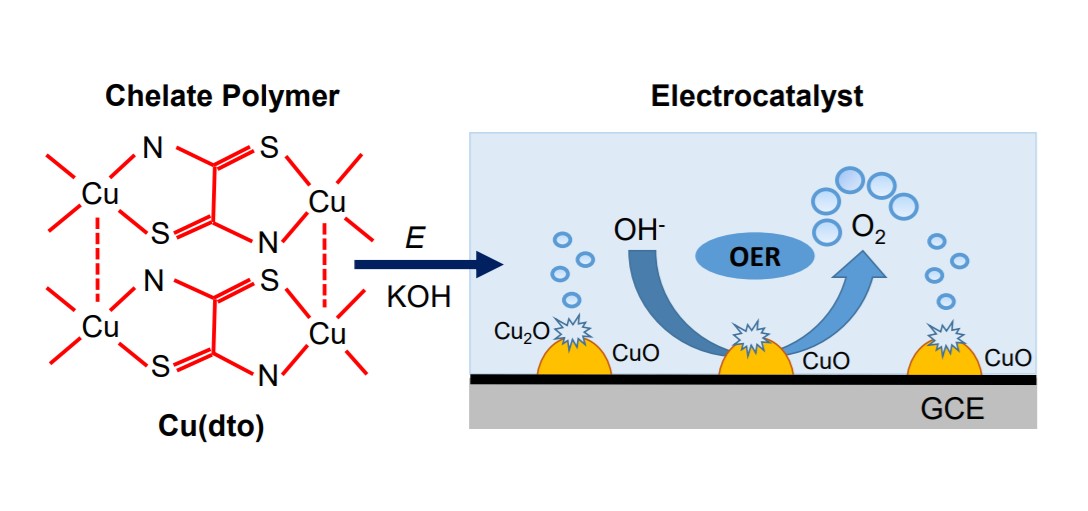
Efficient oxygen evolution reaction (OER) electrocatalysts are highly desired in the field of water electrolysis and rechargeable metal-air batteries. In this study, a chelate polymer, composed of copper (II) and dithiooxamide, was used to derive an efficient catalytic system for OER. Upon potential sweep in 1M KOH, copper (II) centers of the chelate polymer were transformed to CuO and Cu(OH)2. The carbon-dispersed CuO nanostructures formed a nanocomposite which exhibits an enhanced catalytic activity for OER in alkaline media. The nanocomposite catalyst has overpotential of 280 mV (at 1 mA/cm2) and a Tafel slope of 81 mV/dec in 1M KOH solution. It has a seven-fold higher current than IrO2/C electrode, per metal loading. A catalytic cycle is proposed, in which, CuO undergoes electrooxidation to Cu2O3 that further decomposes to CuO with releasing oxygen. This work reveals a new method to produce an active nanocomposite catalyst for OER in alkaline media using a non-noble metal chelate polymer and a porous carbon. This method can be applied to the synthesis of transition metal oxide nanoparticles used in the preparation of composite electrodes for water electrolyzers and can be used to derive cathode materials for aqueous-type metal-air batteries.