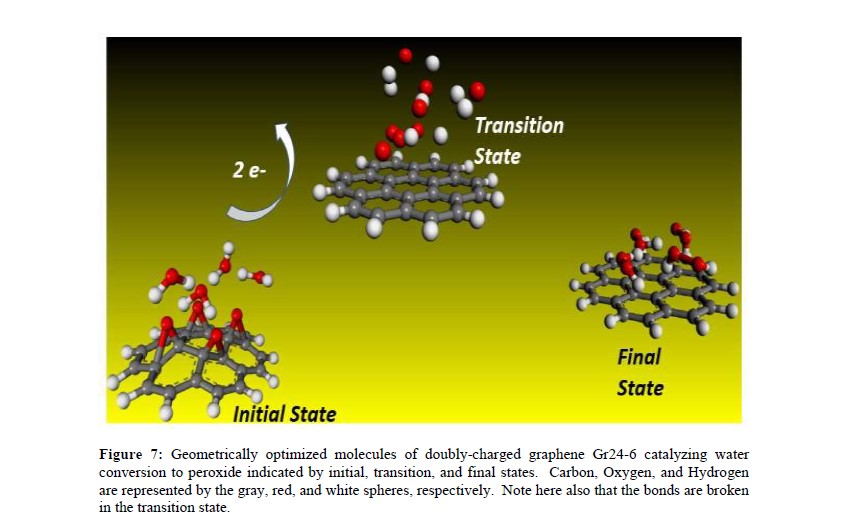
The fundamental mechanism underlying negative-ion catalysis involves bond-strength breaking in the transition state (TS). Doubly-charged atomic/molecular anions are proposed as novel dynamic tunable catalysts and demonstrated in water oxidation to peroxide. Density Functional Theory TS calculations have found tunable energy activation barrier reduction ranging from 0.030 eV to 2.070eV, with Si2ˉ, Pu2ˉ, Pa2ˉ and Sn2ˉ the best catalysts; the radioactive elements usher in new application opportunities. C602ˉ reduces significantly the standard C60ˉ TS energy barrier while graphene increases it, behaving like cationic systems. Rank-ordered catalysts according to their reaction barrier reduction efficiency, variation across charge states and systems reveal their tunable and wide applications, ranging from water purification to biocompatible anti-viral and anti-bacterial sanitation systems.