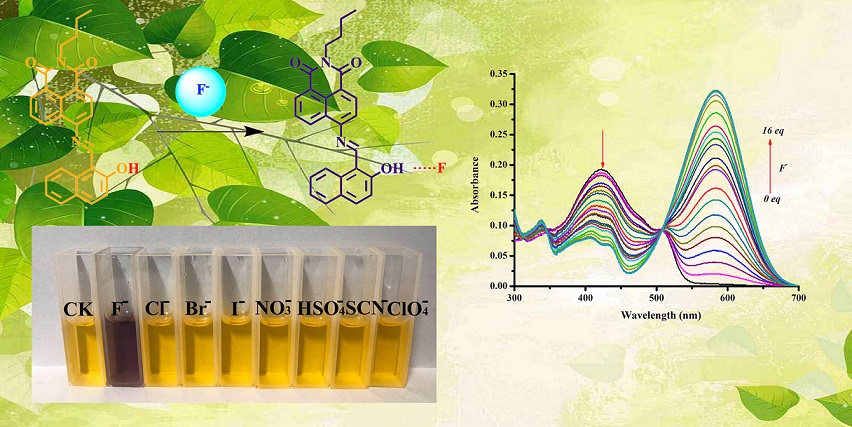
A dual-platform colorimetric and ratiometric chemosensor, namely 6-(2-hydroxynaphthalen)-N-n-butyl-naphthalimide (HNA), based on Schiff base constructed from N-n-butyl-4-amino-1,8-naphthalimide and 2-hydroxy-1-naphthaldehyde has been designed and fabricated for detecting fluoride anion (F−) in DMSO solution. HNA is a colorimetic and ratiometric probe with superior selectivity and sensitivity. The color changes of HNA from yellow to purple was observed by the naked eyes in the presence of F−. The absorbance in the UV-Vis spectra of HNA was decreasing in 422 nm, while gradually increasing in 583 nm by adding F−. The limit of detection (LOD) of HNA for detecting F− is low to 0.61 μM and the binding model of HNA and F− is in 3:2 stoichiometry. Meanwhile, HNA can be used to detect F− in real samples. Finally, the mechanism of HNA for the detection of F− was investigated. The present work indicated that HNA would be a superior potential chemosensor in monitoring F− selectively and sensitively.