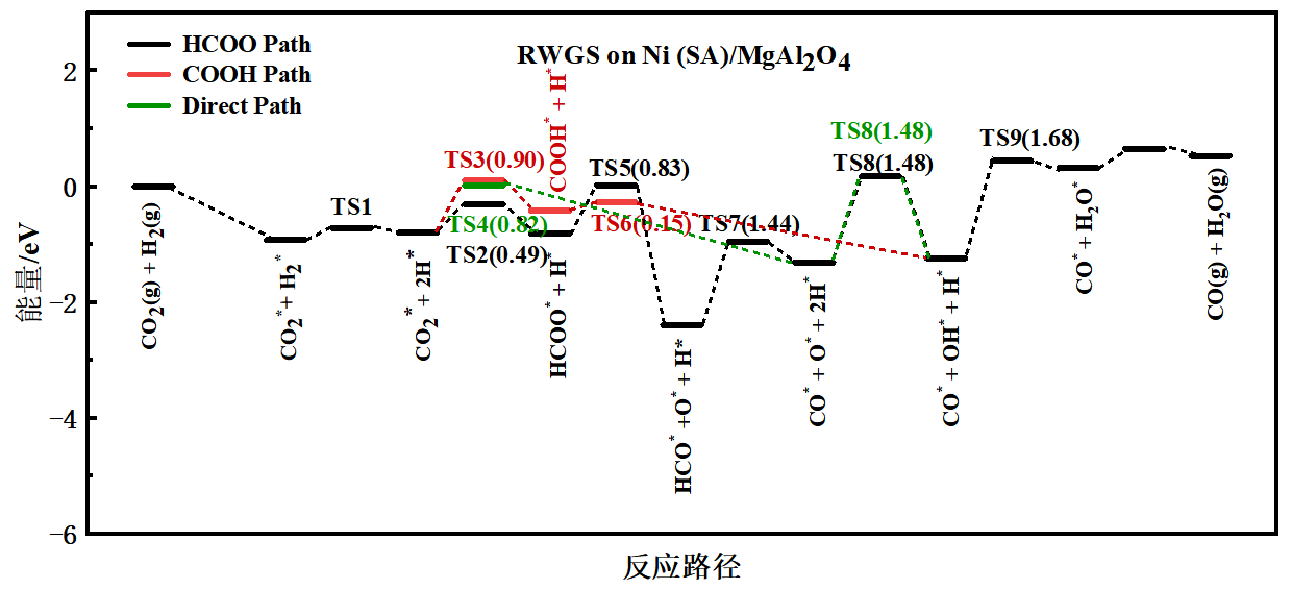
Reverse water gas shift reaction (RWGS) is an important process which plays a vital role in many CO2 utilization related reactions. Noble metals are the most active catalysts in RWGS, but the high price and low reserve strangled their applications. In the present work, we reported a non-transition-metal MgAl2O4 catalyst which showed outstanding activity and stability at high temperatures in the RWGS reaction and improved performance after doping of single atomic Nin+. The catalyst can obtain 46% of CO2 conversion in durability test of 75 h at 800 °C under high weight hourly space velocities (225 000 ml g-1 h-1). The adsorption sites, possible reaction route, and effects of Nin+ single atoms on the (111) surface of MgAl2O4 for RWGS were investigated by in situ DRIFTS and DFT calculations. The results indicated that the rate determining reaction step of RWGS on MgAl2O4 and Ni (SA)/MgAl2O4 were both the reaction of OH* + H* → H2O* + *, but the energy barrier was significantly reduced after introducing single atomic Nin+. Nin+ atoms can increase the hydroxyl coverage on the surface of catalyst and Al3+ sites near the Nin+ ion are considered as the predominant active sites for RWGS reactions.