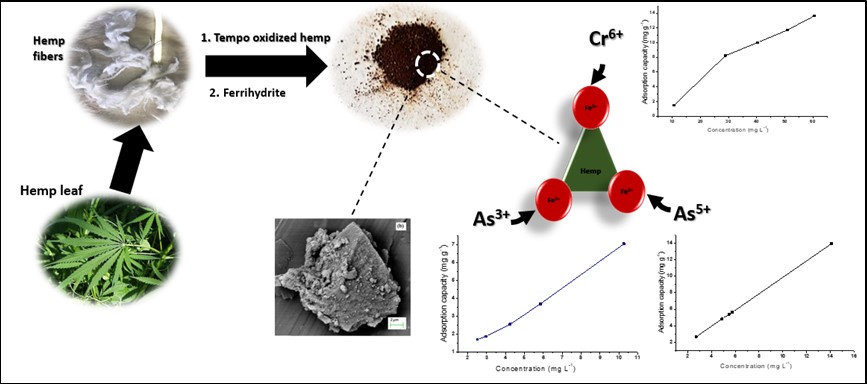
Mining-induced water contamination remains a significant concern in many regions of the world due to the high concentrations of toxic ions often associated with it. In this study, cellulose-supported ferrihydrite composites (CNF-Fe) were prepared by seeding of ferrihydrite nanoparticles on cellulose nanofibres (CNFs) and employed for the removal of As(III), As(V) and Cr(VI) from contaminated water. The adsorbent was characterized by electron microscopy, gas adsorption, point of zero charge (pHPZC), X-ray diffractometry (XRD), as well as infrared and Raman spectroscopy. Compared to parent CNFs, CNF-Fe adsorbents had lower crystallinity and a higher surface area: 218.76 m2 g-1. Further, with a pHPZC of 6.3, CNF-Fe was positively charged at low pH and suitable for adsorption of anions at acidic conditions characteristic of acid mine drainage. In single-ions solutions, the removal efficiency of CNF-Fe was in the order Cr(VI)>As(V)>As(III) (i.e. 0.15, 0.12 and 0.11 mg g-1 respectively). Adsorption kinetics followed the pseudo second-order model and isotherms were best fitted by the Freundlich, Dubinin-Radushkevich, and Temkin models. However, when CNF-Fe was applied to AMD-contaminated water (pH 2.7), Cr(VI) uptake decreased to ~39% which was likely due to competition from sulphate and selenium ions. Nevertheless, the adsorbent displayed regeneration capabilities with ~98% As and ~45% Cr desorbed after 24 hours of treatment. Together, these results suggest that cellulose supported ferrihydrite composites can be applied in treatment of mine drainage-contaminated water in conjunction with pre-treatments that limit SO42- and selenium concentrations.