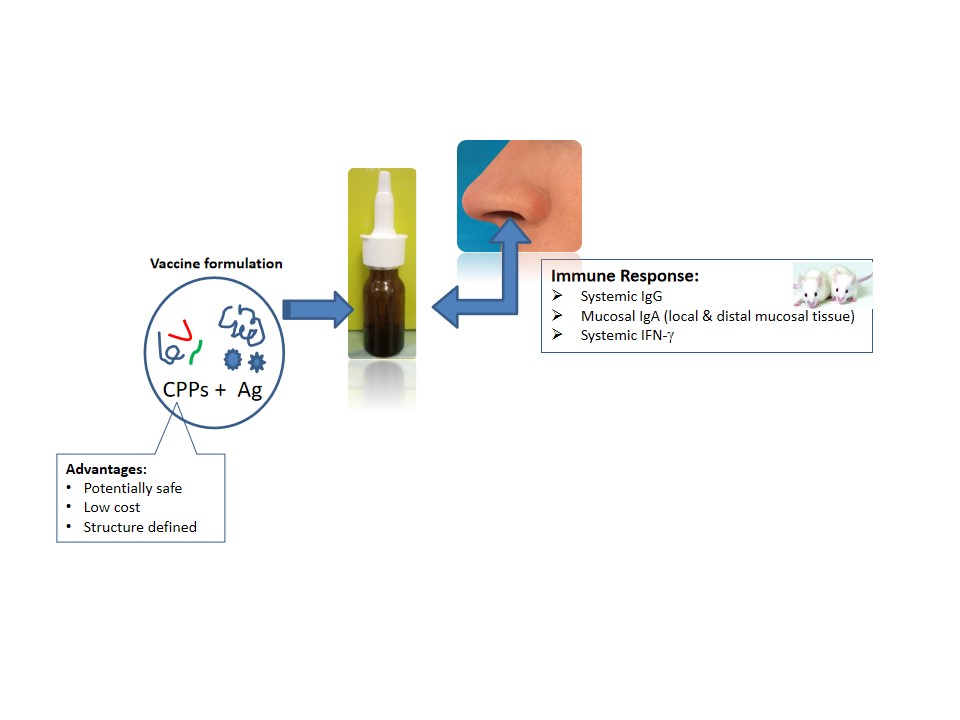
Cell-penetrating peptides (CPPs) have been evaluated as enhancers in drug delivery, their addition in medical formulations favors absorption allowing obtaining the pharmacological effect with lower drug doses. In vaccine formulations their inclusion has been also explored with interesting results. Currently mucosal vaccination constitutes a promising alternative with the main advantage of inducing both systemic and mucosal immune responses, which are crucial for control tumors and infections at mucosal tissues. The known CPP Penetratin was recently evaluated in vaccine formulations designed for nasal administration. The authors demonstrated that this non-covalent linked CPP could improve the antigen-specific systemic and mucosal antibody responses. In the present work we evaluate in Balb/C mice the nasal immune-enhancing effect of four CPPs. Animals were intranasally immunized with CPP and the recombinant hepatitis B surface protein (HBsAg) as model antigen. The IgG antibody response in sera and the mucosal IgA response were measured by ELISA. The IFN-g secretion response at spleen was also evaluated by ELISPOT and ELISA. Among the CPPs studied one novel peptide stand out by its ability to potentiate the humoral and cellular immune response against the co-administered antigen. Considering that the use of mucosal routes is a promising strategy in vaccination against infectious diseases and cancer, which are gaining special relevance nowadays in the development of novel candidates against SARS-CoV-2 and other potential emerging respiratory virus, the searching and development of safe mucosal adjuvants constitute a current need.