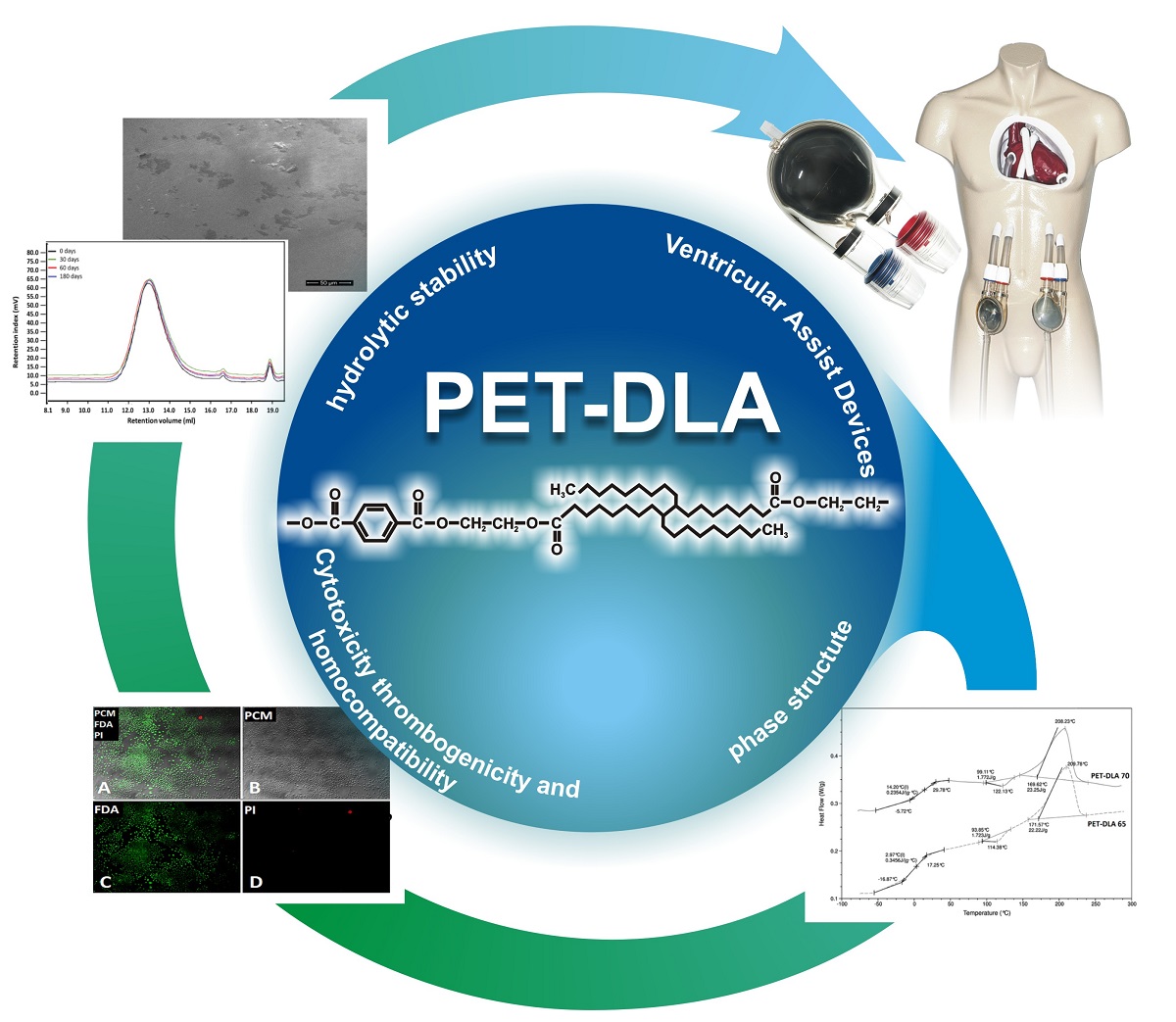
(1) Background: The evaluation of ventricular assist devices requires the usage of biocompatible and chemically stable materials. The commonly used polyurethanes are characterized by versatile properties making them well suited for heart prostheses applications but simultaneously they show low stability in biological environment. (2) Methods: An innovative material - copolymer of poly(ethylene-terephthalate) and dimer linoleic acid - with controlled and reproducible physico-mechanical and biological properties was developed for medical applications. Biocompatibility (cytotoxicity, surface thrombogenicity, hemolysis and biodegradation) were evaluated. All results were compared to medical grade polyurethane currently used in the extracorporeal heart prostheses. (3) Results: No cytotoxicity was observed and no significant decrease of cells density as well as no cells growth reduction was noticed. Thrombogenicity analysis showed that the investigated copolymers have the thrombogenicity potential similar to medical grade polyurethane. No hemolysis was observed (the hemolytic index was under 2% according to ASTM 756-00 standard). These new materials revealed excellent chemical stability in simulated body fluid during 180 days ageing. (4) Conclusions: The biodegradation analysis showed no changes in chemical structure, molecular weight distribution, good thermal stability and no changes in surface morphology. Investigated copolymers revealed excellent biocompatibility and great potential as materials for blood contacting devices.