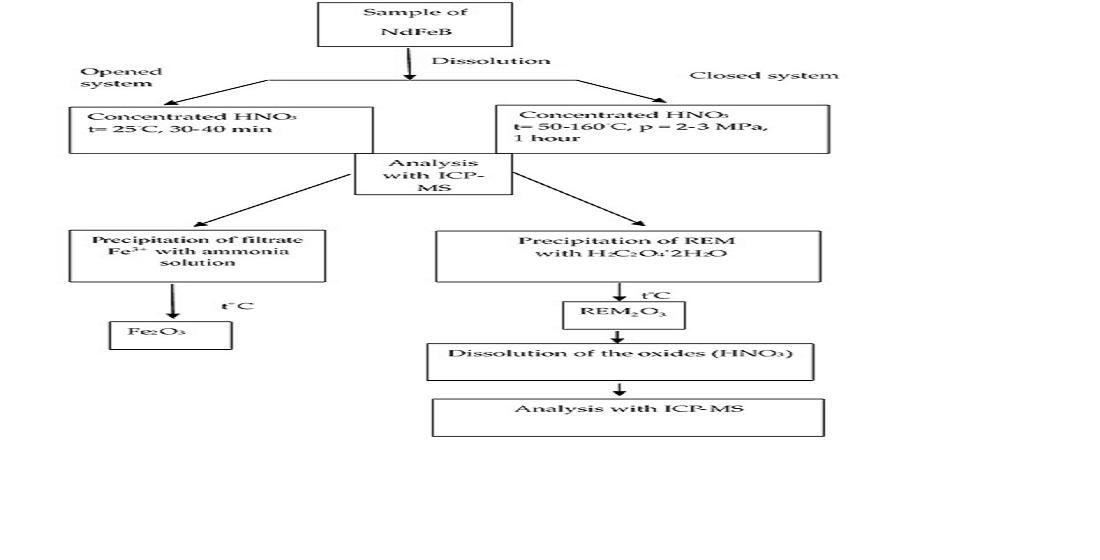
The separation of rare earth metals (REM) from a neodymium magnet has been widely studied in the last year. We have shown that the waste of computer hard disk contains 25.41 % neodymium, 64.09 % iron, and <<1 % boron. To further isolate rare-earth metals, the magnet was acidically dissolved in open and closed systems. In both methods of dissolution was used concentrated nitric acid. The difference between these methods are the conditions of dissolution of magnet. The magnet was dissolved in a microwave sample preparation system at different temperatures and pressures in a closed system. In the open system, the acid dissolution of the magnet conducted at room temperature. 0.2 g of the neodymium magnet sample was taken under two conditions, and the dissolution process in the closed system lasted 1 hour, and in the open system-30-40 minutes. The open system is a non-laborious, simple and cheap method of dissolving the magnet by comparing both systems. Therefore, an open sample preparation system is used for further work. To remove the iron in the magnet, oxalic acid was used and precipitated as oxalates under both conditions. According to the result of the ICP-MS method, it is shown that the neodymium and iron contents in the precipitate are 24.66 % and 0.06 %, respectively. This shows that the iron has almost completely passed to the filtrate. Thus, it is possible to remove the iron from the sample.