which we attribute to the presence of ions (i.e. choline chloride) in glyceline. Our findings have general implications to surfactant self-assembly in DES, solvents that are rich in hydrogen bonding and ions.